Introduction
There is an effort to use beneficial microorganisms to promote plant growth in order to reduce the intensive use of agrochemicals and to contribute to the expansion of sustainable agricultural practices (Vassilev et al., 2001; Cappellari et al., 2013). Three major groups of microorganisms are considered beneficial to plant growth: mycorrhizal fungi, free-living plant growth promoting fungi and plant growth-promoting rhizobacteria (Harman et al., 2004; Malusá et al., 2012). Mycorrhizal fungi include arbuscular mycorrhizae and ectomycorrhizae of multiple fungal clades such as Glomeromycota, Ascomycota, and Basidiomycota (Kuo et al., 2014). Free-living plant growth-promoting fungi include species of the genera Trichoderma, Aspergillus and Phoma (Harman et al., 2004; Salas, 2011). Plant growth promoting rhizobacteria comprise the genera Azospirillum, Azotobacter, Bacillus, Burkholderia, Enterobacter, Klebsiella and Pseudomonas, and some endophytes such as Axoarcus, Gluconacetobacter and Herbaspirillum (Siddiqui, 2006). The action of plant growth promoting microorganisms (PGPM) was studied intensively in the last decade; increment in biomass and fruit yield of various cropped species due to direct and indirect effects was documented (Gupta et al., 2000; Saharan and Nehra, 2011). The most common direct effects are N fixation, phosphate solubilization, siderophore and phytohormone production, and indirect effects include induction of host plant resistance and antagonist against phytopathogen (Martínez-Viveros et al., 2010; Ahemad and Kibret, 2014).
Even though the inoculation of plants with PGPM is carried out commercially in agriculture, the formulation of inoculum with a reliable and consistent effect under field conditions is still problematic (Malusá et al., 2012). Successful formulation of PGPM must overcome difficulties arising from unfavorable soil temperature, humidity, salinization, UV-radiation, soil pH and water stresses (García et al., 2011). A key aspect of successful inoculation technology is to avoid losing activity of PGPM through the use of proper inoculum formulation and selection of an adequate carrier (Malusá et al., 2012). The carrier helps to deliver a suitable amount of microorganism in good physiological condition, which can then provide a suitable microenvironment and assure sufficient shelf life. Among the natural materials utilized as carriers, organic polymers are widely used (Bashan et al., 2002; Yabur et al., 2007). Organic polymers are compounds that cross-link forming a matrix, which temporarily encapsulates or immobilizes microorganisms that are then gradually released during polymer degradation (Malusá et al., 2012). The use of polymer-based encapsulation offers protection against environmental stress and consequently enhances the survival and release of microorganisms into the soil or seed (Guo et al., 2012). Materials used for polymer encapsulation ideally should be inexpensive and easily available, such as carboxymethyl cellulose, guar gum, xanthan gum, alginate and starch (Bhardwaj et al., 2000; Hernández et al., 2011).
The effect of polymer-based encapsulation on performance of PGPM has been evaluated. However there is no systematic analysis on the performance of these microorganisms when encapsulated in a polymer-based matrix. In this context, the present meta-analysis addresses the following questions: 1) does polymer-based encapsulation enhance microorganism performance? 2) is the response of polymer-based encapsulation of PGPM similar for all plant species? 3) what plant structures are most influenced when plants are inoculated with polymer-based encapsulated PGPM? 4) does the hardener or gum concentration of the polymer influence the performance of the encapsulated PGPM?
Materials and Methods
Definition of literature to search
In this meta-analysis we examined the effectiveness of using polymer-based microencapsulated agents on PGPM in different crops. The population was defined as the encapsulated and non-encapsulated microorganisms (rhizobacteria or free-living plant growth promoting fungi) used to promote growth in various crops. In the first round of literature search, studies were included if at least one of the following aspects were addressed as a core question in the work: 1) comparison of performance of polymer-based encapsulated and non-encapsulated PGPM, 2) comparison of different concentration of the polymer-based matrix or hardener for encapsulation were evaluated, and 3) evaluation of the seed germination or plant growth components were evaluated.
