Introduction
The white shrimp (Litopenaeus vannamei) is a commercial penaeid species with a distribution range throughout the Pacific coast of Sonora, Mexico, and the north of Peru (Holthius, 1980), which tolerates a wide range of environmental salinities (~1-40 ppt). At salinities under iso-osmotic point (~26 ppt), penaeid shrimp hyper-regulate solute concentrations in hemolymph, whereas at high salinities they behave as hypo-regulators (Díaz et al., 2001; Chong-Robles et al., 2014).
Osmoregulation in crustaceans involves the adjustment of the osmotic pressure (OP) of the intra- and extracellular fluids (e.g., hemolymph) (Péqueux 1995; Charmantier et al., 2009). In euryhaline species, such as L. vannamei, the extracellular regulation comprises changes in active ion transport, urine production, and permeability of the body surface to water and salts (Henry et al., 2012). The osmoregulation is subjected to a neuroendocrine control. The X-organ/sinus gland complex (XO/SG) located in eyestalks appears essential for the regulation of osmoregulatory mechanisms (Kamemoto, 1976; Charmantier et al., 1984; Mantel, 1985). The crustacean hyperglycemic hormones (CHHs) are the most abundant neuropeptides produced in the XO/SG complex (Webster et al., 2012). Classic gland ablation experiments in lobsters and crayfish have shown that eyestalk removal alters water and ion concentrations in crustaceans, an effect that can be restored by the injection of CHHs purified from SG tissue (Charmantier-Daures et al., 1994; Serrano et al., 2003). Moreover, chromatographic fractions containing CHH raised Na+ influx in isolated gills from crabs (Spanings-Pierrot, 2000). This evidence suggests that CHHs may have a prime role in controlling the osmoregulatory processes in decapod crustaceans.
In L. vannamei, the cDNAs encoding different CHH variants isolated from the eyestalks were cloned and sequenced (Lago-Lestón et al., 2007; Ventura-López et al., 2016). The variants named CHH-B1 and CHH-B2 are originated from the same gene through alternative splicing events (Lago-Lestón et al., 2007). Notably, the expression of CHH variant B1 (CHH-B1) is strongly influenced by environmental salinity and temperature (Lago-Lestón et al., 2007). Moreover, the ability of CHH-B1 peptide to elicit hyperglycemia and hyperlipidemia in shrimp hemolymph has been proven by the injection of recombinant peptides (Sánchez-Castrejón et al., 2008; Camacho-Jiménez et al., 2015, Montiel-Arzate et al., 2020). Administration of recombinant CHH-B1 also has demonstrated to restore the osmoregulatory capacity (OC) of L. vannamei acclimated to hyper-osmotic salinity (Camacho-Jiménez et al., 2017a), suggesting that this variant participates in the response to salinity stress and the hydromineral balance in white shrimp.
The Na+/K+-ATPase (NKA) pump is a main driving force for ion transport in the cells of aquatic organisms, including crustaceans (Lucu and Towle, 2003). In L. vannamei, NKA α-subunit mRNA expression in posterior gills responds to changes in salinity conditions, suggesting its importance for shrimp survival to osmotic stress (Sun et al., 2011). Moreover, we have recently demonstrated that NKA mRNA expression in posterior gills of intact L. vannamei shrimp maintained at iso-osmotic salinity (26 ppt) was significantly up-regulated 3 h post-injection of rCHH-B1. However, the acute transference of shrimp from 26 ppt to hypo-osmotic salinity (8 ppt) showed a significant decrease of the NKA expression 1 h post-injection of rCHH-B1. In contrast, the NKA transcripts were significantly up-regulated 1 h post-injection of rCHH-B1 in animals acutely exposed to hyper-osmotic conditions (45 ppt). These results suggested that CHH-B1 could be directly involved in regulating ion transport mechanisms (Camacho-Jiménez et al., 2018).
Due to the importance of this molecular mechanism for the osmo-ionic regulation, we examined in vivo the dose-dependent effects of rCHH-B1 on the hemolymph OC and its relation with the mRNA expression of the NKA catalytic α-subunit in gills of bilaterally eyestalk-ablated shrimp acclimated to hyper-osmotic salinity.
Material and methods
Animals
L. vannamei post-larvae (PL) were brought to CICESE’s Marine Biotechnology wet laboratory from a shrimp farm located in La Paz, Mexico. The PL were grown to sub-adults in 2000 L reservoirs filled with seawater (35 ± 1 ppt, 28 ± 1°C) under constant aeration. Sub-adult shrimp were individually placed within 3.5 L containers inside 200 L reservoirs with recirculated seawater (35 ppt, 26°C) with aeration. Shrimp were fed once a day with commercial pelleted feed, and remanent food and feces were siphoned from the containers. Animals were maintained under these conditions for 10 days until the assays.
