Introduction
Catalysts are broadly used in the chemical and oil industries to upgrade diverse types of important processes. These industrial catalysts regularly consist of metals supported on porous materials like alumina or silica. During operations, these catalysts deactivate with their periodical use (Jong, Rhoads, Stubbs & Stoelting, 1992), through structural changes, poisoning, or the deposition of external materials, and then sent to on-site or off-site regeneration plants. However, the regeneration of the spent catalysts that are discarded from industrial processes can only be performed for a limited number of times and it is only possible for some of these residues. Thus, when regeneration is not possible because the catalyst can no longer perform its original duty, is referred to as “spent catalyst”, and is considered as a solid waste.
Catalysts are used in a broad range of industrial processes and in elevated amounts, commonly to produce clean fuels and many other valuable products (Marafi, Stanislaus &Furimsky, 2010; Stanislaus, Marafi & Rana, 2010). It has been reported that spent hydroprocessing catalysts are the major solid wastes of refining industries, representing the main contributors to the generation of spent catalysts (Liu,Yu & Zhao, 2005), being annually produced between 150,000-170,000 tonnes of spent hydroprocessing catalysts worldwide (Chiranjeevi, Pragya, Gupta, Gokak & Bhargava, 2016), and the amount will continue to increase as new hydrotreatment processes are needed to meet the growing demand. Spent catalysts have been classified as hazardous residues by the Environmental Protection Agency (EPA) in the USA due to their dangerous self-heating liability and their highly toxic content (Eijsbouts, Battiston & van Leerdam, 2008), caused by the simultaneous presence of metals and other non-metallic elements, such as Al, V, Mo, Co, Ni, As and Fe, and elemental sulfur, carbon and oils, respectively (Mishra, Kim, Ralph, Ahn & Rhee, 2008). Besides, the metals contained in spent catalysts can be leached after disposal due to water action, generating pollution dispersion (Marafi & Stanislaus, 2007; 2008a,b), and/or may react with other environmental components like oxygen, which can cause the release of toxic gases such as H2S, HCN, or NH3 (Noori Felegari, Nematdoust Haghi, Amoabediny, Mousavi & Amouei Torkmahalleh, 2014).
Spent catalysts can be moderately regenerated to be re-used as catalysts for other processes (Kim & Shim, 2008a,b; Shim & Kim, 2010; Bitemirova, Alihanova, Spabekova, Shagrayeva & Ermahanov, 2015), treated before final disposal for the recovery of valuable metals, or directly disposed in landfills as solid wastes, although this latter option may be the least recommended one, due to environmental constraints. Considering the worrying exhaustion of natural resources and the elevated environmental pollution nowadays, the recovery of metals from spent catalysts has been under the scope in the last years, as they represent a source of commercially valuable metals, offering a viable alternative for the recovery of the metallic components contained therein, in order to reduce the amount of disposed waste and promoting the conservation of natural resources.
Recycling of spent catalysts
There have been suggested interesting options for the usage of spent catalysts as raw materials for the production of other valuable products, which may also represent an attractive option for the recycling (instead of disposal) of these types of residues. Diverse materials have been prepared using spent catalysts, such as abrasive components for the ceramic and refractory industries (Zeiringer, 1979), aggregates for concrete production (Stanislaus, Gouda & Al-Fulaij, 1998) or in road bases and sub-bases for construction aplications (Taha, Al-Kamyani,Al-Jabri, Baawain & Al-Shamsi, 2012), production of refractory bricks and cement (Vargas et al., 2018), as a component in asphalt mixtures (Yoo, 1998), anorthite glass-ceramics for application as an electrical insulating material (Su, Chen & Fang, 2001), as a wastewater filtering agent (Sanga & Nishimura, 1976), in combination with activated sludge for biological treatment of wastewater from municipal and industrial sources (Liles & Schwartz, 1976) and properly as catalysts for other applications, including the reduction of nitrogen oxides (Choi, Kunisada, Korai, Mochida & Nakano, 2003). However, most of the processes regarding these recycling options are still under study in a laboratory stage (Marafi & Stanislaus, 2008a).
Traditional techniques for metal extraction from spent catalysts
For the recovery of precious metals, plasma technologies have been assessed in a wide range of spent catalysts, especially to recover Pt group metals from these high metal content residues generated in both automotive and diverse industrial processes, where the same metal recovery procedure can be used to deplete the hazardous properties of the spent catalyst while recovering the metals contained therein for their reutilization (Rui,Wu, Ji & Liu, 2015). Also, hydrometallurgical (treatment in organic and inorganic aqueous medium), pyrometallurgical (heating, roasting), and chelating agent methods for the treatment of spent catalysts and metal recovery are available, and were reviewed in detail by Akcil, Vegliò, Ferella, Okudan &Tuncuk (2015). Although these conventional approaches confer an economic advantage, they generate large volumes of potentially hazardous wastes and emission of harmful gases (Llanos & Lacave, 1986), which involve high costs and environmental risks. Thus, new alternatives are needed to develop eco-friendly solutions associated with the treatment of this kind of residues (Marafi & Stanislaus, 2008a,b).
