Introduction
Experiences in dryland reforestation recognize the importance of seedling quality, and particularly, belowground components (root architecture and morphology) of woody species for water stress resistance (Padilla and Pugnaire 2007; Negreros-Castillo, Apodaca-Martinez and Mize, 2010; West et al., 2012; Ovalle, Ginocchio, Arellano and Valenzuela, 2017). In xerophytic species, the ability of seedlings to survive drought periods after outplanting largely depends on roots developed during the nursery phase. Therefore, nursery practices should be designed to promote proper root development to optimize the acquisition of limited site resources after outplanting (Luis et al., 2009). Suitable plant containers for proper root system development are a key aspect in nursery production (Chirino, Vilagrosa, Hernández, Matos and Vallejo, 2008; Mariotti et al., 2015). Seedling root development is affected by shape, size, color, and container material (Dumroese and Landis, 2015). For example, a gain up to 40% in root biomass has been found when container volume is doubled (Poorter, Buhler, Van Dusschoten, Climent and Postma, 2012). In general, the propagation of native woody species in semiarid ecosystems utilizes black polyethylene bags with volumes as low as 400 mL (Aghai, Pinto and Davis, 2014). These containers do not promote lateral root self-pruning at early growth stages; therefore result in root deformation and restricted shoot growth and root depth (Dominguez-Lerena et al., 2006) and unsuitable shoot/root biomass ratios that negatively impact the water economy of seedlings (Tsakaldimi, Zagas, Tsitsoni and Ganatsas, 2005). This is particularly relevant in tree species adapted to dry environments that have fast growing taproots and long lateral and superficial roots (Ovalle, Arellano and Ginocchio, 2015). Therefore, to obtain adequate morpho-type seedlings, a container with basal root pruning and suitable size (volume) and shape (diameter and depth) should be used to prevent spiral taproot formation and to improve both generation of lateral roots, and root distribution (Chirino et al., 2008; Ovalle, Arellano, Ginocchio and Becerra, 2016; De la Fuente, Ovalle, Arellano and Ginocchio, 2017).
Objectives
The aim of the present study is to compare responses of root morphology to changes in size and shape of containers in both a deep-rooting (A. caven) and shallow-rooting species (B. linearis), two pioneer and nurse plants frequently used in reforestation programs in the semiarid Mediterranean-type conditions of central Chile.
Meterials and methods
Plant material and experimental growing conditions
Native woody species A. caven (Mol) Mol. (Fabaceae) and B. linearis (Ruiz et Pav) Pers. (Asteraceae) were chosen for their contrasting root morphologies: A. caven has a deep root system with a pivotal taproot that allows access to groundwater and B. linearis has a dimorphic root system, which uses both groundwater (main root) and rainfall (Donoso, 1982). Seedlings were produced from seeds collected from individuals growing at the Elqui Province, north-central Chile (29º 49' S-70º 48' W). Seeds of A. caven were subjected to chemical scarification using concentrated sulfuric acid (technical quality) for 120 minutes. Seeds of B. linearis do not need pregerminative treatments. The seeds were sown in A-6 perlite (Harborlite ®) followed by transplantation to container. The experimental containers were filled up to 88% of the total volume with a standard propagation mixture of one part A-6 perlite (Harborlite ®), one-part peat (Kekkilä DSM), and two parts of sandy soil. Average final seedling size (height), before transplant to experimental containers, was 3 cm for shoot, 5 cm for root system, and a pair of well-developed true leaves. Water content of containers was daily checked. They were watered with demineralized water up to 80% field capacity each time the substrate reached 40% field capacity. Seedlings were fertilized twice during the assay with 40 mL of a modified Hoagland’s solution containing 150 mg N L-1, 80 mg P L-1, and 100 mg K L-1 (Harper, Smith and Macnair, 1997). Seedlings were grown for six months, until control seedlings reached 60 cm in height, an average size equivalent to a plant of this species after one nursery season in central Chile. Environmental conditions of the plant growth room were: mean temperature of 23 °C ± 2 °C, relative humidity of 46%, and light intensity of 273 µmol s-1m-2, with a 12/12 h photoperiod.