Collection of database and inclusion criteria
An electronic search was carried out from November 2013 to February 2014. Relevant published papers were retrieved through searches in Google Scholar and top peer review journals indexed in: Only Library, Science Direct, Springer Journal, Taylor & Francis and Wiley Online Library. For the studies search, the following combination of the keywords were used: “microcapsules”, “capsules”, “microorganisms”, “growth”, “promoting”, “germination” “bacteria”, “plant”, “sodium alginate”, “antagonist” and “fungi”.
The literature search comprehensively retrieved all relevant scientific studies reporting the effectiveness of using microencapsulated agents on PGPM for a wide variety of crops. Approximately 117 references were examined based on title, abstracts, and keywords within the first evaluation stage of this study. A subset of 91 irrelevant reports were excluded and 26 potential articles were identified to fulfill the eligibility criteria: 1) studies had to quantify the effects of polymer-based encapsulated and non-encapsulated PGPM on seed germination or plant growth variables (leaf area, leave biomass, root biomass, shot biomass, total biomass, number of leaves, root length and shot length); 2) studies had to include among their treatments the polymer-based encapsulated and the non-encapsulated PGPM, the latter was used as their respective control; 3) studies had to use natural polymers like gums to encapsulate microorganisms; 4) studies had to be written in English and be published in the peer-reviewed literature. We obtained 11 articles published between 2002 and 2012 that satisfied these criteria (Table 1; Appendix A), and from them we selected 41 cases to be evaluated by the meta-analysis.
Methodological assessment and data extraction
Before risk of bias assessment (BA) and data extraction (DE), the papers were selected through abstract screening and confirmed using the full articles. To conduct the DE the appropriate studies were analyzed and data extracted quantitatively. The data needed for analyses (sample size, means, standard deviations, F-test statistics, χ2 or p-value) were reported in the article, either in numerical or graphical form, or could be provided by the authors on request. The studies with more than one study design were duplicated and extracted as separate studies. Many studies reported multiple treatment comparisons, which were extracted as unique treatment comparisons (trials) for analytic purposes. Information extracted from each study included: 1) gum and hardener concentration, (1) type of microencapsulated agent; 2) type of microorganism; 3) type of plant species and; 4) study results. Variables taken included: leaf area (LA), leave biomass (LB), root biomass (RB), shot biomass (SB), total biomass (TB), leave number (LN), root length (RL), shot length (SL). These data were extracted for the control and treated groups.
Meta-analysis
The meta-analysis was carried out with the MetaWin 2.1 statistical program (Rosenberg et al., 2002). For performance of plant growth measures, the effect size was calculated as Hedges’s d, the standardized mean difference (Gurevitch and Hedges, 2001) between encapsulated and non-encapsulated treatment means: d=[(XO-XY)/s] J, where XO designates the mean of plant growth trait in encapsulated treatment, XY is the corresponding mean for the non-encapsulated treatment, s is the pooled standard deviation, and J is the small-sample-size bias correction factor. When the mean and standard deviations needed for calculation of Hedges’s d were presented in graphs, they were taken using ImageJ software (Rasband, 2012). In some studies these data were not available, and univariate statistics (F, χ2 or p-value) were converted into Hedges’s d estimates (Rosenberg et al., 2002).
Analyses were performed using the mixed-effects model, which assumes that studies differ due to sampling error and random variation. Given that these sources of variation are likely to be important in biological data, the mixed-effects model is preferred for these analyses (Gurevitch and Hedges, 2001). Bias-corrected bootstrap 95 % confidence intervals around the effect size were generated from 999 iterations, and effects are considered to be significant when 95 % confidence interval did not include zero. For those mean-effect sizes that were significantly different from zero, we calculated a fail-safe number (nfs) using the weighted method of Rosenberg (2005). This number represents how many additional studies of null effect and mean weight should be added to reduce the significance level of the observer mean effect to 0.05. A fail-safe number greater than 5n+10, where n is the original number of studies in the analysis, is considered robust against publication bias (Rosenthal, 1991). In addition, we examined publication bias by mean of funnel plots (Light and Pillemer, 1984). Funnel plots revealed a low probability that publication bias affected the data set. To test whether patterns differed among the explanatory variables discussed above (type of microorganism, plant species, plant growth structure, plant process, hardener and gum concentration) studies were subdivided into corresponding groups, and between-group heterogeneity was examined using the X2 statistic Qb (Gurevitch and Hedges, 2001). All figures were made using SigmaPlot version 11 from Systat Software.