Microbiological approaches for the treatment of spent catalysts
As it has been previously sustained, biotechnological methods may represent a promising alternative for the treatment of spent catalysts (Noori-Felegari, et al., 2014), due to important microbial properties, like their ability to survive and adapt to elevated metal concentrations, and also to transform solid non-essential metals into soluble and extractable elements that could be recovered (Yang, Qi, Low & Song, 2011; Sahu, Agrawal & Mishra, 2013). In this regard, research has also been performed to develop bio-approaches for the mining industry. To date, several bio-techniques comprised under the term of "biohydrometallurgy" have been investigated, standardized, or even industrially exploited (Mishra, Kim, Ralph, Ahn & Rhee, 2007), including: a) the removal of metals contained in low-grade ores or low-grade mineral resources (Brombacher, Bachofen & Brandl, 1997; Olson, Brierley & Brierley, 2003) and residues (Krebs, Brombacher, Bosshard, Bachofen & Brandl, 1997) by the action of microorganisms, b) the recovery of these metals, and c) the subsequent metal purification steps.
Bioleaching is one of the techniques included in biohydro-metallurgical applications (Asghari, Mousavi, Amiri &Tavassoli, 2013), which enables metal recycling by processes similar to the ones found in the natural biogeochemical cycles (Brierley, 2008), being demonstrated its suitability for the successful removal of metals contained in diverse kinds of solid industrial wastes, like fly ash (Burgstaller & Schinner, 1993; Bosshard, Bachofen & Brandl, 1996; Brombacher et al., 1997; Xu, Ramanathan & Ting, 2014), sewage sludge (Chartier & Couillard, 1997), spent batteries (Cerrutti, Curutchet & Donati, 1998), electronic scrap materials (Brandl, Bosshard & Wegmann, 2001), and spent catalysts (Santhiya & Ting, 2005; Marafi & Stanislaus, 2008b). Besides, bioleaching has also been applied for the bioremediation of contaminated soils (Chen & Lin, 2004; Gadd, 2004) and sediments (Beolchini, Rocchetti, Regoli & Dell’ Anno, 2010b). It is important to mention that bioleaching approaches can be considered as more eco-friendly techniques, whose development is important to attenuate the negative environmental impacts of the traditional methods applied to date (Mishra et al., 2007), and have been gaining importance due to their following demonstrated advantages in comparison to conventional processes of metal extraction: besides they represent environmental-friendly technologies, they also involve lower costs and lower energy requirements, are simpler and cheaper to perform and maintain, they may operate at environmental pressure and non-excessive temperatures, they present higher efficiencies in terms of heavy metal removal and non-strict requirements of raw material composition, they have been successfully applied at industrial scale for low grade ores (concentration of metals < 0.5 wt %) and are applicable for highly contaminated materials. In addition, these approaches do not generate hazardous emissions (Akcil et al., 2015). Above all, no chemical reagents are needed for the bioleaching process, as these processes are biologically induced with no requirement of a continuous delivery of other raw materials to the processing plant, which implies a reduction in the environmental and economical impacts, as it has been stated the diminished production of carbon emissions due to transportation, and also that raw materials represent a significant part (52.2%) of chemical leaching costs for spent hydrogenation catalysts (Yang et al., 2011). It has also been established that carbon emissions have the major contribution, together with energy, on the impact of these chemical leaching procedures, in terms of global warming potential (Beolchini, Fonti, Dell’Anno, Rocchetti &Vegliò, 2012).
During bioleaching processes, the leached and recovered highly valuable metals may be recycled and re-used as secondary raw materials (Bosshard et al., 1996; Brandl et al., 2001). Thus, a lot of the large-scale bioleaching industrial facilities are located in developing countries, mainly due to two important factors: 1) the significant mineral reserves and mining industries they have; and 2) the simplicity and low-cost requirements of bioleaching techniques (DaSilva, 1981; Gentina & Acevedo, 1985; Warhurst, 1985; Acharya, 1990; Acevedo, Gentina & Bustos, 1993; Acevedo, 2002). This is the case of Mexico, and also of countries like Chile, Indonesia, Peru and Zambia. In the specific case of Mexico, the company Peñoles S.A. has established an integrated process consisting of bioleaching, solvent extraction and electrowinning, successfully generating 500 kg of Cu per day (Acevedo, 2002).