Experimental design and treatments
A randomized experimental design was conducted with five treatments consisting of different types of containers for seedling production (Fig. 1). Sixteen replicates per treatment and species were considered. The control treatment (C) was a 12 cm × 15 cm black polyethylene bag (440 mL), commonly used in Chilean nurseries. The remaining four containers (T1, T2, T3, and T4) were made with PVC tubes (polyvinyl chloride) (Table 1). To favor basal and lateral air pruning of primary and secondary roots, a 2 mm × 2 mm plastic mesh was glued in the bottom of the PVC tube and slots were made along each PVC container (2 cm below and above the end of the tubes). PVC containers were left suspended (in contact with air) on a plastic structure. Seedling density was 82 plants per square meter. Distribution of treatments in the plastic structure was completely randomized. More details of the experimental design were presented by De la Fuente et al. (2017).

Figure 1 Photographs of the different size and shape of experimental containers used in the present study. All photos taken in November 2012 by Luz M. de la Fuente.
Table 1 General characteristics of experimental containers.
| Container | Volume (mL) | Length (cm) | Diameter (cm) | |
|---|---|---|---|---|
| Treatment | Material | |||
| C C | Black polyethylene bag | 440 | 10 | 7.5 |
| T1 | PVC | 440 | 10 | 7.5 |
| T2 | PVC | 440 | 35 | 4.0 |
| T3 | PVC | 880 | 20 | 7.5 |
| T4 | PVC | 880 | 45 | 5.0 |
Measurements
Shoot height and stem collar diameter were measured once a month in every seedling (2×5×16=160 seedlings). Six randomly selected plants per treatment were assessed after six months (2×5×6=60 seedlings). Seedling shoot total height, stem collar diameter, shoot and root dry biomass, height/stem collar diameter ratio, and shoot/root dry biomass ratio were measured. To determine dry biomass, the seedlings were carefully separated from substrate and thoroughly washed with tap water and then dried at 45 °C in a forced air oven until constant weight. Root morphology was evaluated based on scanned images (Epson Perfection 4490 Scanner, Nagano, Japan) of the root system. Taproot (A. caven) or main root (B. linearis) length considered the container depth and the presence of spiral roots. Spiral root was defined as that root that had an angle less than 90° (Ortega et al., 2006).
Root growth potential (RGP) test
The ability of roots to form new roots (longer than 1 cm) after seedling outplanting was tested according to the Ritchie (1985) procedure. After the first assay, six randomly selected seedlings from each treatment (2×5×6=60 seedlings) were transplanted into larger PVC tubes filled with A-6 perlite (Harborlite®) and watered twice a week to 100% field capacity with demineralized water. After 28 days of growth under the same plant growth conditions of the first stage assay, each PVC tube was longitudinally opened and divided into 10 cm depth sections. The new root number and biomass (dry weight) produced per section of the A-6 perlite substrate were recorded.
Statistical analysis
Data normality was verified by the Shapiro-Wilks test and homogeneity of variances by the Levene’s test. If requirements were not met, the data was either transformed or analyzed using the nonparametric Kruskal-Wallis test. Independent one-way analysis of variance was performed to evaluate the effect of types of containers and the RGP test on shoot and root growth. For each analysis, treatments with significant differences (P < 0.05) were identified with the Fisher's LSD test. Statistical analyses were carried out using InfoStat® version 2010 (Di Rienzo et al., 2012).
Results
After six months of propagation, A. caven seedlings showed significant differences between containers in shoot height, stem collar diameter, and length of the taproot (Table 2; Fig. 2). Specifically, root length, shoot height and height/stem collar diameter ratio were significantly larger in C compared to T1 (containers of equal shape (10 cm depth) and volume (440 mL)). The taproot in C was three times longer than in T1, but 67% of the plants showed spiral roots. When comparing T1 to T2 (same volume but different depth), T2 had a smaller stem collar diameter and longer taproot. The same pattern was observed when comparing T3 to T4 (Table 2). When comparing plants grown in T1 (440 mL) and T3 (880 mL), no significant differences were observed. However, in thinner containers (T2 and T4) shoot height significantly increased when volume and depth increased (T4) (Table 2).