Results and Discussion
The general response of the PGPM to the polymer-based encapsulation was positive [E ++ (mean effects size) = 0.8700; 95 % Bias CI (confidence interval) =0.02171 to 1.5178], although there was a clear difference among microorganism species (df=11, Q=22.6, p=0.01). The fungus Trichoderma harzianum showed negative effect, and the bacteria Rhodanobacter sp., Anthrobacter sp. and Azospirillum brasilense corrig. Tarrand et al, (1978) showed no response to the polymer-based encapsulation (Figure 1).
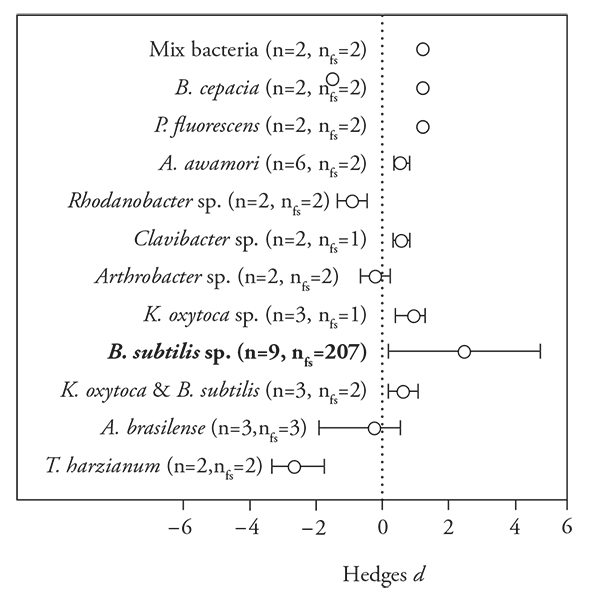
Figure 1 Mean Hedges’s d effect sizes (±95 % bias-corrected confidence intervals), sample sizes, and fail-safe numbers (nfs) for studies comparing polymer-based encapsulated versus non-encapsulated PGPM. Significant patterns occur when the intervals fail to include zero, which is marked with a dotted line. Microorganisms species in bold indicate that the fail-safe number is robust (>5 n + 10).
The effects of polymer-based encapsulated PGPM in various plant species had a positive general tendency [E ++ (mean effects size) = 0.8654; 95 % Bias CI (confidence interval) =0.3093 to 1.5350]. Differential effects among plant species were not detected (df=6, Q=6.6, p=0.35), but the magnitude of the effect showed some variation (Figure 2). For example, in Triticum sp., Vigna radiata and Gossypium sp. there was a positive trend in the response, whereas in buffalo grass [Buchloe dactyloides (Nutt.) Engelm], big salbrush [Atriplex lentiformis (Torr.) S. Watts], lettuce (Lactuca sativa L. 1753) and tomato (Solanum lycopersicum L.), there was no response (Figure 2).