Microorganisms used for the biotreatment of spent catalysts
During the growth of microorganisms, some formed metabolites may be useful to perform the extraction of valuable metals from waste materials, due to their acidic nature or their complex formation capability. As illustrated in Figure 1, the ability of diverse microorganisms to remove and leach metals contained in solid materials may be due to: a) the transformation of organic or inorganic acids; b) oxidation and reduction reactions; and c) the production of complexing agents. Metals can be leached either directly, by the physical contact between microorganisms and solid materials, or indirectly, by the bacterial oxidation of an element (for example Fe2+ to Fe3+), which catalyses metal solubilization as an electron carrier (Krebs et al., 1997). Specifically, diverse microorganisms have been analyzed to determine their metal removal capabilities from spent catalysts, comprising bacteria, archaea and fungi. The compiled results reported in this respect have been previously addressed by Lee & Pandey (2012), Srichandan, Kim, Gahan & Akcil (2013), Mishra & Rhee (2014) and Akcil et al. (2015). Additionaly to previous compendiums, Table I presents an upgrade of the results reported to date. Furthermore, the removal abilities and characteristics of the diverse microorganisms that have been used for this purpose are described below.
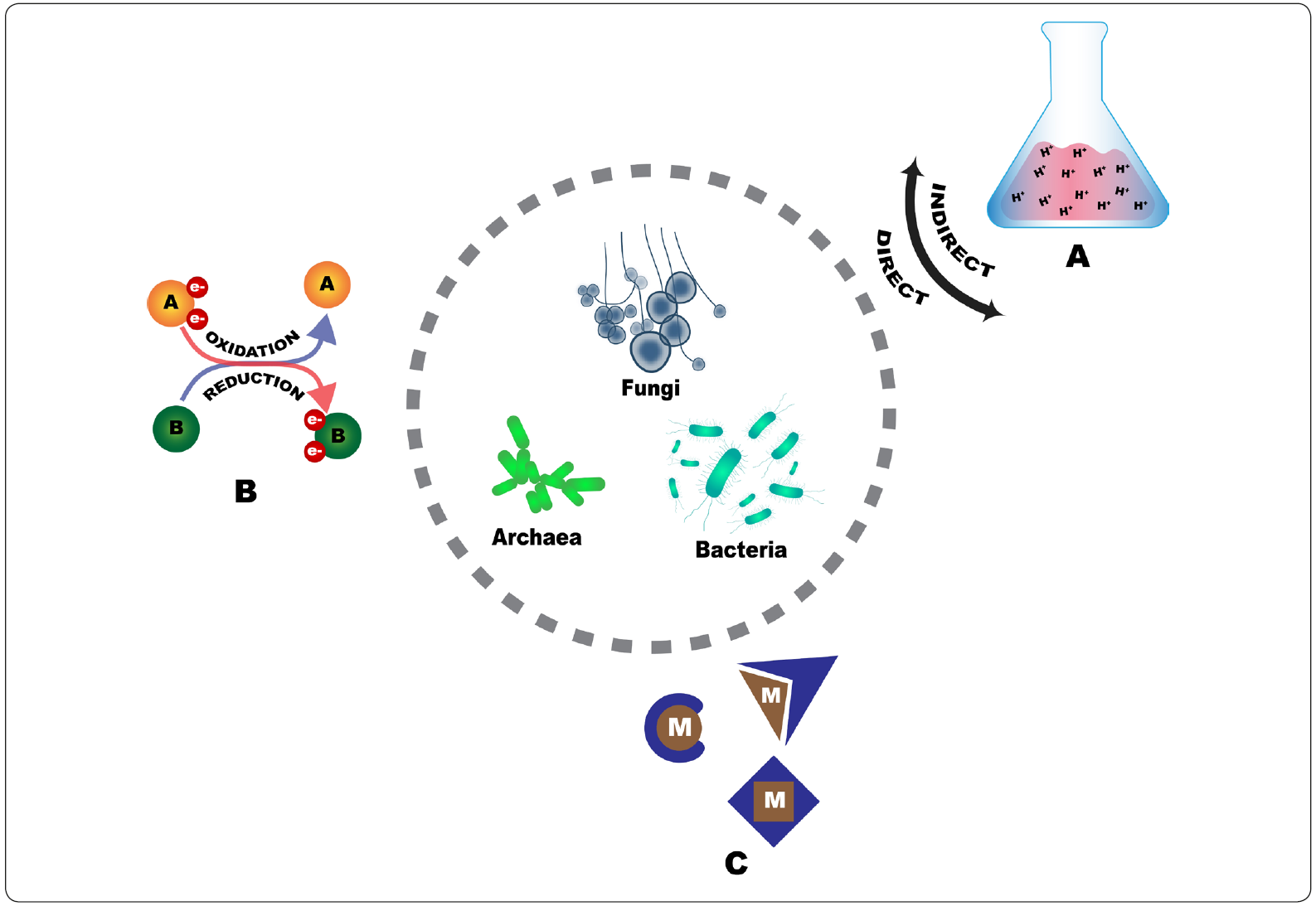
Figure 1 Mechanisms of metal bio-removal by microorganisms: A) Production of acids for direct or indirect biolixiviation; B) Oxidation and reduction reactions; or C) Production of metal (M) complexing agents. Figure designed by the authors.