Table 2 Morphological parameters evaluated in Acacia caven and Baccharis linearis seedlings after six months of propagation in different containers (treatments).
| Morphological parameter | Treatment * | F; P-value | ||||
|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | T4 | ||
| Acacia caven | ||||||
| Shoot height (cm) | 63.41 ± 3.69 c | 49.96 ± 3.76 ab | 40.99 ± 2.60 a | 55.50 ± 4.37 bc | 52.69 ± 3.37 b | F = 5.13 P <0.05 |
| Stem collar diameter (mm) | 4.02 ± 0.11 b | 3.91 ± 0.16 b | 3.25 ± 0.09 a | 3.96 ± 0.13 b | 3.55 ± 0.11 a | F = 7.10 P <0.05 |
| Shoot height/Stem collar diameter | 157.63 ± 8.10 b | 126.19 ± 7.38 a | 125.69 ± 6.79 a | 139.25 ± 8.92 ab | 147.88 ± 8.19 ab | F = 3.06 P <0.05 |
| Shoot biomass (g, dry weight) | 1.65 ± 0.31 | 1.47 ± 0.21 | 0.82 ± 0.11 | 1.93 ± 0.38 | 1.57 ± 0.22 | F = 2.44 P = 0.073 |
| Root biomass (g, dry weight) | 1.11 ± 0.14 | 1.20 ± 0.27 | 0.72 ± 0.10 | 1.38 ± 0.24 | 1.32 ± 0.17 | F = 1.80 P = 0.161 |
| Shoot biomass/Root biomass | 1.44 ± 0.15 | 1.34 ± 0.16 | 1.14 ± 0.09 | 1.41 ± 0.14 | 1.19 ± 0.08 | F = 1.01 P = 0.422 |
| Taproot length (cm) | 18.78 ± 3.53 b | 6.87 ± 0.28 a | 26.13 ± 0.85 bc | 12.57 ± 0.52 ab | 33.65 ± 0.51 c | F = 24.20 P <0.05 |
| Plants with spiral roots (%) | 67 | 0 | 0 | 0 | 0 | |
| Baccharis linearis | ||||||
| Shoot height (cm) | 47.88 ± 1.57 bc | 43.92 ± 1.55 ab | 39.91 ± 1.22 a | 55.94 ± 2.07 d | 53.16 ± 3.40 cd | F = 31.73 P <0.05 |
| Stem collar diameter (mm) | 4.04 ± 0.13 b | 3.97 ± 0.12 b | 3.04 ± 0.08 a | 4.71 ± 0.13 c | 3.79 ± 0.11 b | F = 31.74 P <0.05 |
| Shoot height/Stem collar diameter | 120.50 ± 5.66 ab | 111.69 ± 4.42 a | 132.00 ± 4.65 bc | 120.56 ± 6.13 ab | 140.81 ± 8.92 c | F = 3.39 P <0.05 |
| Shoot biomass (g, dry weight) | 1.92 ± 0.20 bc | 1.71 ± 0.22 b | 0.93 ± 0.08 a | 3.15 ± 0.22 d | 2.30 ± 0.17 c | F = 19.37 P <0.05 |
| Root biomass (g, dry weight) | 1.08 ± 0.09 cd | 0.83 ± 0.03 b | 0.45 ± 0.07 a | 1.25 ± 0.08 d | 0.89 ± 0.06 bc | F = 17.46 P <0.05 |
| Shoot biomass/Root biomass | 1.82 ± 0.23 a | 2.08 ± 0.29 ab | 2.16 ± 0.15 abc | 2.54 ± 0.13 bc | 2.58 ± 0.10 c | F = 3.06 P <0.05 |
| Main root length (cm) | 5.94 ± 0.74 a | 5.28 ± 0.27 a | 28.80 ± 1.27 c | 11.98 ± 0.65 b | 30.47 ± 0.87 c | F = 217.56 P <0.05 |
| Plants with spiral roots (%) | 0 | 0 | 0 | 0 | 0 | |
Mean ± standard error values are presented; different letters indicate significant differences among treatments.
