1. Introduction
The Colombian Andes, located in the northwest of South America, are divided into three mountain ranges separated by valleys, namely from east to west: the Eastern Cordillera, the Magdalena River Valley, the Central Cordillera, the Cauca River Valley, and the Western Cordillera (Figure 1A ). The western flank of the Central Cordillera, the Cauca River Valley, the Western Cordillera, and the Baudó Range make up the geographic region called “Western Colombia” (Restrepo and Toussaint, 1973; Bourgois et al., 1987; Moreno-Sánchez and Pardo-Trujillo, 2003). Due to its high potential for precious metals, mainly gold, silver and Platinum-Group Elements (PGE), this region has been largely explored since pre-hispanic and colonial times.
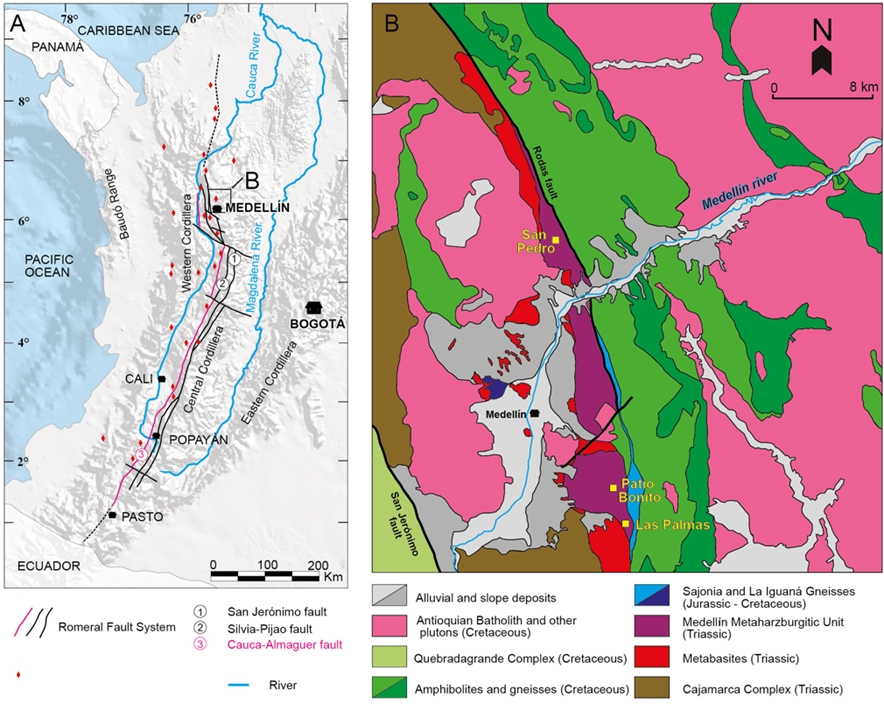
Figure 1 (A) Distribution map of peridotite bodies and mafic-ultramafic rock associations in Colombia (modified from Correa-Martínez, 2007; Gómez et al., 2015). (B) Geological map of the Medellín region (modified from García-Casco et al., 2020 and references therein). The location of the studied samples is shown in the map.
Western Colombia is characterized by the occurrence of several ultramafic bodies (Figure 1A, some of them interpreted as: (1) fragments of an ophiolite mantle sequence (Restrepo and Toussaint, 1973; Álvarez, 1987; Bourgois et al., 1987; Correa-Martínez, 2007) and (2) mantle portions of the Caribbean-Colombian oceanic plateau (Nivia, 1987; Kerr et al., 1996; Serrano, 2009). The main ultramafic body of the Central Cordillera of Colombia is historically known as the Medellin Dunite (e.g., Restrepo and Toussaint, 1984; Álvarez, 1987). It is located in the western flank of the Central Cordillera, northeast of Medellín (Department of Antioquia; Figure 1A) and is formed by peridotites metamorphosed at amphibolite facies conditions (ca. 600 ºC, <6 kbar; Restrepo, 2008; García-Casco et al., 2020 and references therein). This ultramafic body has been interpreted as the mantle section of the so-called Aburrá Ophiolite (Correa-Martínez, 2007). Using a bulk-rock major element geochemical approach, García-Casco et al. (2020) inferred that this ultramafic body is mainly harzburgitic, and that the term Medellin Metaharzburgitic Unit (MMU) is more appropriate than the historical “Medellin Dunite” term. The MMU hosts the best-known chromian spinel mineralization of Colombia, including the large deposit of Patio Bonito, located in the southern area of the ultramafic body (Figure 1B). This ore deposit contains refractory grade chromian spinel (Al-rich; Proenza et al., 2004a; Correa-Martínez, 2007).
The origin of the MMU and its associated Cr-PGE mineralization has been a matter of debate for various decades (e.g., Restrepo and Toussaint, 1973, 1984; Restrepo, 1986, 2008; Álvarez, 1987; Correa-Martínez and Nilson, 2003; Proenza et al., 2004a; Pereira et al., 2006; Correa-Martínez, 2007; García-Casco et al., 2020 and references therein). Proenza et al. (2004a) and Correa-Martínez (2007) suggested that the MMU represents a fragment of oceanic lithospheric mantle formed in a supra-subduction environment. Pereira et al. (2006) documented a high PGE content in two samples of metadunite (up to 1.2 ppm), mainly of Pt, Pd, and Rh, suggesting a primary magmatic origin of the PGE content, chromian spinel and pentlandite. Accessory chromian spinel in ultramafic rocks provides valuable information about the petrogenesis of the host rock. Nevertheless, to date, there are no detailed studies of the textural and compositional characteristics of the accessory chromian spinel of the MMU, as well as its alteration.
This paper focuses on the study of the chromitite bodies hosted in the MMU. We have studied mineralogical, petrological and geochemical characteristics of chromitites and associated metaperidotites from Las Palmas, Patio Bonito and San Pedro, which cover the southern and northern sections of the ultramafic body (Figures 1B and 2).
2. Geological setting
2.1. Mafic/Ultramafic rock associations in The Colombian Andes
The central part of the Colombian Andes (Central Cordillera) comprises a metamorphic basement overlain by Mesozoic and Cenozoic sedimentary successions intruded by plutons of different ages, ranging from the Triassic to the Neogene (Feininger et al., 1972; Vinasco et al., 2006). In the western part of the Central Cordillera, there is an important tectonic boundary between Cretaceous oceanic crust to the west and the Paleozoic metamorphic continental basement to the east, called the Romeral Shear Zone (Figure 1A; Case et al., 1971; McCourt et al., 1984; Nivia, 1996; Vinasco, 2019). This complex shear zone consists of three main faults (from E to W): San Jerónimo, Silvia-Pijao and Cauca-Almaguer (Maya and González, 1995). The Cauca-Almaguer fault is considered to be the tectonic boundary between the Cretaceous rocks of oceanic affinity and the Paleozoic basement of the Central Cordillera (Nivia, 1996, 2001; Moreno-Sánchez and Pardo-Trujillo, 2003; López et al., 2009). A series of mafic-ultramafic bodies of different ages lie on both sides of the Cauca-Almaguer fault (Figure 1A) (Restrepo and Toussaint, 1973; Bourgois et al., 1987; Nivia, 1993; Moreno-Sánchez and Pardo-Trujillo, 2003).
The mafic-ultramafic bodies located to the west of the Cauca-Almaguer fault are associated with volcano-sedimentary rocks of oceanic affinity and are mainly considered to be fragments of a Cretaceous oceanic plateau (Nivia, 1987, 1996; Kerr et al., 1996; Serrano, 2009). Nivia (1993) grouped these bodies into the Western Cretaceous Lithospheric Province. On the other hand, the majority of the mafic-ultramafic bodies and complexes located to the east of the Cauca-Almaguer fault are interpreted as of ophiolitic origin (Restrepo and Toussaint, 1973; Álvarez, 1987; Bourgois et al., 1987; Correa-Martínez and Martens, 2000).
The Aburrá Ophiolite (Correa-Martínez, 2007; Figure 1B) is one of the mafic-ultramafic units that crops out to the east of the Cauca-Almaguer fault. It lies to the west of the low-grade metamorphic rocks of the Cajamarca Complex (Maya and González, 1995) that represents the paleo-continental margin. The Aburrá Ophiolite comprises ultramafic rocks that form the Medellin Metaharzburgitic Unit (MMU) and several mafic units, which include the El Picacho Metagabbros and the Espadera-Chupadero Amphibolites (Correa-Martínez and Martens, 2000; Correa-Martínez and Nilson, 2003; Correa-Martínez et al., 2004, 2005; Correa-Martínez, 2007; Restrepo, 2008). The contacts between the different units of the Aburrá Ophiolite are tectonic and the MMU overthrusts the Espadera-Chupadero amphibolites (Figure 1B) (Restrepo and Toussaint, 1973; Rodríguez et al., 2016).
2.2. Medellin metaharzburgitic unit (MMU)
The MMU covers approximately 71 km2 and represents the main ultramafic body of ophiolitic affinity in the Colombian Central Cordillera (Figure 1B), which is in fault contact with Jurassic Sajonia mylontic gneisses and Cretaceous amphibolites (Figure 1B; Restrepo, 2008; Rodríguez et al., 2016). This unit has been of special interest for chromium mining and exploration since it is a host for several small bodies of chromitite that were exploited over various decades (e.g., Botero-Restrepo, 1945; Hall, et al., 1970; Álvarez, 1987). Restrepo and Toussaint (1973) interpreted the ultramafic rocks of the MMU as part of an obducted ophiolite. Correa-Martínez and Nilson (2003) considered this unit to have formed in a subduction zone environment, and suggested that the origin of the MMU can be related to a Triassic back-arc basin. However, the age and the geodynamic setting of the ophiolite formation still remains a subject of debate (see Rodríguez et al., 2016; Spikings and Paul, 2019; García-Casco et al., 2020 and references therein). Correa-Martínez (2007) obtained an age of 216.6 ± 0.36 Ma (Late Triassic) in zircons from a plagiogranite dike intruding the El Picacho metagabbros, which was interpreted as the minimum age for the formation of the ophiolite oceanic crust. In addition, the age of emplacement of the Aburrá ophiolite onto the continent is still unknown, but a minimum pre-Cretaceous age is constrained by the intrusion of several apophysis of the Cretaceous Antioquia Batholith (Figure 1B) (Feininger and Botero, 1982; Correa-Martínez, 2007; Rodríguez et al., 2016).
The MMU comprises mainly metaharzburgites and minor metadunites, containing tremolite, talc, chlorite, fine-grained recrystallized olivine, serpentine group minerals, magnetite, and chromian spinel, and to a lesser extent, carbonates, anthophyllite and relicts of magmatic olivine, chromian spinel and orthopyroxene (e.g., Álvarez, 1987; Restrepo and Toussaint, 1984; Correa-Martínez, 2007; Restrepo, 2008), indicating medium-grade metamorphic conditions (ca. 600 ºC). García-Casco et al. (2020) analyzed two possible geodynamic settings in order to explain the metamorphism of the MMU and associated metabasites: i) ocean-floor metamorphism (e.g., Correa-Martínez, 2007), and ii) intra- back-arc subduction-initiation metamorphism, which supposes a new tectonic scenario for the MMU (García-Casco et al., 2020).