Table I Metal removal from spent catalysts by different microorganisms.
| Microorganism | Removal efficiency (%) | Spent catalyst type | Reference | ||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Ni | Mo | V | |||
| Archaea | |||||||
| Acidianus brierleyi | 67 | 100 | 100 | 100 | -a | Hydrotreating catalyst | Bharadwaj & Ting, 2013 |
| Acidianus brierleyi | 35 | - | 69 | 83 | - | Hydrocracking catalyst | Gerayeli et al., 2013 |
| Fungi | |||||||
| Acremonium sp. | - | - | 21 | - | 23.5 | Hydrocracking catalyst | Gómez-Ramírez et al., 2015b |
| Aspergillus niger | 30 | 23 | 9 | - | 36 | Fluid catalytic cracking catalyst | Aung & Ting, 2005 |
| Aspergillus niger | 54.5 | - | 58.2 | 82.3 | - | Refinery processing catalyst | Santhiya & Ting, 2005 |
| Aspergillus niger | 65.2 | - | 78.5 | 82.4 | - | Refinery processing catalyst | Santhiya & Ting, 2006 |
| Aspergillus niger | 13.9 | - | 45.8 | 99.5 | - | Hydrocracking catalyst | Amiri et al., 2012 |
| Penicillium sp. | - | - | 0.0 | - | 24 | Hydrocracking catalyst | Gómez-Ramírez et al., 2015b |
| Penicillium simplicissimum | 25 | 100 | 66.4 | 92.7 | - | Hydrocracking catalyst | Amiri et al., 2011 |
| Rhodotorula mucilaginosa | - | - | 87 | - | 48 | Petroleum catalyst | Arenas-Isaac et al., 2017 |
| Rhodotorula mucilaginosa | - | - | 9.4 | - | 2 | Hydrocracking catalyst | Gómez-Ramírez et al.,2014 |
| Bacteria | |||||||
| Acidithiobacillus spp. | - | - | 85 | 26 | 92 | Hydroprocessing catalyst | Kim et al., 2008 |
| Acidithiobacillus spp. | - | - | 88 | 46 | 95 | Petroleum catalyst | Pradhan et al., 2009 |
| Acidithiobacillus thiooxidans | - | - | 88.3 | 58 | 32.3 | Refinery catalyst | Mishra et al., 2007 |
| Acidithiobacillus thiooxidans | - | - | 88 | 46 | 95 | Hydroprocessing catalyst | Mishra et al., 2008 |
| Acidithiobacillus thiooxidans | 2.4 | - | 16 | 95 | - | Naphta hydrotreating catalyst | Gholami et al., 2015 |
| Acidithiobacillus thiooxidans | 5.7 | 0.8 | 0.0 | 0.0 | - | Hydroprocessing catalyst | Rivas-Castillo et al., 2018 |
| Acidithiobacillus thiooxidans | 0.4 | 0.8 | 0.1 | 0.0 | - | Automotive catalyst | Rivas-Castillo et al., 2018 |
| A. thiooxidans and A. ferrooxidans | 10.0 | - | 58.6 | 5.8 | 33.4 | Refinery catalyst | Pathak et al., 2015 |
| A. thiooxidans, A. ferrooxidans and L. ferrooxidans | - | - | 83 | 40 | 90 | Hydroprocessing catalyst | Beolchini et al., 2010a,b, 2012 |
| Acidithiobacillus spp. and Sulfobacillus thermosulfidooxidans | 38 | - | 97 | - | 91 | Petroleum catalyst | Srichandan et al., 2014 |
| Bacillus megaterium | - | - | 10 | - | 6.5 | Hydrocracking catalyst | Arenas-Isaac et al., 2017 |
| Bacillus megaterium | 0.0 | - | 22.6 | 6.0 | 46.4 | Hydrocracking catalyst | Rivas-Castillo et al., 2017a |
| Bacillus megaterium | 0.8 | - | 0.5 | - | 1.6 | Petroleum catalyst | Rivas-Castillo et al., 2019 |
| Cupriavidus metallidurans | 0.0 | 0.0 | 0.0 | 17.5 | 15.9 | Petroleum catalyst | Rivas-Castillo et al., 2017b |
| Microbacterium liquefaciens | - | - | 40.6 | - | 9.3 | Petroleum catalyst | Rojas-Avelizapa et al., 2015 |
| Microbacterium liquefaciens | - | - | 45 | - | 25 | Petroleum catalyst | Gómez-Ramírez et al., 2015a |
| Microbacterium spp. | - | - | 51 | - | 41.4 | Petroleum catalyst | Gómez-Ramírez et al., 2015a |
a- Not Determined.