* Treatment codes according to table 1.
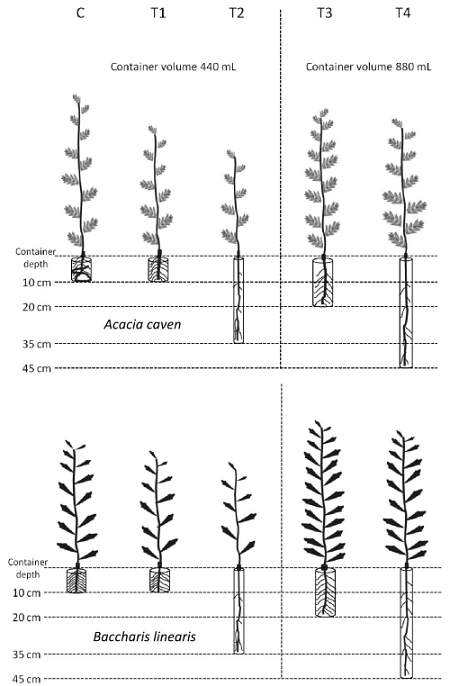
Figure 2 Scaled graphical representation of some morphological parameters obtained on seedlings of Acacia caven and Baccharis linearis grown in different types of containers at the end of experimental propagation stage (treatment codes according to table 1).
Baccharis linearis seedlings showed significant differences between containers in all morphological parameters evaluated (Table 2). When comparing C and T1, C was significantly greater in root biomass. Comparing T1 and T2 (both 440 mL, but T2 had shorter depth), the stem collar diameter, shoot biomass, and root biomass were greater in the T1 container. The same occurred when comparing T3 to T4 (Table 2). However, these differences were more evident in plants from smaller containers. T1 seedlings produced approximately 100% more biomass than T2, while T3 plants only produced 50% more biomass than T4. Regarding the main root length, significant differences were also observed; plants from the deepest containers, T2 (35 cm) and T4 (45 cm), presented the longest roots, with no significant differences between them (Table 2). When comparing T1 (440 mL) to T3 (880 mL), the plants grown in T3 were taller, had greater stem collar diameter, increased biomass, and increased length of the main root. Seedlings grown in T3 generated 85% more shoot biomass and 50% more root biomass than plants grown in T1. Shoot biomass in T4 increased 150% more than those in T2 (Table 2).
Root growth potential test
Seedlings of A. caven showed no significant differences in production of total root biomass (F = 1.26; P = 0.3112) and in total number of new roots (F = 1.50; P = 0.2328) (Table 3). Although there were no significant differences in total root biomass production and number of roots, distribution of these parameters varied with container depth (Fig. 3). The roots in shallower containers (C and T1) colonized and concentrated at shallower depths, reaching 30 cm. Roots of T3 plants colonized up to 40 cm and T4 up to 70 cm, while roots of T2 plants, which had half the volume of T3 and T4, colonized up to 60 cm in depth (Fig. 3). For B. linearis, total biomass (F = 9.15, P < 0.05) and total number of new roots (F = 8.35, P < 0.05) showed significant differences among treatments (Table 3). The lowest total root biomass and lowest number of roots were produced in plants of smaller containers (C, T1, and T2), with no significant differences among them. The largest increases in total root biomass and total number of roots were observed in larger containers, T3 and T4; T3 biomass was significantly greater than T4 (Fig. 3).
Table 3 Total dry root biomass (mg) and new roots after the root growth potential tests (RGP). Mean ± standard error values are presented; different letters indicate significant differences among treatments.
| Treatment * | Acacia caven | Baccharis linearis | ||
|---|---|---|---|---|
| Root biomass | Root number | Root biomass | Root number | |
| C | 115.9 ± 29.7 | 71 ± 15.0 | 160.9 ± 25.9 a,b | 102 ± 17.0 a |
| T1 | 87.8 ± 11.8 | 71 ± 5.0 | 190.8 ± 19.9 a,b | 149 ± 15.0 a |
| T2 | 77 ± 12.2 | 60 ± 9.0 | 112.4 ± 21.5 a | 141 ± 20.0 a |
| T3 | 109.9 ± 28.0 | 94 ± 16.0 | 334.2 ± 32.7 c | 268 ± 35.0 b |
| T4 | 147.1 ± 31.2 | 111 ± 29.0 | 233.7 ± 35.3 b | 240 ± 29.0 b |
| F and P-values | F = 1.26; P = 0.311 | F = 1.50; P = 0.233 | F = 9.15; P < 0.05 | F = 8.35; P < 0.05 |
* Treatment codes according to table 1.