2.3. The chromitite bodies
Chromitite bodies occur mainly as centimetric to metric pods, but also as dikes, lenses, and disseminated schlieren (Álvarez, 1987), showing massive to disseminated textures. Chromitites usually show sharp contacts with the enclosing strongly serpentinized dunite (metadunite) and are concordant to subconcordant with the foliation of the host ultramafic rock (Correa-Martínez, 2007). The ore bodies are small, containing a maximum tonnage estimate of ~20000 tons of ore. They were exploited during the 1970’s-1980’s by metallurgical and glass industries (Correa-Martínez, 2007). The Patio Bonito deposit was the largest in the area, with 30 m length and up to 7 m width (Álvarez, 1987). Artisanal mining activity was reactivated in the 2000’s with the opening of small mines and quarries in the northern and southern bodies (Correa-Martínez, 2007).
3. Studied samples and analytical techniques
A total of 19 representative samples from Las Palmas, San Pedro and Patio Bonito outcrops (Figure 2) were selected to be studied on polished thin sections using optical microscopy (transmitted and reflected light). These include metaharzburgites and metadunites from Las Palmas, San Pedro and Patio Bonito also chromitites at San Pedro and Patio Bonito. The textural study of the chromian spinel alteration and the mineralogical characterization of the fine grain phases was carried out with Scanning Electron Microscopy with Energy-Dispersive X-Ray Spectroscopy (SEM-EDS) using the ESEM Quanta 200 FEI, XTE 325/D8395 at the Serveis Científics i Tecnològics (CCiTUB), Universitat de Barcelona. For the mineral chemistry analyses, the JEOL JXA-8230 electron microprobe was used at the same institution. Operating conditions were set to an acceleration voltage of 20 kV and a beam current of 20 nA. The elements were acquired using the analyzing crystals: LiF for Fe, Mn, and Ni; TAP for Mg and Al; PET for Cr, V, and Ti. The standards used were chromian spinel (Cr, Al, Fe), periclase (Mg), rhodonite (Mn), nickel oxide (Ni), rutile (Ti), metallic vanadium (V), albite (Na), wollastonite (Ca), and orthoclase (Si, K).
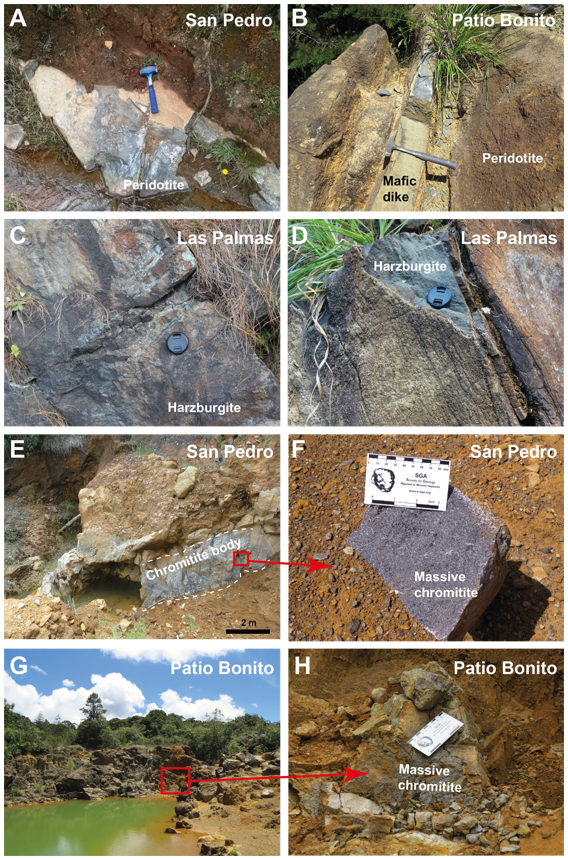
Figure 2 Field photographs of San Pedro, Patio Bonito, and Las Palmas localities. (A) Metaperidotite outcrop in the San Pedro locality. (B) Metaperidotite outcrop crosscut by a mafic dike or sill in the Patio Bonito locality. (C) and (D) Metaharzburgite outcrops in the Las Palmas locality. (E) Chromitite body in the San Pedro locality. (F) Massive chromitite hand-specimen from the San Pedro locality. (G) General view of the Patio Bonito Mine. (H) Zoom on the massive chromitite in the Patio Bonito locality.
Whole rock analyses of peridotite samples were carried out in the Centro de Instrumentación Científica (CIC), Universidad de Granada. Major elements and Zr were analyzed using a Philips Magix Pro (PW-2440) X Ray Fluorescence (XRF). Trace elements abundances (except Zr) were obtained by ICP Mass Spectrometry (ICP-MS). Details of the analytic protocols have been described by Lázaro et al. (2014). Results are shown in Table 1. Platinum-Group Elements analyses were performed by ICP-MS method at Genalysis Laboratory Services Pty. Ltd. in Maddington (Australia). The detection limits were 1 ppb for Rh and 2 ppb for Os, Ir, Ru, Pt, and Pd. The details of the analytical procedure can be found in Gervilla et al. (2005) and the results are shown in Table 2.
Table 1 Whole rock analyses of the Medellin Metaharzburgitic Unit (MMU) metaperidotites.
| Sample | LP-1 | LP-4 | LP-7 | SP-1 |
| Locality | Las Palmas | Las Palmas | Las Palmas | San Pedro |
| Typeofrock | Metaharzburgite | Metaharzburgite | Metadunite | Metaharzburgite |
| SiO2 (wt%) | 39.59 | 40.60 | 31.18 | 38.93 |
| TiO2 | 0.02 | 0.06 | 0.30 | 0.04 |
| Al2O3 | 0.84 | 0.96 | 5.27 | 0.77 |
| Fe2O3T | 8.61 | 8.02 | 10.80 | 7.58 |
| MnO | 0.13 | 0.12 | 0.12 | 0.12 |
| MgO | 41.20 | 40.36 | 37.20 | 39.92 |
| CaO | 0.57 | 0.74 | 0.07 | 0.53 |
| Na2O | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| K2O | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| P2O5 | 0.01 | 0.01 | 0.01 | 0.01 |
| LOI | 8.17 | 8.63 | 9.79 | 11.29 |
| Total | 99.14 | 99.50 | 94.74 | 99.19 |
| Sample | LP-1 | LP-4 | LP-7 | SP-1 |
| Li (ppm) | 1.1 | 0.9 | 2.3 | 1.5 |
| Rb | 0.80 | 0.37 | 0.38 | 0.60 |
| Cs | 0.071 | 0.008 | 0.037 | 0.027 |
| Be | 0.19 | 0.19 | 0.19 | 0.19 |
| Sr | 2.7 | 0.9 | 2.8 | 1.7 |
| Ba | 1.49 | 0.46 | 0.47 | 0.83 |
| Sc | 6.2 | 7.3 | 8.4 | 7.4 |
| V | 28.9 | 122.5 | 30.5 | 28.6 |
| Cr | 2231 | 12771 | 1488 | 1558 |
| Co | 101 | 129 | 98 | 109 |
| Ni | 2241 | 3593 | 1927 | 2260 |
| Cu | 1.3 | 113 | 2.6 | 19 |
| Sample | LP-1 | LP-4 | LP-7 | SP-1 |
| Locality | Las Palmas | Las Palmas | Las Palmas | San Pedro |
| Type of rock | Metaharzburgite | Metadunite | Metaharzburgite | Metaharzburgite |
| Zn | 44 | 134 | 33 | 42 |
| Ga | 0.7 | 5.3 | 0.9 | 0.7 |
| Yb | 0.48 | 0.08 | 0.79 | 0.26 |
| Nb | 0.45 | 0.28 | 0.12 | 0.09 |
| Ta | 0.14 | 0.11 | 0.11 | 0.11 |
| Zr (XRF) | 8.8 | 5.8 | 5.6 | 7.0 |
| Zr (ICPMS) | 1.1 | 0.6 | 4.5 | 0.6 |
| Mo | 0.14 | 0.18 | 0.13 | 0.16 |
| Sn | 0.36 | 0.34 | 0.31 | 0.32 |
| Tl | 0.005 | 0.007 | 0.003 | 0.004 |
| Pb | 0.49 | 0.15 | 0.19 | 0.29 |
| U | 0.016 | 0.008 | 0.009 | 0.006 |
| Th | 0.203 | 0.054 | 0.041 | 0.024 |
| La | 0.084 | 0.043 | 0.080 | 0.056 |
| Ce | 0.174 | 0.078 | 0.239 | 0.098 |
| Pr | 0.027 | 0.011 | 0.039 | 0.014 |
| Nd | 0.143 | 0.031 | 0.183 | 0.072 |
| Sm | 0.047 | 0.007 | 0.085 | 0.020 |
| Eu | 0.019 | 0.002 | 0.016 | 0.006 |
| Gd | 0.052 | 0.007 | 0.088 | 0.019 |
| Tb | 0.008 | 0.001 | 0.017 | 0.004 |
| Dy | 0.063 | 0.007 | 0.124 | 0.027 |
| Ho | 0.013 | 0.002 | 0.026 | 0.009 |
| Er | 0.043 | 0.007 | 0.072 | 0.023 |
| Tm | 0.01 | 0.002 | 0.017 | 0.007 |
| Yb | 0.061 | 0.016 | 0.098 | 0.045 |
| Lu | 0.008 | 0.003 | 0.015 | 0.008 |
| Hf | 0.52 | 0.37 | 0.39 | 0.31 |
b.d.l. = below detection limit.
4. Results
4.1. Petrography
4.1.1. Ultramafic rocks
The metaperidotite samples (Figure 2) correspond to metaharzburgites (>40% Ol and >5% Opx) and metadunites (>90% Ol), both partially serpentinized (Figures 3A to 3D). The preserved primary textures are typical of mantle tectonites, predominantly porphyroclastic/granoblastic textures, and minerals show undulose extinction and kink-bands. The ultramafic rocks contain relicts of mantle-derived olivine, chromian spinel, and minor orthopyroxene. However, much of the mineral assemblage is secondary and includes secondary fine-grained olivine, tremolite, chlorite, talc, serpentine, secondary chromian spinel, and minor carbonates and anthophyllite (García-Casco et al., 2020 and references therein).