Archaea
Some reports have already suggested the potential of thermophilic microorganisms for the bioleaching of spent catalysts (Deveci, Akcil & Alp, 2004). The sulfur-oxidizing extreme thermophile Acidianus brierleyi, which grows best in pH 1-2 and temperature 60-70 °C, has been identified with a good potential to perform the recovery of metals contained in minerals (Konishi, Tokushige, Asai & Suzuki, 2001). Also, it was shown that when exposed to the presence of spent hydrotreating catalysts, in a pulp density between 0.6-1% (w/v), A. brierleyi is capable of sustaining growth, and furthermore, metal solubility was observed in the ranges of 35-67% Al, 100% Fe, 69-100% Ni, and 83-100% Mo (Bharadwaj & Ting, 2013; Gerayeli, Ghojavand, Mousavi, Yaghmaei & Amiri, 2013). These authors, also demonstrated that Ni and Mo bioleaching using this microorganism was more effective than chemical leaching using commercial sulfuric acid (Bharadwaj & Ting, 2013).
Fungi
Besides their intrinsic removal capabilities, especially the incremented tolerance of microbial strains isolated from extremely polluted environments, some microorganisms possess the ability to survive to high concentrations of toxic heavy metals, by adaptation or mutation processes (Konishi et al., 2001; Valix & Loon, 2003; Bharadwaj & Ting, 2013; Gerayeli et al., 2013), which may confer them with exceptional survival advantages. For this reason, some researchers have inquired around this idea in order to obtain heavy metal-tolerant fungal strains, including descendants from Penicillium funiculosum, Aspergillus foetidus and Penicillium simplicissimum, specifically for the bioleaching of Ni laterite ores and low-grade ore materials (Valix & Loon, 2003; Santhiya & Ting, 2006; Liu et al., 2008); and Acremonium spp. and Penicillium spp. strains isolated from a high metal content soil have also been assessed for their metal removal capabilities from an hydrotreating spent catalyst (Gómez-Ramírez, Plata-González, Fierros-Romero & Rojas-Avelipaza, 2015b). Amiri, Yaghmaei & Mousavi (2011) adapted the fungus P. simplicissimum to the metals Ni, Mo, Fe, and W, which were known to be present in a W-rich spent hydrocracking catalyst, and then performed a spent catalyst bioleaching assay using one-step and two-step processes, as well as assessing leaching efficiencies using the spent medium, at pulp densities between 1-5% (w/v). They reported the optimum removal efficiencies of 25% Al, 100% Fe, 66.4% Ni, 92.7% Mo, and 100% W, and also stated that an optimized two-step bioleaching process may be a suitable alternative to conventional treatment methods. As well, Santhiya & Ting (2006) performed the adaptation of Aspergillus niger to Ni, Mo and Al in order to assess the tolerance increment of this fungus to a spent refinery processing catalyst, observing that the Ni:Mo:Al-adapted strain extracted 78.5% Ni, 82.4% Mo and 65.2% Al, which represented higher Al and Ni removals compared to the ones with the non-adapted culture, demonstrating that adaptation may be a promising approach for the biotreatment of spent catalysts and high metal content wastes.
A.niger is one of the most widely used fungus for bioleaching approaches (Santhiya & Ting, 2005), and has also been used in the production of organic acids, such as citric acid (Grewal & Kalra, 1995), oxalic acid (Strasser et al., 1994) and gluconic acid (Dronawat, Svihla & Hanley, 1995), which can be used as lixiviants of heavy metals contained in ore materials and solid wastes (Bosshard et al., 1996; Groudev, Spasova, Georgiev & Nicolova, 2014). Results showed that the presence of a spent catalyst may cause a decrease in the biomass yield of this fungus but an increase in its oxalic acid secretion (Santhiya & Ting, 2005). The extraction of metals by A. niger from diverse spent catalysts in the presence of pulp densities between 1-3% (w/v) were in the range of 13.9-54.5% Al, 9-58.2% Ni, 82.3-99.5% Mo, and 36% V (Aung & Ting, 2005; Santhiya & Ting, 2005; Amiri, Mousavi, Yaghmaei & Barati, 2012). Besides, it was also demonstrated the lixiviation ability of Fe (23%), and Sb (64%) by A. niger from a spent catalyst, also reporting that its metal extraction efficiency tends to decrease with increased pulp density, and as in the case of other microbial bioleaching processes (Bharadwaj & Ting, 2013), this biotechnological approach allowed higher metal extraction yields than chemical leaching (Aung & Ting, 2005).
The yeast Rhodotorula mucilaginosa has been also tested for its metal removal capability, and it has been reported that a strain isolated from a filter plant of a Cu mine located in the Northwest of Argentina is capable of accumulating up to 44 % of Cu from a medium supplemented with 0.5 mM CuSO4 (Villegas, Amoroso & Figueroa, 2005). Also, a report has been published where the heavy metal-resistant R .mucilaginosa strain UANL-001L, isolated from the Northeast region of Mexico, presented a Minimum Inhibitory Concentration (MIC) of 1000 mg/L to Zn and Pb, and MICs between 600 and 800 mg/L to Cr (III and VI), Cu, Cd and Ni. Also, this strain can produce an exopolysaccharide (EPS) during growth, which production is enhanced by the presence of metals like Zn (II), Pb (II), Cr (VI), Cu (II), Ni (II) and Cd (II) (Garza-González et al., 2016). Besides, another R. mucilaginosa strain, coded as MV-9K-4, was isolated from a high metal content site in Guanajuato, Mexico, and when exposed to a spent catalyst at 16% (w/v) pulp density, presented the ability to remove 87% Ni and 48% V, being one of the most relevant strains in terms of its Ni and V removal capability of all the strains tested that were isolated in-situ from different mining sites (Arenas-Isaac et al., 2017).