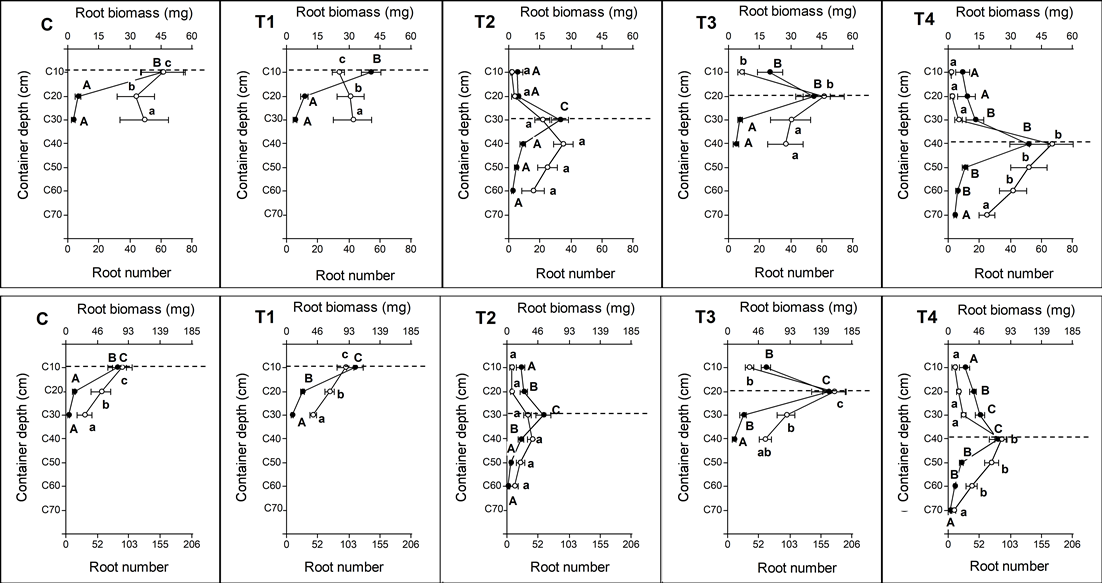
Different letters indicate significant differences among treatments for each section of the containers. The dotted line indicates the depth where the base of the first stage assay root plug was located.
Figure 3 Distribution of new root biomass (white circles and lowercase letters) and new root number (filled circles and capital letters) as result of the RGP test. Upper graphs: Acacia caven; Lower graphs. Baccharis linearis (codes according to Table 1).
Discussion
Results of this study demonstrate that change in size and shape of containers has significant impact on morphological responses of seedlings of A. caven and B. linearis, with greater influence on B. linearis. For B. linearis, the increased volume of containers had a positive effect on shoot biomass, which may be associated with increased nutrients and water availability because there exist a strong relation between the container size and the resources availability into the container (Landis, Steinfeld and Dumroese, 2010).
The plant growth differential response to containers type and size has been broadly described by a number of authors in woody species of semiarid environments, and is related to different root growth strategies presented by each particular species (Romero, Fisher and Mexal, 1986, Dominguez-Lerena et al., 2006; Chirino et al., 2008; Mariotti et al., 2015). However, also container size itself could determine changes in plant growth through decrease in photosynthesis activity per unit leaf area, independently of the woody species (Poorter et al., 2012).
When a plant has a taproot it does not normally restart its growth in depth once outplanted and in some cases the plant simply dies (Dominguez-Lerena et al., 2006). Then, the importance of growing seedling with longer taproot, through adequate container design, led to an increase in root hydraulic conductance and number of new roots colonizing the deepest layers and, consequently improve the plant ability to avoid water-stress after outplanting (Chirino et al., 2008). In the case of B. linearis, spiral roots were not detected, as it does not have a strong taproot, but a dual system, which has a rooting pattern that depends on the ease of penetration into the substrate.