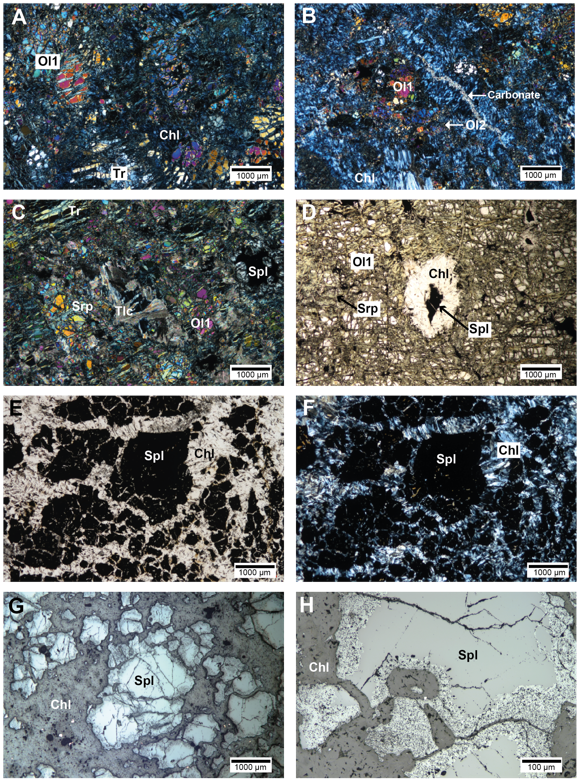
Figure 3 Microphotographs of metaharzburgites, metadunites and chromitites from the MMU. (A) Metaharzburgite with tremolite (Tr) and relict olivine (Ol1) replaced by serpentine and chlorite (Chl). Transmitted light and crossed nicols. (B) Metaharzburgite with relict olivine (Ol1), secondary olivine (Ol2), chlorite (Chl) and late carbonate veins. Transmitted light and crossed nicols. (C) Metadunite showing magmatic olivine (Ol1) altered to serpentine (Srp) and iddingsite. Talc (Tlc) and accessory spinel (Spl) are also observed. Transmitted light and crossed nicols. (D) Metadunite with olivine (Ol1) partially altered to serpentine. Chromian spinel (Spl) is surrounded by a decussate chlorite (Chl) corona. Transmitted light and parallel nicols. (E) Chromitite with coarse chromian spinel (Spl) crystals in a chlorite matrix (Chl). Transmitted light and parallel nicols. (F) Same as E in transmitted light and crossed nicols. (G) Chromitite showing strongly altered and fractured chromian spinel (Spl) crystals surrounded by chlorite (Chl). Reflected light. (H) Chromitite showing alteration rims in chromian spinel (Spl) crystals in chromitite. Reflected light.
Metaharzburgites: Metaharzburgites show pseudomorphic mesh and bastite textures, where mantle olivine is replaced by serpentine and iddingsite along rims and fractures, and pyroxene by serpentine and tremolite (Figure 3A and 3B). There are two generations of olivine: primary olivine (Ol1), which forms 100-500 μm porphyroclasts, and secondary recrystallized olivine (Ol2) that forms smaller (< 20 μm) rounded grains (Figure 3B). Primary olivine is variably deformed and oriented, showing undulose extinction and kink-bands, and is also found as relict inclusions in pyroxene pseudomorphs. Non-pseudomorphic textures, characterized by intergrowths of antigorite, tremolite and secondary olivine are also observed (Figure 3A). Talc and chlorite replace mainly serpentine.
Chromian spinel (600-800 μm) is a common accessory phase in the MMU metaperidotites. Three types of chromian spinel can be distinguished petrographically, and can be classified according to the textural classification proposed by Gervilla et al. (2012): type I partially altered chromian spinel (Figures 4A and 4B), type II porous chromian spinel, which is completely altered and contains abundant chlorite within the porosity (Figures 4C and 4D), and type III homogeneous chromian spinel (Figures 4E and 4F). Both types I and II chromian spinel in the MMU are surrounded by a decussate chlorite corona.
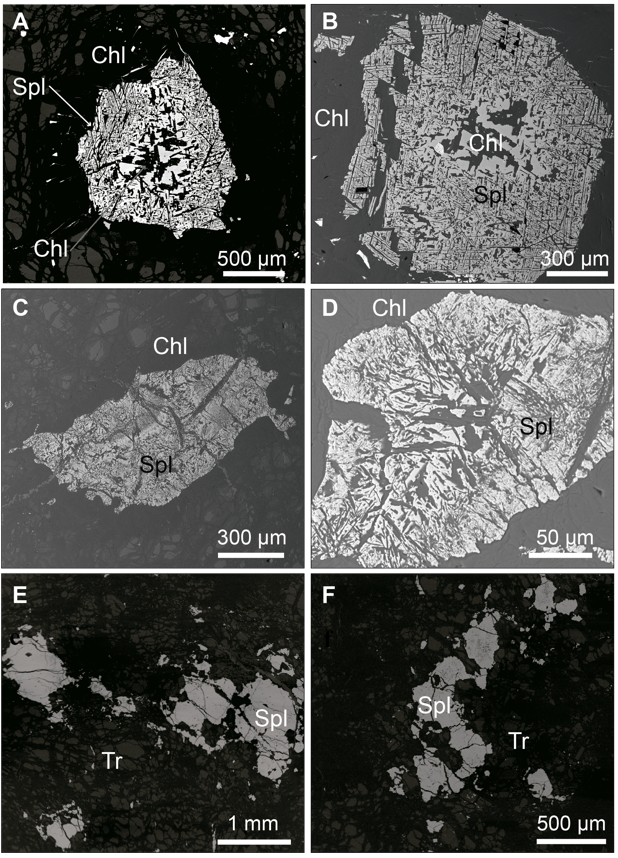
Figure 4 Back-scattered electron images (BSE) of the accessory chromian spinel in MMU. (A) and (B) Type I chromian spinel (Spl) (Al-rich) in metaperidotite. Chlorite (Chl) is present as inclusions within the chromian spinel that follow the (111) crystallographic planes and as a corona surrounding the spinel. (C) and (D) Type II chromian spinel (Cr-Fe2+-rich, and Al-Mg-depleted) with chlorite inclusions and a chlorite halo surrounding the spinel. (E) and (F) Type III chromian spinel (Fe3+-rich) intergrown with tremolite (Tr).
Metadunites: Metadunites are less abundant than metaharzburgites in the MMU, and have been observed in Las Palmas and San Pedro localities (Figure 1B). In San Pedro, metadunites occur as envelopes around chromitite bodies. Metadunites show pseudomorphic mesh textures, where serpentine replaces primary olivine porphyroclasts along grain boundaries and fractures (Figure 3C). Primary mantle olivine forms crystals ~100 μm in size and secondary olivine forms smaller (~10 to 20 μm) crystals. Tremolite and talc are found as overgrowths in serpentine and filling thin veinlets (Figure 3C).
Accessory chromian spinel (0.6 - 1 mm) shows unaltered cores surrounded by rims of porous chromian spinel with chlorite following the altered chromian spinel (111) crystallographic planes (Figure 4A and 4B). This partially altered chromian spinel corresponds to type I spinel according to the classification of Gervilla et al. (2012). A decussate chlorite corona surrounds the type II chromian spinel (Figure 3D). Chlorite crystals reach up to 500 μm in size and neither primary pyroxene nor pseudomorphs after pyroxene are observed.
4.1.2. Chromitites
Chromitite samples from San Pedro and Patio Bonito localities (Figures 3E to 3H) exhibit massive (>80% vol. chromian spinel) and semi-massive (60-80% vol. chromian spinel) textures. Massive chromitites consists of large (0.5-0.8 cm) chromian spinel crystal aggregates, which have unaltered cores surrounded by thin alteration rims of ferrian chromian spinel with abundant chlorite inclusions (Figure 3G), also showing typical pull-apart textures. Semi-massive chromitites are made up of smaller chromian spinel grains (up to 0.2 cm) that are strongly fractured and altered along rims to ferrian chromian spinel (Figure 3H), locally forming brecciated textures. Chlorite is the predominant intergranular mineral accompanied by minor serpentine, rutile, ilmenite, and titanite. Mineral inclusions observed within chromian spinel include olivine, chlorite, serpentine, ilmenite, rutile, and amphibole. In both chromitite bodies, chromian spinel alteration rims are highly porous and the voids are filled by chlorite and minor sulfides. The voids within the alteration rims in the less altered chromian spinel crystals are rounded (Figure 3H), whereas the ones in the more altered crystals have coarse irregular or acicular-shaped voids (Figures 4A to 4D).
4.2. Whole rock geochemistry
4.2.1. Metaperidotites
The LOI values of the MMU metaperidotites range from 8.17 to 11.29 wt% (Table 1). The Al2O3 contents (0.84 - 0.96 wt%) are rather low (except for sample LP-7), SiO2 ranges between 31.18 and 40.60 wt%, Fe2O3 between 7.58 and 10.80 wt%, MgO between 37.20 to 41.20 wt%, and CaO and TiO2 contents are low (0.07 - 0.74 wt% and 0.02 - 0.30 wt% respectively) (Table 1). One metadunite from Las Palmas (sample LP-7) shows the lowest contents of SiO2 and CaO (31.18 wt% and 0.07 wt% respectively), and the highest contents of TiO2 (0.30 wt%), Al2O3 (5.27 wt%), and Fe2O3 (10.80 wt%) (Table 1).
Chondrite-normalized rare earth element (REE) patterns (Figure 5A) are almost flat or U-shaped. The metaharzburgite sample from San Pedro (SP-1) shows a positive Ho anomaly and a negative Er anomaly. One metaharzburgite sample from Las Palmas (LP-4) is clearly depleted in REEs when compared to the other samples. The metadunite (LP-7), which is already particular in terms of major elements, shows a noticeable negative Eu anomaly, not observed in the other samples.
In the multielemental diagram normalized to the primitive mantle (Figure 5B) metaperidotites are typically depleted in large-ion lithophile elements (LILE) and slightly enriched in high field strength elements (HFSE). All samples show positive Th and Pb anomalies.
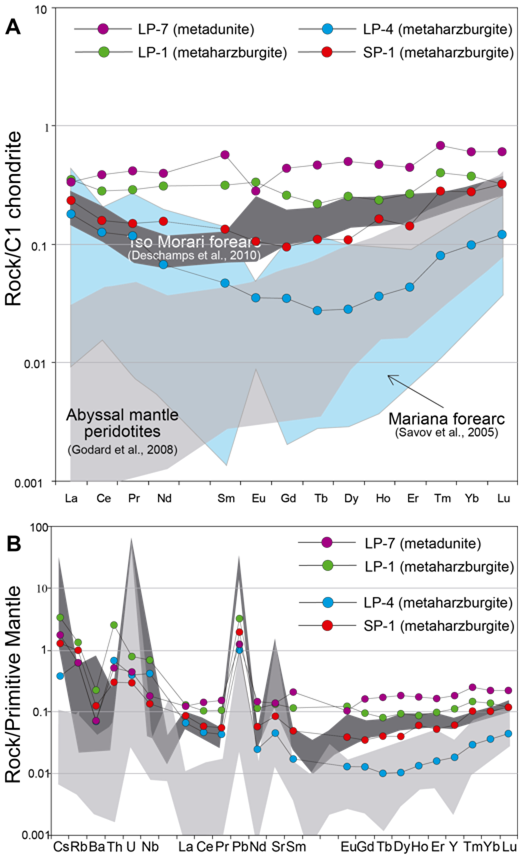
Figure 5 Whole rock geochemistry of the Las Palmas (LP-1, LP-4 and LP-7) and San Pedro (SP-1) metaperidotites. (A) Chondrite-normalized REE patterns. (B) Multi-elemental diagram normalized to the primitive mantle. The fields for abyssal mantle peridotites (Godard et al., 2008) and hydrated mantle wedge serpentinites (Tso Morari, Himalaya, Deschamps et al., 2010; Mariana Forearc, Savov et al., 2005) are projected for comparison. Normalizing values were taken from McDonough and Sun (1995).