Bacteria
Most of the studies about the biotreatment and extraction of valuable metals from spent catalysts have been focused on the use of the acidophilic sulfur-oxidizing bacteria Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans (Rohwerder, Gehrke, Kinzler & Sand, 2003; Beolchini, Fonti, Ferella &Vegliò, 2010a; Hong & Valix, 2014) in liquid and column systems (Pathak, Srichandan & Kim, 2019), mainly because they present biolixiviating properties. Besides, these microorganisms are autotrophic and tolerate high concentrations of heavy metals. The usefulness of Acidithiobacillus species for metal solubilization from ores and solid wastes is closely related to their ability to acidify their habitat by the production of special metabolic byproducts as leaching agents, like sulfuric acid and sulfur-oxidation intermediates (Sand, Gehrke, Jozsa & Schippers, 2001).
A. thiooxidans and A. ferrooxidans have been previously reported with the ability to reduce V (V) to V (IV) in the presence of elemental sulphur (Brandl et al., 2001; Bredberg, Karlsson & Holst, 2004), and studies have also demonstrated the applicability of these sulfur-oxidizing bacteria for the release of metals contained in different spent catalysts at diverse pulp densities, being capable of removing Ni, V and Mo in the ranges of 0.1-99%, 25-95%, and 25-95%, respectively (Mishra et al., 2007, 2008; Pradhan, Mishra, Kim, Chaudhury & Lee, 2009; Gholami, Borghei & Mousavi, 2011; Gholami, Razeghi & Ghasemi, 2015; Pathak, Srichandan & Kim, 2015; Ferreira, Sérvulo, Ferreira & Oliveira, 2016; Rivas-Castillo, Gómez-Ramírez, Rodríguez-Pozos & Rojas-Avelipaza, 2018). Both bacterial species, A. thiooxidans and A. ferrooxidans, seem to present similar leaching kinetics under the same conditions of pH, nutrient concentration, pulp density, particle size and temperature, and their dissolution kinetics were reported to be higher for Mo than for Ni and V (Pradhan et al., 2009). Also, Acidithiobacillus spp. Al and Co removal capabilities were reported between 0.4-89% and 83-96%, respectively (Gholami et al., 2011, 2015; Pathak et al., 2015; Sharma et al., 2015; Ferreira et al., 2016; Rivas-Castillo et al., 2018). Both Mishra et al. (2008) and Pradhan et al. (2009) reported that two-step processes may be the most suitable to increase the bioleaching efficiencies of Acidithiobacillus spp., due to the general advantages of two-stage processes, as that the independent generation of the lixiviating agent separates the bioprocess from the chemical process, making it possible to optimize each step independently in order to maximize productivity (Mishra et al., 2008), and they also stated that higher waste concentrations can be treated with a two-step procedure, instead of a one-step process, to increase metal removal yields (Johnson, 2013).
Likewise, investigations with acidophilic bacteria have been conducted using mixed cultures of Acidithiobacillus spp., A. thiooxidans and A. ferrooxidans (Kim, Mishra, Park, Ahn & Ralph, 2008), and A. ferrooxidans, A. thiooxidans and Letosphirilum ferrooxidans (Beolchini et al., 2012), grown in the presence of a broad range of pulp densities, in the range of 0.15-10% (w/v) of spent catalysts. Results showed the removal of Ni, Mo and V to the extent of 83-85%, 26-40% and 90-92%, respectively, emphasizing the potential of this type of microorganisms to remove significant amounts of Ni and V, and a less amount of Mo. Furthermore, assays have been made to determine the leaching potential of moderate thermophilic bacteria using a mixed consortium of moderate thermophilic iron and sulphur oxidizers: Sulfobacillus thermosulfidooxidans, Acidithiobacillus caldus, A. ferrooxidans, and A. thiooxidans, in the presence of 10% (w/v) pulp density of a spent catalyst, where higher recoveries of Ni (92-97%) and V (81 - 91%) were obtained, whereas leaching of Al (23-38%) was lowest in all the assessed particle sizes of the spent catalysts, suggesting that bioleaching using a consortium of moderate thermophilic microorganisms may be also an efficient process for the recovery of metals from spent catalysts (Srichandan et al., 2014).