Diverse approaches have been proposed for recommending better plant containers. Dominguez-Lerena et al. (2006) suggested a minimum volume of 300 mL for seedlings destined to dry environmental conditions, while Poorter et al. (2012) suggested a container size which the plant biomass does not exceed 1 g L-1. In our study, all treatments exceeded that threshold, resulting 3.5 g L-1 for large containers (880 mL) and 6.5 g L-1 for small containers (440 mL). This later implies the need to further progress in improving container design to reach an adequate balance between size and plant quality.
Another important operational issue is the cost of production of seedlings in large containers (Puértolas, Jacobs, Benito and Peñuelas, 2012; Dumroese and Landis, 2015). From this point of view, smaller containers (440 mL) could be more appropriate for the cultivation of A. caven and B. linearis; of these, T2 has the greatest advantages in that it has smaller diameter and therefore uses less space in the nursery. Smaller container also results in less planting cost due to reduced soil movement (Puértolas et al., 2012). Plants of elongated containers could present root plug disintegrate (Peñuelas and Ocaña, 1996); however, these containers did not evidence this problem during the process of transplantation to the PCR assay.
Both A. caven and B. linearis species are commonly used for restoration plans of drylands due their strong water-resistance ability (Donoso, 1982). This implies that under dry conditions is more convenient to get a larger seedling size (Cuesta et al., 2010), which is achieved using large containers (Poorter et al., 2012; Mariotti et al., 2015). The ecophysiological basis that explains the better responses of large seedlings under limited water availability is because larger seedlings had higher nitrogen remobilizable rates, which promote higher growth of new roots for water absorption (Cuesta et al., 2010).
Container shape, at constant volume, was evaluated by comparing T1 versus T2 (both 440 mL) and T3 versus T4 (both 880 mL). Elongated containers caused a decline in stem collar diameter in both species tested. In B. linearis, elongated containers caused decreased shoot and root biomass; this may be due to the longer openings in these types of containers. However, this behavior has been also observed in elongated containers that do not have slots (Korndörfer, Mosena and Dillenburg, 2008). Also, this response has been observed in other woody species where the root length is determined by the container length (Dominguez-Lerena et al., 2006; Peman, Gil-Pelegrin and Voltas, 2006). There is evidence that the root plug of an elongated container has greater contact surface with the soil, and consequently could promote a quick restart of root growth (Grossnickle, 2005).
Both species showed the capacity to develop new roots (RGP test). This capacity is essential for improving seedling survival after outplanting (Tsakaldimi et al., 2005; Trubat, Cortina and Vilagrosa, 2010). In addition, the RGP test showed that new roots were developed on sides of the root plug, which are potentially better surfaces for water absorption and nutrient uptake. Instead, thicker root growth restarted at the base of the root plug, which improves anchorage and absorbs more water from deeper soil layers. The RGP test suggested that seedling roots grown in elongated containers (T2 and T4) have advantage over shorter containers (C and T3). The reason is that under field conditions, the plants from elongated containers would colonize the deepest soil strata quicker than plants from short containers. This would help the "thick" roots to reach the deep-water reserves (Canadell and Zedler, 1995), which represent a safer water source in semiarid environments (Ehleringer and Dawson, 1992).
Conclusions
The main conclusion of the present study is that both container size and shape, in combination with the species, affect the initial plant growth. Elongated containers produced seedlings with smaller shoots but with a longer taproot or main root, a proper morphological characteristic for dryland plants. Containers that favor basal root pruning avoid taproot deformation in A. caven seedlings, a practice that is not necessary for B. linearis due to its dual root system. The increase in container volume (size) only increased the biomass of B. linearis, therefore, using a container of 440 mL and deeper than 35 cm seems more suitable for growing A. caven and B. linearis seedlings











 nueva página del texto (beta)
nueva página del texto (beta)