4.2.2. Chromitites: PGE geochemistry
Whole rock PGE contents normalized to chondritic values for the massive chromitites associated with the MMU (Figure 6) show a general PGE depletion, around 100 times lower than chondritic values. The San Pedro chromitites have ΣPGE < 41 ppb, Ru being the most abundant element (17 - 18 ppb). The Patio Bonito chromitites have ΣPGE < 37 ppb, Ru also being the most abundant element (7 - 14 ppb), but with a significantly lower content than in San Pedro. The chondrite-normalized PGE patterns (Figure 6) are characterized by comparable Os and Ir values, positive Ru anomalies, and a negative slope from Ru to Pd, which is typical for podiform chromitites (e.g., Leblanc, 1991). These patterns are similar to those from the Al-rich chromitites from Tehuitzingo, México (Proenza et al., 2004b).
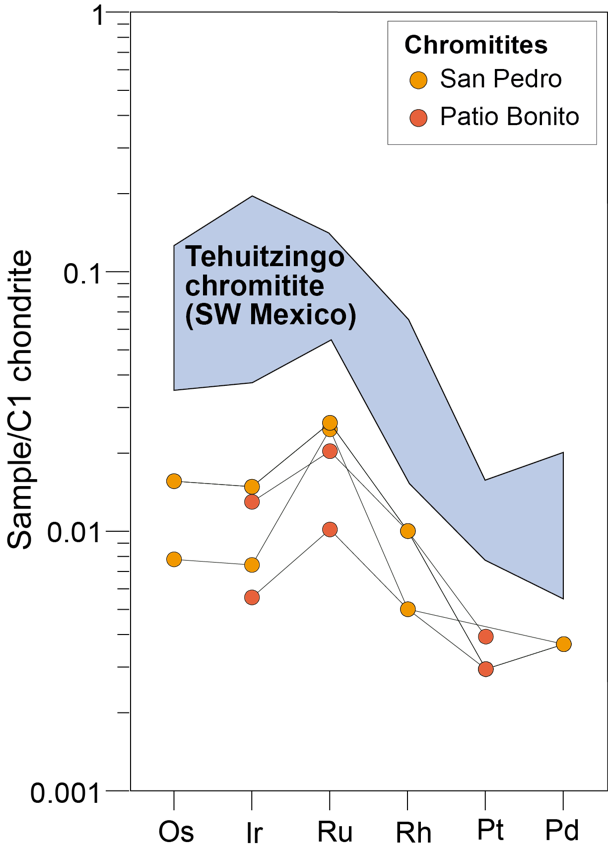
Figure 6 Chondrite-normalized platinum group element (PGE) patterns for the San Pedro and Patio Bonito chromitites. Normalizing chondritic values are from Naldrett and Duke (1980). The field for the Tehuitzingo chromitites (México) is from Proenza et al. (2004b).
4.3. Mineral chemistry
4.3.1. Chromian spinel
Accessory chromian spinel in the metaperidotites has been divided into 3 textural groups. The compositional variations regarding the Fe3+, Cr and Al contents are directly related to the textural classification defined petrographically (Figures 7A, 7B, 8 and Table 3).
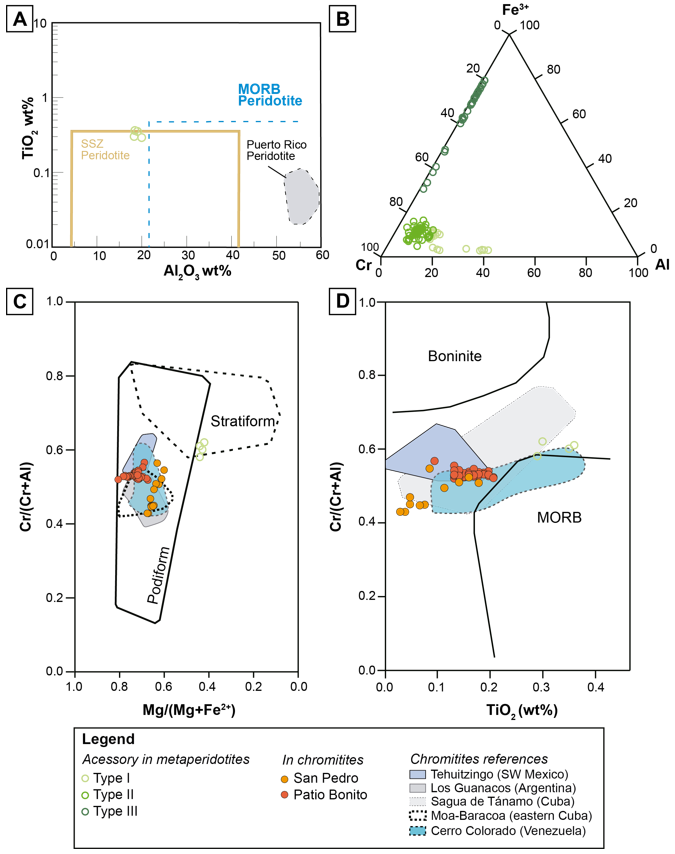
Figure 7 Mineral chemistry of accessory chromian spinel in the metaperidotites and in the chromitite bodies of the MMU. (A) TiO2 (wt%) vs. Al2O3 (wt%) diagram for type I chromian spinel. Fields for supra-subduction zone (SSZ) and MORB-type peridotites are from Kamenetsky et al. (2001), and the field for the Puerto Rico Peridotite is from Marchesi et al. (2011). (B) Cr, Fe3+ and Al compositions for chromian spinel of the MMU. (C) Cr# [Cr/(Cr+Al)] vs. Mg# [Mg/(Mg+Fe)] diagram for chromian spinel of the San Pedro and Patio Bonito chromitites, and primary chromian spinel for the metaperidotites. The podiform and stratiform fields are after Irvine (1967) and Leblanc and Nicolas (1992) respectively, the Moa Baracoa and Sagua de Tánamo (Cuba) fields are from Proenza et al. (1999), Tehuitzingo (México) is from Proenza et al. (2004b), Los Guanacos (Argentina) is from Proenza et al. (2008), and Cerro Colorado (Venezuela) is from Mendi et al. (2020). (D) Cr# vs. TiO2 (wt%) for the San Pedro and Patio Bonito chromitites, and type I chromian spinel in the metaperidotites. Fields for boninites and MORB are from Arai (1992), references are the same as in (C).
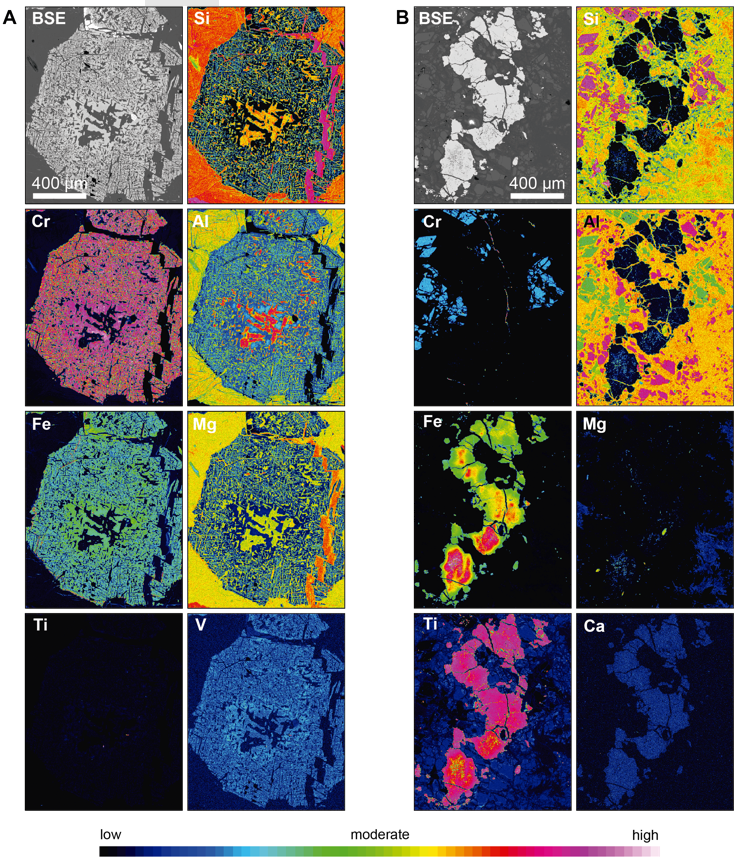
Figure 8 Element distribution maps for accessory chromian spinel in the MMU. (A) Type I chromian spinel from Las Palmas (Sample LP-7). Note the high Al, Fe, and Cr contents in the core that gradually decreases towards the rims. The Al-rich cores are surrounded by a high-V halo. (B) Type III chromian spinel from the Las Palmas metaperidotite (Sample CO-7). The Fe enrichment is related with the Cr depletion, whereas Al is almost absent.
Table 3 Representative electron microprobe analyses of accessory chromian spinel in the metaperidotites.