Besides sulfuric acid, which is the mainly acid found in bioleaching processes due to the metabolism of Acidithiobacillus species (Sand et al., 2001; Rawlings, 2002), other organic acids may be produced by bacterial and fungal metabolisms, that may also promote metal removal from solid materials by acidification or complex and chelate formations (Burgstaller & Schinner, 1993). One of these cases is the solubilization of metals by Hydrogen Cyanide (HCN), which may be produced during microbial growth (Faramarzi & Brandl, 2006). As cyanide forms water-soluble metal complexes of high chemical stability, it may be a promising strategy for the recovery of metals that are removed from solid residues (Brandl, Lehmann, Faramarzi & Martinelli, 2008; Motaghed, Mousavi, Rastegar & Shojaosadati, 2014). However, it is known that working with cyanide compounds present the inconvenience of HCN volatilization, which is a potent hazardous gas (Luque-Almagro, Moreno-Vivián & Roldán, 2016). It has been reported that B. megaterium strain PTCC 1656 may produce HCN when grown under glycine-rich conditions (Faramarzi, Stagars, Pensini, Krebs & Brandl, 2004; Faramarzi & Brandl, 2006).Thus, this strain was grown under these conditions in the presence of a spent refinery catalyst rich in Pt and Re at pulp densities of 1-10% (w/v), showing that after 7 days in the presence of 4% (w/v) pulp density of the residue, the maximum extraction for Pt and Re corresponded to 15.7% and 98%, respectively (Motaghed et al., 2014).
To address the hypothesis that native microorganisms from high metal content sites may present evolutionary advantages in reference to resistance and metal removal capabilities in the presence of spent catalysts, Arenas-Isaac et al. (2017) performed an in-situ sampling in four different mining sites in Guanajuato, Mexico, and demonstrated that all isolates recovered from these locations presented tolerance limits greater than 200 ppm for Ni and V. Moreover, when the strain coded as MV-9K-2, identified as Bacillus megaterium, was exposed to a spent catalyst at a pulp density of 16% (w/v), it was able to remove 2541.7 mg/kg of Ni and 3750 mg/kg of V, corresponding to 10% and 6.5% of each metal, respectively, showing the enhanced potential of MV-9K-2 for Ni and V removal from high metal content residues (Arenas-Isaac et al., 2017). Also, B. megaterium strain MNSH1-9K-1, which was isolated during the same in-situ sampling, has been identified for its ability to remove up to 0.8% Al, 0.5% Ni, 46.4% V and 6% Mo from high metal content spent catalysts (Rivas-Castillo, Orona-Tamayo, Gómez-Ramírez & Rojas-Avelipaza, 2017a; Rivas-Castillo, Guatemala-Cisneros, Gómez-Ramírez & Rojas-Avelipaza, 2019).
Another in-situ isolated microorganism is Cupriavidus (Wautersia, Ralstonia, Alcaligenes) metallidurans strain CH34, which is widely known for its multiple heavy metal resistance and for possessing a proven capability for simultaneous heavy metal accumulation. When in contact with a spent catalyst, this strain was able to remove 2111.20 ± 251.81 mg/kg of V and 931.56 ± 95.38 mg/kg of Mo, representing the 15.93% and 17.58% of each metal content in the residue, respectively (Rivas-Castillo et al., 2017b). On the other hand, it has been reported that Microbacterium spp. have been found in metal contaminated sites, and some isolates present enhanced resistance to As (Kaushik et al., 2012), and resistance and removal capabilities for U (Islam & Sar 2016). Also, some Microbacterium spp. strains were isolated in-situ from high metal content sites in Guanajuato, Mexico (Arenas-Isaac et al., 2017), and three isolates, namely Microbacterium liquefaciens MNSH2-PHGII-2, Microbacterium oxydans MNSH2-PHGII-1, and Microbacterium oxydans MV-PHGII-2 were evaluated on their potential for Ni and V removal contained in different spent catalysts, at pulp densities of 8 and 16% (w/v). Results showed that these strains present the ability to remove Ni (16-45.4%) and V (9.5-41.4%) contained in the high metal content residues, varying in their Ni and V removal capabilities between the strains isolated from the same site, or even between the strains of the same specie isolated from different sites (Arenas-Isaac et al., 2017; Gómez-Ramírez, Flores-Martínez, López-Hernández & Rojas-Avelipaza, 2014; Gómez-Ramírez, Montero-Álvarez, Tobón-Avilés, Fierros-Romero & Rojas-Avelipaza, 2015a). Furthermore, M. liquefaciens strain MNSH2-PHGII-2 was assessed for its ability to remove Ni and V from a spent catalyst at 80% (w/v) pulp density in a glass-column system at laboratory conditions, showing a removal capability of 40.6% and 9.3% for Ni and V, respectively (Rojas-Avelizapa, Gómez-Ramírez & Alamilla-Martínez, 2015).