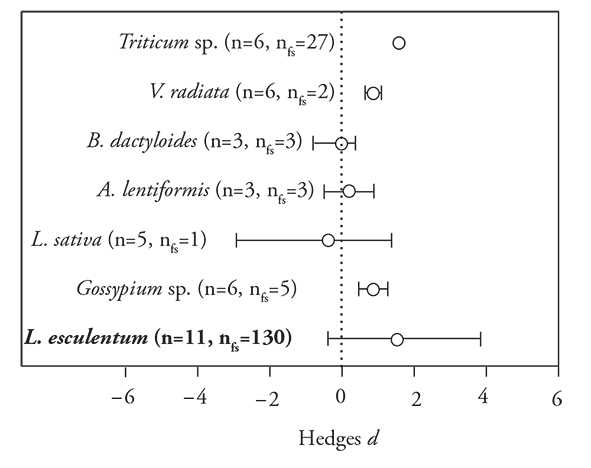
Figure 2 Mean Hedges’s d effect sizes (±95 % bias-corrected confidence intervals), sample sizes, and fail-safe numbers (nfs) for studies comparing the effects of polymer-based encapsulated versus non-encapsulated PGPM in different plant species. Significant patterns occur when the intervals fail to include zero, which is marked with a dotted line. Plant species in bold indicate that the fail-safe number is robust (> 5 n + 10).
Plant growth was separated into the analysis of different plant structures, as well as the plant process. For plant growth, the effect of polymer-based encapsulation of PGPM was positive in 67.69 % of species used in this study [E ++ (mean effects size) = 0.6769; 95 % Bias CI (confidence interval) =0.1008 to 1.3027]. The magnitude of increase in growth, however, was different among plant structures (df=4, Q=13.91, p=0.007) in which only the root mass and shoot length showed a significant effect by inoculation of polymer-based encapsulated relative to free-cell PGPM; while total plant mass, shoot mass, and root length showed no apparent effect (Figure 3a).
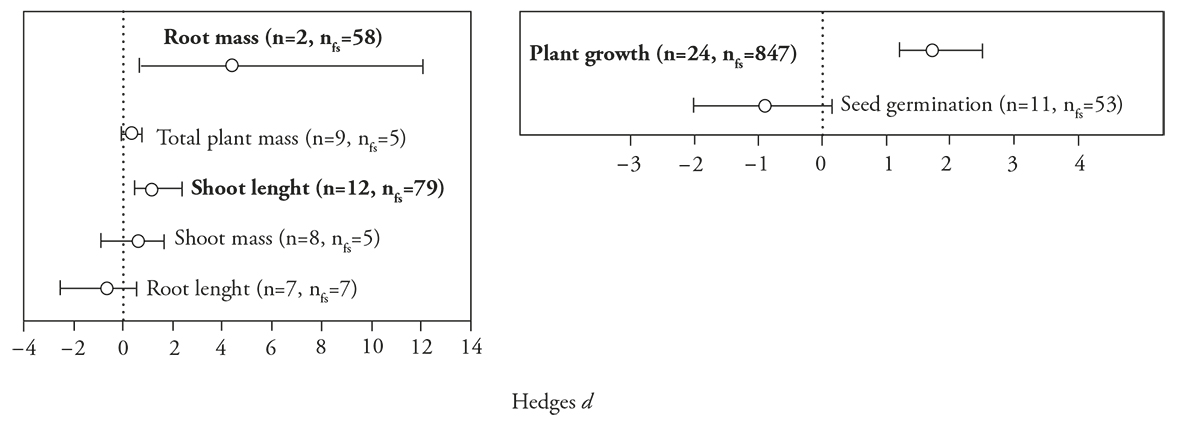
Figure 3 Mean Hedges’s d effect sizes (±95 % bias-corrected confidence intervals), sample sizes, and fail-safe numbers (nfs) for studies comparing the effect of polymer-encapsulated versus non-encapsulated PGPM on growth of different plant structures (a) and plant process (b). Significant patterns occur when the intervals fail to include zero, which is marked with a dotted line. Plant structures and process in bold indicate that the fail-safe number is robust (> 5 n + 10).
The response of plant process was categorical in comparison with plant growth and seed germination. However, the plant structures and plant process were not collinear. A trend of positive effect of polymer-based encapsulated PGPM was in general found [E ++ (mean effects size) = 0.9680; 95 % Bias CI (confidence interval) = 0.3714 to 1.6551]. The response on plant growth was highly positive, while on seed germination there was no response (df=1, Q=21.42, p ≤0.0001) (Figure 3b).