| Type I | Type II | Type III | |||||||
| Area | Las Palmas | Las Palmas | Las Palmas | Las Palmas | Las Palmas | San Pedro | Patio Bonito | Patio Bonito | Patio Bonito |
| >Sample | LP-7 | LP-7 | LP-7 | LP-7 | LP-7 | SP-001 | COL-6 | COL-7 | COL-6 |
| >SiO2 (wt.%) | 0.05 | b.d.l. | 0.07 | b.d.l. | 0.10 | 0.07 | 0.08 | 0.08 | 0.05 |
| >TiO2 | 0.29 | 0.35 | 0.30 | 0.78 | 0.88 | 1.13 | 0.29 | 0.31 | 0.29 |
| >Al2O3 | 19.97 | 19.03 | 18.29 | 6.82 | 3.67 | 2.19 | 0.24 | 0.10 | b.d.l. |
| >Cr2O3 | 41.91 | 42.76 | 44.09 | 52.42 | 54.54 | 53.73 | 28.67 | 17.49 | 11.03 |
| >V2O3 | 0.16 | 0.17 | 0.14 | 0.25 | 0.33 | 0.33 | 0.16 | 0.16 | 0.11 |
| >Fe2O3 | 5.37 | 5.77 | 5.26 | - | - | - | 39.22 | 50.72 | 56.79 |
| >FeO | 21.44 | 21.54 | 21.86 | 32.69 | 34.88 | 38.16 | 27.40 | 28.17 | 28.92 |
| >MnO | 0.34 | 0.36 | 0.32 | 0.58 | 0.62 | 0.70 | 0.58 | 0.32 | 0.26 |
| >ZnO | 0.87 | 0.68 | 0.79 | 0.50 | 0.57 | 0.63 | 0.24 | 0.07 | 0.08 |
| >MgO | 8.44 | 8.39 | 8.10 | 5.08 | 3.54 | 2.55 | 2.19 | 1.81 | 1.18 |
| >NiO | 0.12 | 0.05 | 0.05 | 0.04 | b.d.l. | 0.09 | 0.48 | 0.66 | 0.81 |
| >Total | 98.96 | 99.11 | 99.27 | 99.15 | 99.14 | 99.58 | 99.55 | 99.89 | 99.52 |
| > | Type I | Type II | Type III | ||||||
| >Ti (a.p.f.u.) | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.02 |
| >Al | 0.80 | 0.76 | 0.73 | 0.28 | 0.15 | 0.09 | 0.02 | 0.01 | 0.00 |
| >Cr | 1.12 | 1.15 | 1.19 | 1.44 | 1.54 | 1.53 | 1.34 | 1.00 | 0.73 |
| >V | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| >Fe3+ | 0.06 | 0.06 | 0.05 | 0.24 | 0.24 | 0.31 | 0.60 | 0.94 | 1.22 |
| >Fe2+ | 0.55 | 0.55 | 0.57 | 0.72 | 0.80 | 0.84 | 0.76 | 0.77 | 0.80 |
| >Mn | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 |
| >Zn | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.00 | 0.00 |
| >Mg | 0.43 | 0.43 | 0.41 | 0.26 | 0.19 | 0.14 | 0.19 | 0.20 | 0.15 |
| >Ni | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.00 | 0.02 | 0.04 | 0.05 |
| > | Type I | Type II | Type III | ||||||
| >Cr# | 0.58 | 0.60 | 0.62 | 0.84 | 0.91 | 0.94 | 0.99 | 0.99 | 1.00 |
| >Mg# | 0.44 | 0.43 | 0.42 | 0.27 | 0.19 | 0.14 | 0.20 | 0.20 | 0.16 |
| >Fe3+# | 0.03 | 0.03 | 0.03 | 0.12 | 0.12 | 0.16 | 0.30 | 0.48 | 0.63 |
b.d.l. = below detection limit.
Type I chromian spinel (partially altered) has Al-rich cores (Figures 7A and 8A, Table 3) that represent the primary composition. These cores are characterized by Cr# [Cr/(Cr+Al) atomic ratio] ranging from 0.58 to 0.62, Mg# [Mg/(Mg+Al) atomic ratio] from 0.42 to 0.44, Fe3+# [Fe3+/(Fe3++Cr+Al) atomic ratio] ≤ 0.03, TiO2≤ 0.36 wt%, MnO from 0.32 to 0.42 wt%, and ZnO from 0.68 to 0.87 wt%. This composition is typical of accessory spinel in mantle rocks at supra-subduction zones (Kamenetsky et al., 2001) (Figure 7A).
Type II chromian spinel (porous) is Al and Mg-depleted and enriched in Cr, Fe2+, Ti and Mn, when compared to Type I chromian spinel. The composition is characterized by Cr# that ranges from 0.84 to 0.96, Mg# from 0.13 to 0.27, Fe3+# from 0.05 to 0.16, TiO2from 0.42 to 1.57 wt%, MnO from 0.39 to 0.76 wt%, and ZnO from 0.37 to 0.66 wt% (Figure 7B, Table 3).
Type III chromian spinel (homogenous) is Fe3+-rich (Figures 7B and 8B, Table 3) and has Cr# that ranges from 0.98 to 1.00, Mg# from 0.09 to 0.22, Fe3# from 0.30 to 0.79, TiO2from 0.27 to 0.45 wt%, MnO from 0.16 to 0.58 wt%, and ZnO from 0.04 to 0.28 wt%.
Unaltered chromian spinel cores from San Pedro chromitites has Cr# ranging from 0.43 to 0.58, Mg# from 0.54 to 0.68, TiO2 from 0.02 to 0.5 wt%, Fe3+# <0.035, MnO < 0.27 wt%, V2O3 < 0.22 wt%, ZnO < 0.17 wt%, and NiO < 0.23 wt% (Figures 7C, 7D, Table 4). In contrast, unaltered chromian spinel cores from Patio Bonito chromitites exhibit higher Cr# (0.51 - 0.63) and Mg# (0.67-0.80), similar TiO2 (0.05 to 0.5 wt%) and NiO (<0.21 wt%) but lower Fe3+# (<0.018 wt%) and slightly higher MnO (0.11-0.49 wt%), V2O3 (<0.26 wt%), and ZnO (<0.37 wt%) (Figures 7C, 7D and Table 4).
Table 4 Representative electron microprobe chromian spinel analyses from the chromitite bodies in the MMU.
| Locality | San Pedro | Patio Bonito | ||||||||
| chr1 | chr2 | chr3 | chr4 | chr5 | chr1 | chr2 | chr3 | chr4 | chr5 | |
| SiO2 (wt.%) | 0.09 | 0.03 | 0.07 | 0.03 | 0.06 | 0.14 | 0.09 | 0.08 | 0.10 | 0.10 |
| TiO2 | 0.03 | 0.04 | 0.05 | 0.07 | 0.08 | 0.14 | 0.22 | 0.15 | 0.16 | 0.18 |
| Al2O3 | 32.74 | 32.49 | 31.33 | 31.31 | 31.23 | 27.06 | 26.81 | 26.63 | 26.58 | 26.49 |
| Cr2O3 | 36.56 | 36.28 | 38.01 | 37.53 | 37.71 | 43.38 | 43.21 | 43.03 | 43.37 | 43.99 |
| V2O3 | 0.09 | 0.13 | 0.19 | 0.10 | 0.19 | 0.12 | 0.11 | 0.15 | 0.21 | 0.14 |
| Fe2O3 | 2.07 | 2.63 | 1.87 | 2.15 | 2.26 | 0.82 | 3.13 | 2.64 | 0.95 | 1.85 |
| FeO | 13.40 | 13.54 | 13.92 | 13.89 | 14.02 | 13.11 | 8.65 | 11.98 | 12.89 | 9.74 |
| MnO | 0.14 | 0.20 | 0.19 | 0.16 | 0.18 | 0.17 | 0.11 | 0.20 | 0.19 | 0.13 |
| ZnO | 0.00 | 0.02 | 0.05 | 0.15 | 0.08 | b.d.l. | 0.05 | 0.07 | 0.12 | 0.11 |
| MgO | 15.70 | 15.51 | 15.13 | 15.05 | 15.09 | 15.10 | 18.16 | 15.93 | 15.07 | 17.25 |
| NiO | 0.08 | b.d.l. | 0.11 | 0.02 | 0.05 | 0.16 | 0.14 | 0.14 | 0.18 | 0.20 |
| Total | 100.89 | 100.89 | 100.94 | 100.47 | 100.93 | 100.20 | 100.68 | 101.00 | 99.82 | 100.18 |
| chr1 | chr2 | chr3 | chr4 | chr5 | chr1 | chr2 | chr3 | chr4 | chr5 | |
| Ti (a.p.f.u.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 1.11 | 1.11 | 1.07 | 1.08 | 1.07 | 0.95 | 0.94 | 0.94 | 0.94 | 0.93 |
| Cr | 0.83 | 0.83 | 0.87 | 0.87 | 0.87 | 1.03 | 1.02 | 1.02 | 1.03 | 1.04 |
| V | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Fe3+ | 0.05 | 0.06 | 0.04 | 0.05 | 0.05 | 0.00 | 0.02 | 0.02 | 0.01 | 0.01 |
| Fe2+ | 0.32 | 0.33 | 0.34 | 0.34 | 0.34 | 0.33 | 0.19 | 0.28 | 0.32 | 0.23 |
| Mn | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Mg | 0.68 | 0.67 | 0.66 | 0.66 | 0.66 | 0.67 | 0.81 | 0.71 | 0.68 | 0.77 |
| Ni | 0.00 | - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| chr1 | chr2 | chr3 | chr4 | chr5 | chr1 | chr2 | chr3 | chr4 | chr5 | |
| Cr# | 0.43 | 0.43 | 0.45 | 0.45 | 0.45 | 0.52 | 0.52 | 0.52 | 0.52 | 0.53 |
| Mg# | 0.68 | 0.67 | 0.66 | 0.66 | 0.66 | 0.67 | 0.81 | 0.72 | 0.68 | 0.77 |
| Fe3+# | 0.13 | 0.15 | 0.11 | 0.12 | 0.13 | 0.05 | 0.25 | 0.17 | 0.06 | 0.15 |
b.d.l. = below detection limit.
4.3.2. Olivine
Olivine is only present in the metaperidotites and its composition ranges from Fo89.7 to Fo93.7 (average of Fo90.5), and NiO from 0.19 to 0.51 wt%, which is within the range of mantle olivine (e.g., Takahashi et al., 1987). Even though petrographically it was possible to distinguish primary mantle-derived olivine and secondary metamorphic olivine, all the analyzed crystals show similar composition (Table 5). Figure 9A shows a scattering of olivine compositions between the abyssal peridotites (Moghadam et al., 2015) and the supra-subduction peridotite fields (Ishii et al., 1992).
Table 5 Representative electron microprobe analyses of olivine in the metaperidotites.