Heterogeneity of the spent catalysts used for biotechnological experimentation
It is notorious that the diverse spent catalysts that have been used for metal removal experimentation are originated from different sources, and both their metal compositions and the pulp densities used for this purpose are different among the studies, as it is shown in the data presented in Table II. Besides, the experimental conditions reported differ between the assays, and it has been demonstrated that metal uptake and spent catalyst biotreatment efficiencies may vary with metal and pulp density concentrations, particle size, pH, temperature, incubation time, growth phase of the microorganisms used and inoculum concentration (Srichandan et al., 2014; Fan, Onal Okyay & Rodrigues, 2014; Motaghed et al., 2014). Thus, all these variables may represent an inconvenience for the accurate comparison of the metal removal abilities of the different microorganisms that have been tested. In addition, the removal capabilities are commonly reported in removal percentage, which may be tricking, as they represent the percentage content from varied metal compositions found in the different spent catalysts, and at diverse pulp densities. For example, it is reported that B. megaterium strain MV-9K-2 is able to remove only 10% of Ni from a spent catalyst, which although it may be seen as a low percentage, it represents 2541.7 mg/kg removed from a spent catalyst that contains 24,822 mg/kg of Ni, in contrast to M. liquefaciens strain MNSH2-PHGII-2 that was able to remove 45% of Ni from a spent catalyst that only contains 427.5 mg/kg of Ni (Arenas-Isaac et al., 2017). Also, it has been previously observed that diverse genera of microorganisms present different metal removal preferences, that may also depend on the total metal charge, and the amounts and the types of metals and other components (as hydrocarbons) present in the solid residues (Rivas-Castillo et al., 2017a,b). Thus, the establishment of similar experimental conditions is essential in order to perform a proper comparison of the metal removal efficiencies and metal removal selectivity between different microorganisms.
Table II Compositions and pulp densities of spent catalysts used for biotreatment experimentation.
| Metal composition (wt %) | Pulp densities (% w/v) | Reference | ||||
|---|---|---|---|---|---|---|
| Al | Fe | Ni | Mo | V | ||
| 17.50 | 0.56 | 0.26 | - | 0.39 | 1 - 12 | Aung & Ting, 2005 |
| 19.20 | 49.00 | 2.10 | 8.50 | -a | 1 | Bharadwaj & Ting, 2013 |
| 39.40 | - | 0.06 | 8.00 | - | 0.15 - 4 | Gholami et al., 2011 |
| 10.97 | 0.03 | 2.48 | 3.27 | 5.76 | 8 | Arenas-Isaac et al., 2017 |
| 10.31 | 0.40 | 0.04 | 0.002 | 0.22 | 16 | Gómez-Ramírez et al., 2014 |
| 14.20 | 1.50 | 1.70 | 1.20 | 7.70 | 0.5 - 5 | Mishra et al., 2008 |
| 15.31 | - | 2.70 | 2.34 | 8.76 | 1 | Pathak et al., 2015 |
| 19.50 | 0.30 | 2.00 | 1.40 | 9.00 | 5 - 25 | Pradhan et al., 2009, 2010 |
| 33.30 | - | 6.09 | 13.72 | - | 1 | Santhiya & Ting 2005, 2006 |
| 15.70 | - | 3.06 | 2.03 | 11.30 | 10 | Srichandan et al., 2014 |
| 10.12 | 0.62 | 0.16 | 0.53 | 1.32 | 1 - 10 | Rivas-Castillo et al., 2017a |
| 13.33 | 0.41 | 0.01 | 0.00 | 0.27 | 15 | Rivas-Castillo et al., 2019 |
a- Not Determined.
Conclusions
Technological approaches for the biotreatment of spent catalysts and metal uptake are tending to move from effective chemical and thermal processes to eco-friendly solutions that may be slower, but as effective as the first, or even more effective. The development of cleaner technologies based on biotechnological approaches is becoming increasingly important for the recycling of these materials and for waste minimization, since the controlled microbiological processing of high metal content residues present meaningful advantages besides its ecological nature, as low economical investment and maintainance, and low energy costs. There are already examples of biotechnological approaches successfully implemented at an industrial scale and, hopefully, they will be continuously installed in developing countries in the near future, as these eco-friendly and cheaper procedures may represent clear advantages in countries like Mexico.
The current challenge may be to optimize the leaching rates and metal recoveries with respect to the biotreatment parameters and to the microorganisms used. In this latter respect, one way can be to improve the microbial adaptations to spent catalysts, in order to enhance their resistance and metal removal capabilities; and other, to identify and improve new strains with these metal removal inherent abilities, including the identification and genetic manipulation of molecular targets crucial for metal uptake, which has been scarcely studied for most of the microorganisms that have been tested and identified with relevant potential for the biotreatment of spent catalysts. Besides, detailed analyses about the correlation between the presence and quantity of each metal in spent catalysts, and the affinity of each microorganism for the removal of metal targets, may be of significant importance to optimize the strategies and encourage the scale-up of these processes.











 nueva página del texto (beta)
nueva página del texto (beta)