The concentrations of the hardener produced differential responses in the performance of the polymer-based encapsulated PGPM [E ++ (mean effects size) = 0.8652; 95 % Bias CI (confidence interval) = 0.3273 to 1.5450]. The effect however was not significant (df=4, Q=6.8, p=0.14), but an unimodal response for 1.1, 2 and 5.55 % (low and higher percentage of hardener) of improvement performance of polymer-based encapsulated PGPM was observed (Figure 4a). All gum concentrations, except the highest (3.5 %), showed beneficial effects on the performance of PGPM [E ++ (mean effects size) = 0.8702; 95 % Bias CI (confidence interval) = 0.2977 to 1.5245] (Figure 4b). No differential effects among gum concentrations were observed (df=3, Q=2.18, p=0.53), but the results for the concentration of 2 % were robust (Figure 4b).
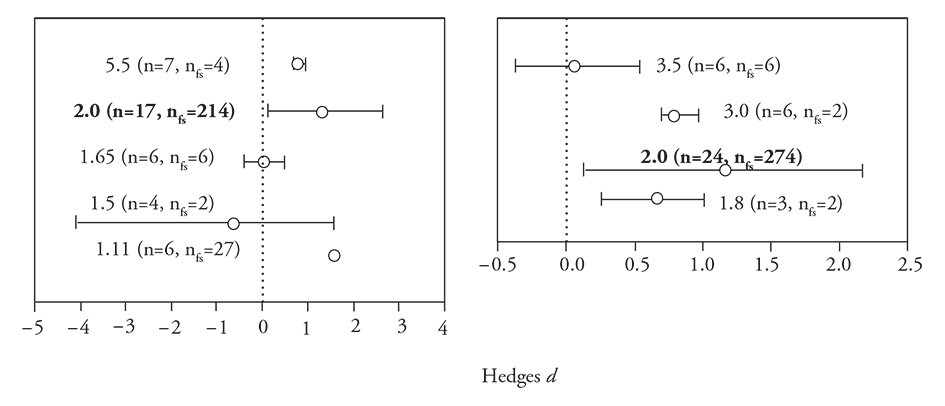
Figure 4 Mean Hedges’s d effect sizes (±95 % bias-corrected confidence intervals), sample sizes, and fail-safe numbers (nfs) for studies comparing hardener (a) and polymeric gum concentration (%) (b) for encapsulating material. Significant patterns occur when the intervals fail to include zero, which is marked with a dotted line. Hardener and polymeric gum concentrations in bold indicate that the fail-safe number is robust (>5n+10).
The present meta-analysis uncovers the effects of polymer-based encapsulation on performance of PGPM. Our main findings are as follows: 1) most of the PGPM included in the study responded positively and similarly in magnitude to polymer-based encapsulation, some of them, however, showed no response, and in the case of Trichoderma harzianum a negative response was observed; 2) the effects of encapsulation on performance of PGPM depended on the plant species inoculated, in this sense, clear trend of positive effects were observed on Triticum sp., Vigna radiate and Gossypium sp.; 3) increases in root mass and shoot length were the most common effects when plants were inoculated with polymer-based encapsulated PGPM; however, seed germination was unaffected when seeds were inoculated with polymer-based encapsulated PGPM; 4) concentration of the hardener and gum influenced the performance of PGPM when encapsulated with positive effects observed when concentrations of both hardener and polymer were 2 %, respectively. It is important to point out that in all cases the results are strongly influenced by the number of studies analyzed. As indicated by the fail safe number, most of our results were not robust enough to draw decisive conclusions (Fragkos et al., 2014), but there was a clear trend on the increase of PGPM performance when encapsulated in a polymer-based matrix. However, Belman and Wolfson (2014) consider that with a low number of articles, the case studies (41) could be a good number for meta-analysis if inclusion criteria were restricted to horticultural crop area. In spite of this, other studies could consider vote-counting techniques (where inclusion criteria used could increase to add studies to the meta-analysis) to strengthen evidence about this item (Butler et al., 2012).