| Mineral | Ol-1 | Ol-1 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-2 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-1 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-1 | Ol-2 | Ol-2 | Ol-2 | Ol-2 |
| Locality | Las Palmas | San Pedro | Patio Bonito | ||||||||||||||||||||||
| Type of rock | dun | dun | dun | dun | dun | dun | dun | dun | dun | dun | dun | dun | dun | per | per | per | per | per | per | per | dun | dun | dun | dun | dun |
| Sample | LP-1 | LP-1 | LP-1 | LP-1 | LP-1 | LP-1 | LP-1 | LP-7 | LP-7 | LP-7 | LP-7 | LP-7 | LP-7 | SP-1 | SP-1 | SP-1 | SP-1 | SP-1 | SP-1 | SP-1 | CO-7 | CO-7 | CO-7 | CO-7 | CO-7 |
| SiO2 (wt.%) | 40.72 | 40.87 | 41.30 | 40.86 | 41.03 | 41.04 | 41.14 | 41.45 | 41.21 | 40.91 | 40.96 | 40.98 | 40.72 | 41.04 | 40.60 | 41.08 | 40.97 | 40.89 | 40.94 | 40.79 | 41.39 | 40.94 | 41.07 | 41.21 | 40.96 |
| TiO2 | b.d.l. | 0.04 | b.d.l. | 0.02 | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.04 | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.03 | 0.02 | b.d.l. | b.d.l. | 0.02 | 0.03 | b.d.l. | b.d.l. | 0.05 |
| Al2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Cr2O3 | 0.03 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.02 | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | 0.02 |
| V2O3 | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.03 | b.d.l. | 0.02 | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.04 | b.d.l. | b.d.l. | b.d.l. |
| FeO | 9.52 | 9.55 | 9.69 | 9.69 | 9.73 | 9.65 | 9.69 | 8.98 | 8.41 | 9.57 | 9.40 | 9.98 | 9.65 | 9.22 | 9.47 | 8.48 | 8.56 | 8.71 | 9.52 | 9.59 | 8.66 | 8.81 | 8.79 | 8.68 | 8.76 |
| MnO | 0.15 | 0.18 | 0.18 | 0.12 | 0.17 | 0.15 | 0.17 | 0.12 | 0.17 | 0.13 | 0.16 | 0.12 | 0.11 | 0.15 | 0.15 | 0.13 | 0.12 | 0.14 | 0.16 | 0.16 | 0.17 | 0.18 | 0.16 | 0.18 | 0.17 |
| ZnO | 0.02 | 0.05 | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | 0.04 | 0.03 | b.d.l. | b.d.l. | 0.05 | 0.08 | b.d.l. | 0.02 | b.d.l. | 0.03 | b.d.l. | 0.04 | b.d.l. | b.d.l. | 0.03 | b.d.l. | b.d.l. | 0.03 |
| MgO | 48.67 | 48.71 | 48.55 | 48.33 | 48.70 | 48.62 | 48.42 | 49.97 | 49.90 | 49.29 | 49.08 | 48.93 | 48.98 | 49.48 | 49.33 | 49.83 | 49.84 | 49.83 | 48.93 | 49.09 | 49.70 | 49.74 | 49.59 | 49.53 | 49.46 |
| NiO | 0.33 | 0.34 | 0.37 | 0.34 | 0.32 | 0.29 | 0.34 | 0.26 | 0.29 | 0.34 | 0.32 | 0.19 | 0.26 | 0.38 | 0.37 | 0.37 | 0.38 | 0.36 | 0.35 | 0.33 | 0.38 | 0.37 | 0.38 | 0.38 | 0.42 |
| CaO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.04 | b.d.l. | b.d.l. | b.d.l. | 0.03 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.02 |
| Na2O | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.03 | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| K2O | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Total | 99.46 | 99.77 | 100.11 | 99.36 | 99.98 | 99.81 | 99.78 | 100.84 | 100.08 | 100.29 | 99.96 | 100.29 | 99.87 | 100.33 | 99.97 | 99.94 | 99.94 | 100.00 | 99.98 | 100.00 | 100.32 | 100.17 | 100.04 | 100.01 | 99.89 |
| Mineral | Ol-1 | Ol-1 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-2 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-1 | Ol-1 | Ol-1 | Ol-2 | Ol-2 | Ol-1 | Ol-2 | Ol-2 | Ol-2 | Ol-2 |
| Si (a.p.f.u.) | 1.003 | 1.004 | 1.010 | 1.007 | 1.005 | 1.007 | 1.010 | 1.003 | 1.003 | 0.999 | 1.003 | 1.002 | 0.999 | 1.000 | 0.994 | 1.002 | 1.000 | 0.998 | 1.003 | 0.999 | 1.006 | 0.998 | 1.002 | 1.005 | 1.001 |
| Ti | - | 0.001 | - | 0.000 | - | 0.000 | - | - | - | - | - | 0.001 | - | 0.000 | - | - | 0.000 | 0.000 | - | - | 0.000 | 0.000 | - | - | 0.001 |
| Al | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cr | 0.001 | - | - | - | - | - | - | 0.000 | 0.000 | - | 0.000 | - | - | 0.000 | - | - | - | - | - | - | - | - | 0.000 | - | 0.000 |
| V | - | 0.000 | - | - | - | - | - | - | - | - | - | - | 0.001 | - | 0.000 | 0.000 | - | - | - | - | - | 0.001 | - | - | - |
| Fe2+ | 0.196 | 0.196 | 0.198 | 0.200 | 0.199 | 0.198 | 0.199 | 0.182 | 0.171 | 0.194 | 0.192 | 0.204 | 0.198 | 0.188 | 0.182 | 0.173 | 0.175 | 0.173 | 0.195 | 0.194 | 0.176 | 0.177 | 0.179 | 0.177 | 0.179 |
| Mn | 0.003 | 0.004 | 0.004 | 0.003 | 0.004 | 0.003 | 0.003 | 0.002 | 0.003 | 0.003 | 0.003 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.004 | 0.003 | 0.004 | 0.004 |
| Zn | 0.000 | 0.001 | - | - | - | 0.000 | - | 0.001 | 0.001 | - | - | 0.001 | 0.001 | - | 0.000 | - | 0.001 | - | 0.001 | - | - | 0.001 | - | - | 0.000 |
| Mg | 1.787 | 1.783 | 1.770 | 1.776 | 1.779 | 1.778 | 1.771 | 1.803 | 1.811 | 1.795 | 1.791 | 1.783 | 1.792 | 1.798 | 1.800 | 1.812 | 1.813 | 1.812 | 1.787 | 1.793 | 1.801 | 1.808 | 1.804 | 1.801 | 1.803 |
| Ni | 0.006 | 0.007 | 0.007 | 0.007 | 0.006 | 0.006 | 0.007 | 0.005 | 0.006 | 0.007 | 0.006 | 0.004 | 0.005 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.008 |
| Ca | - | - | - | - | - | - | - | - | 0.001 | - | - | - | 0.001 | - | - | - | - | - | - | - | - | 0.001 | - | - | 0.000 |
| Na | - | - | - | - | - | - | - | - | - | - | 0.001 | - | - | - | - | - | - | 0.002 | - | 0.001 | - | - | - | - | - |
| K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| O=4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fo | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.91 | 0.91 | 0.90 | 0.91 | 0.90 | 0.90 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.90 | 0.90 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 |
b.d.l. = below detection limit.
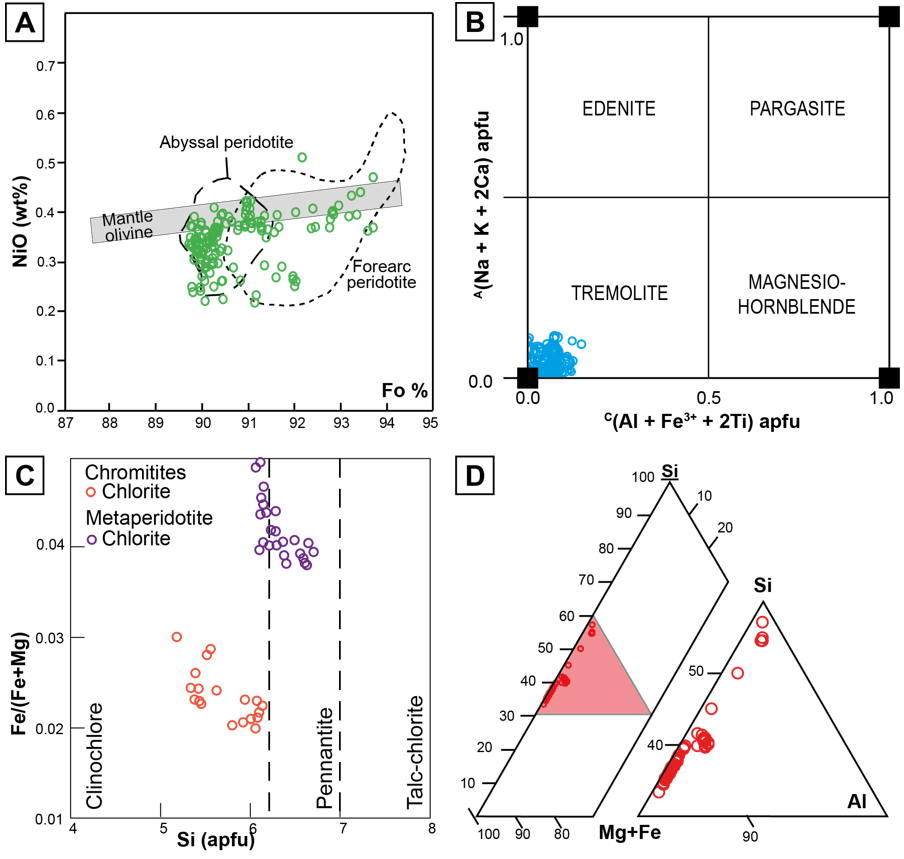
Figure 9 (A) NiO (wt%) vs. Forsterite content (Fo) in olivine from the metaperidotites of the MMU. Fields for mantle olivine are from Takahashi et al. (1987), fore-arc peridotite from Ishii et al. (1992) and abyssal peridotite from Moghadam et al. (2015). (B) Amphibole classification diagram according to Hawthorne et al. (2012), variations of A(Na + K + 2Ca) a.p.f.u. vs. C(Al + Fe3+ + 2Ti) a.p.f.u. (C) Fe/(Fe + Mg) vs. Si a.p.f.u. classification diagram for chlorite according to Hey (1954), for the metaperidotites and chromitites of the MMU. (D) Si, Al, Fe + Mg distribution diagram for serpentine of the MMU metaperidotites.
4.3.3. Tremolite
Amphibole is only present in the metaharzburgites and it is more abundant in samples that contain Type-III chromian spinel. The studied crystals belong to the calcium subgroup (calculations following Locock, 2014) according to the classification scheme of Hawthorne et al. (2012), and have Mg# [Mg/(Mg+Fe2+) atomic ratio] ranging from 0.95 to 0.99 (Table 6). These calcium amphiboles (CaB = 1.196 - 2 a.p.f.u; NaB = 0.001 - 0.19) classify as tremolite (Figure 9B). Analyses yielded very low Ti (<0.02 a.p.f.u.), Mn (<0.06 a.p.f.u.) and K (<0.007 a.p.f.u.) contents, and have variable Si (7.20 - 8.02), AlTOT (0.016 - 0.131), Mg (3.76 - 4.96), FeTOT (0.14 - 0.39), Ca (1.19 - 2.00), and Na (0.001 - 0.19) contents (Table 6). The vacancy in position A is between 0.69 and 1.00 a.p.f.u.
Table 6 Representative electron microprobe analyses of tremolite, chlorite and serpentine in the metaperidotites and chromitites.
| Mineral | Amp | Amp | Amp | Amp | Amp | Amp | Amp | Amp | Chl | Chl | Chl | Chl | Chl | Chl | Chl | Chl | Srp | Srp | Srp | Srp | Srp | Srp | Srp | Srp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | Las Palmas | San Pedro | Patio Bonito | Las Palmas | San Pedro | Patio Bonito | Las Palmas | San Pedro | Patio Bonito | |||||||||||||||
| Type of rock | dun | dun | dun | per | per | dun | dun | dun | dun | dun | dun | dun | per | per | chr | per | dun | dun | dun | dun | per | per | dun | dun |
| Sample | LP-1 | LP-1 | LP-1 | SP-1 | SP-1 | CO-7 | CO-7 | CO-7 | LP-1 | LP-1 | LP-7 | LP-7 | SP-1 | SP-1 | CO-4 | CO-6 | LP-7 | LP-7 | LP-7 | LP-7 | SP-1 | SP-1 | CO-7 | CO-7 |
| SiO2 (wt.%) | 58.17 | 57.73 | 57.68 | 57.73 | 57.77 | 57.61 | 56.72 | 58.48 | 33.62 | 33.15 | 32.26 | 32.38 | 34.41 | 34.20 | 27.91 | 27.91 | 35.54 | 35.15 | 34.83 | 35.12 | 38.87 | 36.28 | 36.85 | 36.15 |
| TiO2 | 0.05 | 0.08 | 0.05 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.06 | 0.04 | 0.10 | 0.09 | b.d.l. | b.d.l. | 0.07 | 0.07 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.04 |
| Al2O3 | 0.22 | 0.38 | 0.33 | 0.36 | 0.33 | 0.43 | 0.49 | 0.25 | 14.09 | 14.59 | 16.16 | 16.23 | 12.75 | 13.22 | 22.17 | 22.17 | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Cr2O3 | 0.02 | 0.08 | 0.36 | 0.05 | 0.10 | 0.03 | 0.07 | 0.04 | 2.67 | 2.15 | 1.81 | 1.71 | 1.71 | 1.43 | 1.24 | 1.24 | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| V2O3 | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.05 | 0.05 | 0.02 | b.d.l. | 0.02 | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. |
| FeO | 1.81 | 1.98 | 1.86 | 1.89 | 1.68 | 1.37 | 1.50 | 1.28 | 2.44 | 2.47 | 2.56 | 2.68 | 2.29 | 2.35 | 1.79 | 1.79 | 5.92 | 6.35 | 6.13 | 5.66 | 4.98 | 5.95 | 5.66 | 6.03 |
| MnO | 0.09 | 0.12 | 0.06 | 0.11 | 0.06 | 0.04 | 0.03 | 0.07 | 0.03 | 0.02 | 0.03 | 0.04 | 0.02 | b.d.l. | b.d.l. | b.d.l. | 0.11 | 0.12 | 0.13 | 0.09 | 0.07 | 0.07 | 0.08 | 0.05 |
| ZnO | 0.04 | 0.02 | 0.08 | b.d.l. | b.d.l. | b.d.l. | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.03 | 0.02 | 0.03 | b.d.l. | 0.04 | 0.03 |
| MgO | 23.36 | 23.57 | 23.11 | 23.44 | 23.41 | 23.90 | 25.00 | 23.10 | 33.55 | 33.13 | 32.80 | 32.39 | 34.21 | 33.99 | 33.56 | 33.56 | 39.40 | 40.20 | 41.37 | 40.84 | 39.13 | 38.82 | 40.96 | 40.60 |
| NiO | 0.05 | 0.08 | 0.07 | 0.06 | 0.11 | 0.12 | 0.09 | 0.07 | 0.13 | 0.14 | 0.13 | 0.15 | 0.21 | 0.21 | b.d.l. | b.d.l. | 0.17 | 0.20 | 0.21 | 0.22 | 0.13 | 0.34 | 0.36 | 0.35 |
| CaO | 12.74 | 12.27 | 12.41 | 11.96 | 12.88 | 13.41 | 12.89 | 13.43 | b.d.l. | 0.02 | 0.03 | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.05 | 0.04 | 0.02 | b.d.l. | 0.04 | 0.03 | b.d.l. | 0.05 |
| Na2O | 0.27 | 0.34 | 0.35 | 1.05 | 0.49 | 0.32 | 0.24 | 0.22 | 0.02 | b.d.l. | b.d.l. | b.d.l. | 0.05 | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.03 | b.d.l. | b.d.l. |
| K2O | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| H2O | 2.18 | 2.18 | 2.17 | 2.18 | 2.18 | 2.19 | 2.18 | 2.19 | 12.65 | 12.54 | 12.56 | 12.53 | 12.56 | 12.54 | 12.75 | 12.75 | 11.78 | 11.87 | 11.94 | 11.87 | 12.19 | 11.85 | 12.19 | 12.07 |
| Total | 99.01 | 98.82 | 98.54 | 98.85 | 99.02 | 99.42 | 99.25 | 99.16 | 99.31 | 98.31 | 98.47 | 98.23 | 98.24 | 98.02 | 99.51 | 99.51 | 92.99 | 93.95 | 94.70 | 93.84 | 95.46 | 93.40 | 96.14 | 95.37 |
| Mineral | Amp | Amp | Amp | Amp | Amp | Amp | Amp | Amp | Chl | Chl | Chl | Chl | Chl | Chl | Chl | Chl | Srp | Srp | Srp | Srp | Srp | Srp | Srp | Srp |
| Si (a.p.f.u.) | 7.997 | 7.950 | 7.975 | 7.954 | 7.951 | 7.905 | 7.807 | 8.018 | 6.375 | 6.341 | 6.161 | 6.196 | 6.573 | 6.542 | 5.249 | 5.249 | 3.617 | 3.552 | 3.498 | 3.550 | 3.825 | 3.672 | 3.626 | 3.593 |
| Ti | 0.005 | 0.008 | 0.005 | - | - | - | - | 0.002 | 0.008 | 0.006 | 0.014 | 0.013 | - | - | 0.010 | 0.010 | - | - | - | - | - | - | - | - |
| Al | 0.036 | 0.062 | 0.054 | 0.058 | 0.054 | 0.070 | 0.079 | 0.040 | 3.149 | 3.289 | 3.637 | 3.660 | 2.871 | 2.980 | 4.914 | 4.914 | 0.003 | - | - | - | - | - | - | - |
| Cr | 0.002 | 0.008 | 0.039 | 0.006 | 0.011 | 0.003 | 0.007 | 0.004 | 0.400 | 0.325 | 0.273 | 0.259 | 0.258 | 0.217 | 0.184 | 0.184 | - | 0.001 | - | - | - | - | - | - |
| V | - | - | 0.002 | - | - | - | - | 0.002 | 0.007 | 0.008 | 0.004 | - | 0.003 | 0.005 | - | - | - | - | - | - | - | 0.002 | - | - |
| Fe3+ | 0.020 | 0.063 | 0.024 | 0.071 | 0.029 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.282 | 0.282 | 0.504 | 0.537 | 0.515 | 0.478 | 0.350 | 0.504 | 0.466 | 0.501 |
| Fe2+ | 0.188 | 0.165 | 0.191 | 0.147 | 0.165 | 0.157 | 0.173 | 0.147 | 0.387 | 0.395 | 0.409 | 0.429 | 0.366 | 0.376 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.059 | 0.000 | 0.000 | 0.000 |
| Mn | 0.011 | 0.014 | 0.007 | 0.013 | 0.007 | 0.004 | 0.004 | 0.009 | 0.005 | 0.004 | 0.005 | 0.006 | 0.004 | - | - | - | 0.009 | 0.010 | 0.011 | 0.008 | 0.006 | 0.006 | 0.006 | 0.005 |
| Zn | 0.005 | 0.002 | 0.008 | - | - | - | 0.004 | - | - | - | - | - | - | - | - | - | - | - | 0.002 | 0.002 | 0.002 | - | 0.003 | 0.002 |
| Mg | 4.787 | 4.839 | 4.763 | 4.814 | 4.803 | 4.889 | 5.130 | 4.722 | 9.483 | 9.448 | 9.338 | 9.240 | 9.743 | 9.692 | 9.409 | 9.409 | 5.978 | 6.057 | 6.194 | 6.153 | 5.740 | 5.857 | 6.009 | 6.015 |
| Ni | 0.006 | 0.009 | 0.007 | 0.007 | 0.012 | 0.013 | 0.009 | 0.008 | 0.020 | 0.022 | 0.020 | 0.023 | 0.033 | 0.033 | - | - | 0.014 | 0.016 | 0.017 | 0.018 | 0.010 | 0.028 | 0.028 | 0.028 |
| Ca | 1.877 | 1.810 | 1.838 | 1.766 | 1.899 | 1.971 | 1.901 | 1.973 | - | 0.003 | 0.005 | 0.004 | - | - | - | - | 0.005 | 0.004 | 0.003 | - | 0.004 | 0.003 | - | 0.005 |
| Na | 0.073 | 0.091 | 0.094 | 0.280 | 0.129 | 0.085 | 0.063 | 0.057 | 0.008 | - | - | - | 0.017 | 0.013 | - | - | - | - | 0.004 | - | - | 0.005 | - | - |
| K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| H | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| O | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Mg# | 0.962 | 0.967 | 0.961 | 0.970 | 0.967 | 0.969 | 0.967 | 0.970 | 0.961 | 0.960 | 0.958 | 0.956 | 0.964 | 0.963 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.990 | 1.000 | 1.000 | 1.000 |
4.3.4. Chlorite
Chlorite composition in the metaharzburgites corresponds to clinochlore and high-Si pennantite (Figure 9C) according to the classification by Hey (1954). SiO2 content ranges from 27.91 to 35.21 wt% (up to 6.7 Si a.p.f.u.) and FeO from 1.79 to 3.37 wt% (up to 0.49 Fe2+ a.p.f.u.). The Fe/(Fe + Mg) ratio ranges between 0.03 and 0.05, also the Cr2O3 content is low (<3.2 wt%) (Table 6).
Chlorite in the chromitites corresponds to clinochlore (Figure 9C), with SiO2 ranging from 27.07 to 32.61 wt%, FeO from 1.22 to 1.79 wt% and Fe/(Fe + Mg) ratio between 0.02 and 0.03. These values are lower than those from chlorite found in metaperidotites. The Cr2O3 content is also low (<2.61 wt%).
4.3.5. Serpentine
Serpentine composition (Figure 9D) ranges from 41.54 to 45.98 wt% of SiO2, FeO from 2.85 to 4.51 wt%, Al2O3 from 0.53 to 2.50 wt%, and Cr2O3 from 0.03 to 0.47 wt% (Table 6). The highest SiO2 values are observed in serpentine from metaperidotites with type III accessory chromian spinel, where chlorite is rare.
5. Discussion
5.1. Tectonic setting of the MMU peridotites
Ophiolitic peridotites are spatially and temporally related with first-order tectonic and magmatic global events, which, together with mantle processes, control the development of different types of oceanic lithosphere (“ophiolites”) in various tectonic settings (Dilek and Flower, 2003; Dilek and Furnes, 2014 and references therein). In general, oceanic ultramafic rocks of ophiolitic affinity can be classified as: (i) subduction zone (supra-subduction zone or volcanic arc) related ophiolites, which might be influenced by the dehydration of the subducting plate, associated metasomatic processes, and repetitive episodes of partial melting of metasomatized peridotites, and (ii) subduction unrelated ophiolites (oceanic ridges, MOR or plumes), which are linked to mantle diapirism, heat advection and melting in the asthenospheric mantle and/or at deeper levels.
The MMU protoliths are predominantly harzburgites and dunites with tectonite textures and are similar to abyssal ocean ridge and supra-subduction zone peridotites. The Cr# of the accessory chromian spinel in metaperidotites (0.58 - 0.62, unaltered cores from Type-I chromian spinel) from the MMU overlap those of supra-subduction peridotites from ophiolites (Figure 7A; Pearce et al., 2000; Marchesi et al., 2016 and references therein).