Our analysis showed that in most of the PGPM species (67 %) the effects of using polymer-based encapsulating agent were positive compared to the effects of non-encapsulated PGPM. About one quarter of the species, however, showed no response to the encapsulation. It is important one case (Trichoderma harzianum) where the effect of the encapsulation was negative, which is due to bacteria having a better germination process than fungi, when used individually (El-Katatny, 2010).
The reason why not all PGPM showed positive response to the encapsulation might be related to two aspects: 1) there are species of microorganisms that naturally have a resistance per se to the harsh environment when exposed to these conditions; thus, adding a polymer-based matrix does not help to enhance the performance, specially for microorganisms that form biofilms and those that have the ability to recognize and cope with physical and chemical stressful conditions of a particular environment (Santos et al., 2010); 2) the polymer may have affected the viability or release of cells. This was observed in studies where different types of gums and species of PGPM were evaluated (Tittabutr et al., 2007).
We also observed that the response of the polymer-based encapsulated PGPM depended on, to a certain degree, the plant species evaluated. Surprisingly, in the species Lycopersicon esculentum, where several studies have been carried out, there was no positive effect of encapsulating PGPM. In this case, the germination levels influenced plant growth thus masking their positive effect following meta-analysis. This occurs because during seed germination the plant requires an immediate response from PGPM, however the onset of encapsulated PGPM action is often delayed due to the fact that the majority of the polymeric capsules are designed for prolonged release (Serban et al., 2010).
The difference in response among plant species might be attributed to aspects of the interaction between the microorganisms and plant roots. Various studies point out the importance of the association of microorganisms to plant when interacting in the rhizosphere (Saharan and Nehra, 2011). Thus, the polymer-based matrix might influence recognition of beneficial microorganisms by the plant roots, either by affecting physiological aspects of the association or by affecting the plant root system (Salamoni et al., 2010).
In our analysis we also found that when PGPM were encapsulated, plant growth was enhanced, in two ways: root biomass and shoot length. Surprisingly, we observed no effects on seed germination. This may suggest that a possible interaction of the polymer-based matrix with substrate or soil where seeds were deposited for germination could have affected the germination process and consequently the establishment of an ideal niche for the PGPM (Bashan et al., 2014). For these types of studies the soil, environmental conditions and seed characteristics must be taken into account when establishing the seeds that were coated with a polymer-based PGPM (Farnsworth, 2000). Even though we found no effect of the polymer-based encapsulation of PGPM on seed germination endpoints evaluated by this study, other aspects of seed germination might have received the benefits, such as increased seed viability under storage (Sivakumar et al., 2014).
As for our last question regarding the influence of the hardener and gum on the performance of polymer-based encapsulated PGPM, in both cases, concentrations of 2 % had clear trends of positive effects. The encapsulation of PGPM protects them from mechanical stress and harsh environmental conditions, like pH, dryness and UV (Mortazavian et al., 2007). However, when the concentrations of the hardener or the polymeric gum are inappropriate, the release and viability of the microorganisms are compromised, which in turn significantly affects the colonization of the rhizosphere by the PGPM (John et al., 2011).
Conclusions
Our analysis of literature about the effects of polymer-based encapsulation on performance of PGPM revealed differential responses in the type of microorganism, plant species, and hardener and gum concentrations evaluated. We detected specific responses for each of these groups, allowing us to describe the most important patterns. The PGPM Bacillus subtilis showed a strong positive effect when encapsulated in a polymer-based matrix. Plant species did not show clear pattern of responses, but there was a trend of positive effect in Triticum sp., Vigna radiate and Gossypium sp. Stronger positive responses were detected on root mass and shoot length, but we detected no responses on seed germination. Finally, we found that 2 % concentration for the hardener and the polymeric gum provided significant positive effects when encapsulating PGPM.











 texto en
texto en 