The REE patterns of the studied metaperidotites compare well with those related with supra-subduction zone peridotites (Savov et al., 2005; Marchesi et al., 2006; Deschamps et al., 2010) and differ from those of abyssal peridotites (MOR-type) (Godard et al., 2008), characterized by positive LREE to HREE slopes (Figure 5A). The REE patterns (almost flat or U-shaped) are consistent with melt percolation reactions, common in supra-subduction zones due to volatile-rich fluid infiltration produced by slab dehydration (Proenza et al., 1999; Pearce et al., 2000; Marchesi et al., 2006, 2016). Also, primitive mantle-normalized trace element patterns (Figure 5B) of the stBudied MMU metaperidotites are similar to supra-subduction zone peridotites from the Tso Morari fore-arc (Savov et al., 2005).
On the other hand, the MMU hosts the major chromian spinel deposits described for Colombia. Several authors have suggested that podiform chromitites are predominantly formed within supra-subduction zone settings (Pearce et al., 1984; Roberts, 1988; Proenza et al., 1999; González-Jiménez et al., 2012, 2014a and references therein). Consequently, the ultramafic rocks in Medellín probably formed in a supra-subduction zone setting, where ophiolites show typical island arc signatures but have oceanic crust structures clearly formed by expansion processes related to a subduction zone (e.g.,: fore-arc, intra-arc, and back-arc basins). This interpretation agrees with the work by Correa-Martínez (2007), who considers the Aburrá Ophiolite an oceanic back-arc basin formed during the Triassic and later tectonically emplaced during Jurassic time.
5.2. Origin of the chromian spinel mineralization
The studied chromitite samples from Patio Bonito and San Pedro deposits are Al-rich (Figures 7C and 7D) and strongly PGE-depleted (Figure 6). Al-rich chromitites are usually found within the mantle-crust transition zone, or Moho Transition Zone (MTZ), near levels of layered gabbros at the base of the crust in ophiolite complexes (Leblanc and Violette, 1983; Proenza et al., 1999, González-Jiménez et al., 2014a; Mendi et al., 2020). The primary cores of chromian spinel forming the chromitite bodies associated with the MMU exhibit compositions that plot between the fields defined for chromian spinel from mid-ocean ridge basalts (MORB; Dick and Bullen, 1984) and boninite-like lavas (Figure 7D) (Arai, 1992). These compositions are similar to other Al-rich ophiolitic chromitites in central and southern America and the Caribbean region (Figures 7C and 7D) such as Moa-Baracoa, Cuba (Proenza et al., 1999), Tehuitzingo, México (Proenza et al., 2004b), Los Guanacos, Argentina (Proenza et al., 2008), and Cerro Colorado, Venezuela (Mendi et al., 2020).
The composition of the melt in equilibrium with unaltered (magmatic) chromian spinel cores of the Patio Bonito and San Pedro chromitites has been calculated using the following equations (Maurel and Maurel, 1982; Zaccarini et al., 2011):
The calculations give average values of Al2O3melt = 15.75 wt%, TiO2melt = 0.41wt% and (FeO/MgO)melt = 0.72 wt%, and plot overlapping the field described for tholeiitic magmas (MORB or BABB) in the FeO/MgO (melt) vs. Al2O3 (melt) binary diagram (Figure 10). These types of MORB/BABB magmas are characteristic for back-arc basins, a geodynamic environment consistent with the previously proposed geological setting of the MMU by other authors that is based on the study of the host peridotites (Correa-Martínez, 2007; Restrepo, 2008; García-Casco et al., 2020). The low PGE content in the chromitites is also consistent with the MORB/BABB composition of the parental magmas. PGE content in chromitite is strongly related with the parental magma composition: PGE-rich Cr-rich chromitites usually crystallize from boninitic magmas, whereas PGE-poor Al-rich chromitites (e.g., Colombian chromitites) crystallize from tholeiitic magmas. This is related to the fact that boninitic magmas are S-undersaturated and have higher PGE-contents than tholeiitic magmas, which are S-saturated (Hamlyn et al., 1985; Zhou et al., 1998; González-Jiménez et al., 2014b).
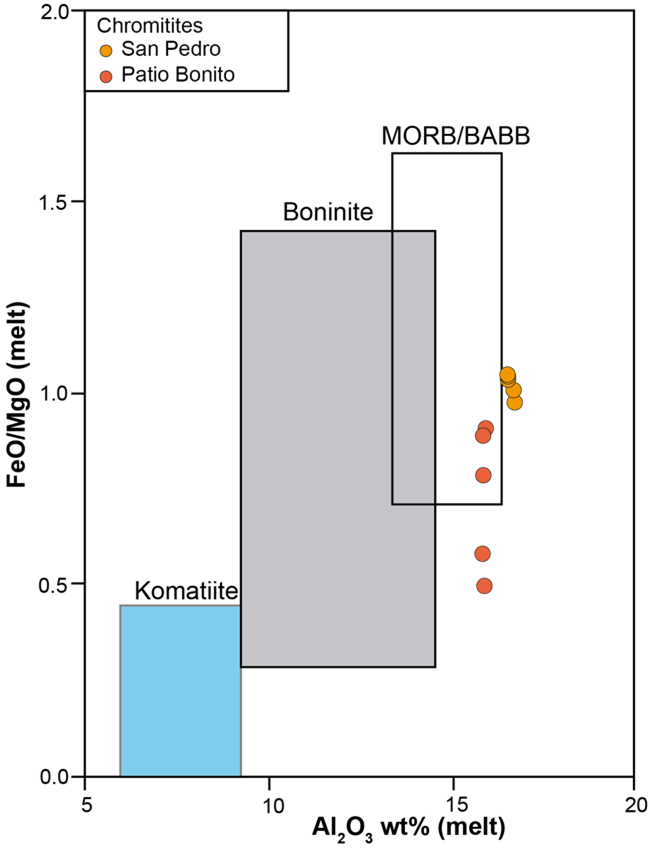
Figure 10 Composition diagram [(FeO/MgO)melt vs. (Al2O3)melt (wt%)] of the parental melt in equilibrium with the San Pedro and Patio Bonito chromitites. Melts in equilibrium were calculated using the equations by Maurel and Maurel (1982) and Zaccarini et al. (2011). Fields are from Moghadam et al. (2015).
The mechanism of crystallization of the chromitites within the supra-subduction back-arc mantle may be related with the mingling of MORB/BABB melts with different degrees of fractionation within dunite channels (Arai and Yurimoto, 1995; Melcher et al., 1997; Zhou and Robinson, 1997; Proenza et al., 1999, 2004b; González-Jiménez et al., 2011, 2014a). In this model, infiltrating melt reacts with the host harzburgite, generating secondary dunite in equilibrium with a more differentiated MORB/BABB melt, while the necessary Cr for chromitite formation is provided by the dissolution of Cr-rich pyroxene from the host peridotite. Mixing/mingling of melts with different SiO2 drives supersaturation in Cr in order to crystallize chromian spinel (Arai and Yurimoto, 1995; Melcher et al., 1997), which may be accumulated by bubble flotation in the relatively hydrous melt (Matveev and Ballhaus, 2002), in a self-sustained process to form massive bodies (González-Jiménez et al., 2011, 2014a and references therein).
5.3. Chromian spinel alteration
Textural and compositional variations observed in the chromian spinel of the MMU cannot be explained by magmatic/metamorphic processes at mantle temperatures or pressures. Instead, these variations suggest an origin related to the post-mantle metamorphic evolution of the ultramafic bodies. The metamorphic mineral associations in the metaperidotites (chlorite + tremolite + talc + forsterite) indicate temperatures of 550-700 ºC, at medium pressures up to 6 kbar (García-Casco et al., 2020).
Compositional and textural variations of the studied chromian spinel could be attributed to cooling associated with a metamorphic cycle during the Permian-Triassic period in the Mesozoic paleomargin of South America. The first transformation of chromian spinel, which forms porous chromian spinel (type II), can be explained by chromian spinel dissolution and chlorite precipitation. Chlorite coronas surrounding chromian spinel and the systematic presence of secondary recrystallized olivine, tremolite and talc indicate medium-T metamorphic imprint (amphibolite facies) during cooling (García-Casco et al., 2020). This transformation is characterized by a decrease in Al and Mg coupled with an increase in Cr and Fe2+. At this stage, the residual chromian spinel is enriched in Cr, whereas the Fe3+ content stays invariable. The porous chromian spinel (type II) composition (Mg# vs. TiO2, MnO, ZnO) is typical of chromian spinel metamorphosed at amphibolite facies (Barnes, 2000; Saumur and Hattori, 2013; Colás et al., 2014, 2019). The formation of this secondary chromian spinel can be represented with the following reaction proposed by González-Jiménez et al. (2015):
According to these authors, at temperatures between ~ 700 and ~ 400 ºC and in the presence of SiO2-rich fluids, primary Al-rich chromian spinel reacts with forsterite to produce chlorite and residual porous type II chromian spinel, enriched in Cr and Fe2+.
Homogeneous chromian spinel (type III) is Fe3+-rich in comparison to the other two chromian spinel types (Figure 7B). The Fe3+# values vary from 0.3 to 0.79, and, therefore, this chromian spinel can be classified as ferrian chromian spinel. The formation of ferrian chromian spinel has been interpreted as the reaction product between chromian spinel and secondary magnetite (Barnes, 2000), or between chromian spinel and antigorite (Merlini et al., 2009) during prograde metamorphism. On the other hand, it has also been interpreted as the reaction product between primary chromian spinel and olivine (Gervilla et al., 2012) or between chromian spinel and lizardite (Mellini et al., 2005) during the cooling of an ultramafic body.
The homogenous chromian spinel formation implies the dissolution of already formed Cr-rich spinel, probably at oxidizing conditions through the addition of magnetite to the porous Al-Mg-depleted chromian spinel (type II) during a late hydrothermal process starting at temperatures close to 600 ºC and evolving to temperatures lower than 500 ºC (Gervilla et al., 2012) or 350 ºC (Colás et al., 2019). The composition (Mg# vs. TiO2, MnO, and ZnO) of homogeneous chromian spinel (type III) from the MMU is also typical for metamorphosed chromian spinel in amphibolite facies (Barnes, 2000; Saumur and Hattori, 2013; Colás et al., 2019) with similarities to chromian spinel metamorphosed at greenschist facies.
6. Conclusions
The petrological and geochemical characteristics of the metaperidotites from the MMU indicate that these represent shallow levels of the suboceanic lithospheric mantle related to a supra-subduction zone setting (back-arc basin/incipient arc scenario). The chromian spinel mineralization associated with the MMU is Al-rich (refractory grade) and is strongly depleted in PGE. The most favorable geodynamic setting for such chromitite formation is a back-arc basin, where the chromian spinel crystallizes from a BABB-type tholeiitic magma. Accessory chromian spinel in the metaperidotites is classified as: (i) partially altered chromian spinel with Al-rich cores, (ii) porous Cr-Fe2+-enriched and Al-Mg-depleted chromian spinel, and (iii) homogeneous Fe3+-rich chromian spinel. Textural and compositional variations of accessory chromian spinel in the metaperidotites of the MMU give evidence of the superimposed metamorphic processes of the MMU, which has reached amphibolite facies and later retrograded to the greenschist facies conditions.











 nueva página del texto (beta)
nueva página del texto (beta)