Expression and purification of rCHH-B1
Recombinant CHH-B1 (rCHH-B1) was expressed in the methylotrophic yeast Pichia pastoris and purified by reversed-phase high-performance liquid chromatography (RP-HPLC) as previously reported, with minor modifications (Camacho-Jiménez et al., 2015). Briefly, P. pastoris (strain X-33) with the pPicZαA-CHH-B1a plasmid for CHH-B1 expression integrated into its chromosomes was cultured in YPD medium for 18 h (30 °C and 200 rpm). The YPD culture (0.5 mL) was used to inoculate BMGY medium (500 mL), which was maintained until an OD600= 4. The BMGY culture was centrifuged (2,500 x g, 5 min), and cells were in BMMY medium (100 mL) supplemented with 2% methanol as an inducer for recombinant protein expression. The induction was maintained for 24 h (30 °C, 200 rpm) with methanol supplementation every 12 h. After induction, culture supernatant was collected by centrifugation (2,500 x g, 5 min). Proteins from the supernatant were precipitated with 50% ammonium sulfate, and the concentrate was dialyzed with phosphate-buffered saline (PBS) 1X. The recombinant protein was separated from other dialyzed proteins by RP-HPLC using a C18 column (TSKgel® Octadecyl-4PW 4.6 mm × 150 mm, Tosoh, Tokyo, Japan) and a gradient of acetonitrile (0-55%) with 0.1% TFA. The recovery of recombinant protein was confirmed through Westen blot immunodetection using an anti-CHH-B1/B2 antibody. Proteins recovered after RP-HPLC were separated by Tricine-SDS-PAGE (12.5%), transferred to a nitrocellulose membrane (0.45 µm) (Bio-Rad, San Diego, CA, USA), and then incubated with the rabbit anti-CHH-B1/B2 polyclonal antibody (1:500) (GenScript, Piscataway, NJ, USA) followed by incubation with a peroxidase-conjugated goat anti-rabbit IgG antibody (Sigma-Aldrich, Saint Louis, MO, USA) (1:5000). Visualization of positive bands was done with the 1-Step TMB Blotting reagent (Pierce, Rockford, IL, USA). The BCA Protein Assay Kit (Pierce) was used to determine the concentration of rCHH-B1. The recombinant protein was stored at -80°C until dose-response experiment.
Biological assay and sample collection
A dose-response in vivo assay was done as described by Camacho-Jiménez et al. (2015) using bilaterally eyestalk- ablated shrimp . This experiment had the aprovation from CICESE’s ethical committee. Fifty-four sub-adult shrimp (12.89 ± 2.41 g) were transferred to individual containers (3.5 L) inside tanks (200 L) filled with seawater (35 ± ١ ppt, 26 ± 1 °C) under constant aeration. Daily, animals were fed with shrimp pelleted diet (4% of their wet weight), debris and feces were siphoned from containers, and seawater was totally exchanged. The intermolt stage was calculated as half of the time between two consecutive molting events indicated by exoskeleton deposition in the containers. During the first counted intermolt, one eyestalk was extirpated from each shrimp by cutting and cauterization, whereas the remaining eyestalk was removed at the second registered intermolt stage. Some of the removed eyestalks were collected for sinus gland (SG) extract preparation. SG were dissected from eyestalks, frozen (-80 °C) and homogenized in cold PBS 1X with a pestle. The homogenized sample was centrifuged (10,500 x g, 4 °C, 15 min), and the collected supernatant was lyophilized and resuspended in 50 µL of PBS 1X every 2 glands. The SG extract was stored at -80 °C before activity assay.
Bilaterally eyestalk-ablated shrimp in intermolt were fasted for 24 h before the experiment. To establish the dose-dependent effect of rCHH-B1 on osmoregulation, various doses of rCHH-B1 peptide (5, 10, 50, 100, 250, 500, and 1000 pmol) were diluted in 50 µL of PBS 1X and injected into shrimp through the arthrodial membrane with a 1 mL sterile syringe (31 G). As a negative control, a group of animals was injected with 50 µL of PBS 1X. The positive control group consisted of shrimp injected with a pair of SG extracted in PBS (50 µL). One hour after injection, six shrimp from each dosage treatment and control (n= 6) were sampled. Hemolymph was collected from shrimp with a sterile syringe (1 mL, 27 G) and immediately placed on ice before analysis. After that, posterior gills (~50 mg) were dissected from each animal and immersed in RNA stabilizing solution (25 mM sodium citrate, 10 mM EDTA, 70% (NH4)2SO4 (w/v), pH 5.2). Tissue samples were stored at -80°C before RNA isolation.
Osmoregulatory capacity (OC)
The osmotic pressure (OP) was measured from hemolymph samples with a vapor pressure osmometer (VAPRO 5520, Wescor, South Logan, UT, USA). The OP of the external medium was also determined from seawater in the experimental reservoirs. The osmoregulatory capacity (OC) was calculated by subtracting OP of the external medium from that of hemolymph. Because penaeid shrimp are hyper-regulators of hemolymph OP under hyper-osmotic conditions of salinity, the OC data was expressed as hypo-OC (Lignot et al., 1997).
NKA mRNA quantification by RT-qPCR
The effect of rCHH-B1 on NKA mRNA expression in L. vannamei gills was measured by real-time quantitative PCR (RT-qPCR) according to Camacho-Jiménez et al. (2018) . Gill samples of shrimp from each treatment and control (n= 6) were pooled in groups of two organisms (n= 3) to add ~50 mg of tissue (same amount per shrimp). Total RNA was isolated from gills tissue samples and treated with DNAse I with the Direct-zolTM RNA MiniPrep kit (Zymo Research, Irvine, CA, USA). Total RNA (~1 μg) was reverse-transcribed for cDNA synthesis with SuperScript® III Reverse Transcriptase and oligo (dT)20 primer (Invitrogen, Life Technologies, Carlsbad, CA, USA). A specific fragment for NKA catalytic α-subunit (122 bp) was amplified by RT-qPCR in a StepOnePlusTM Real-Time PCR System (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). The reaction mix (10 μL) included 5 µL of Power SYBR® Green PCR Mix (Applied Biosystems, Life Technologies), cDNA (25 ng), 0.1 μM of LvαATP_F forward primer (5'-AGCAAGGCCATCAACGATCT-3') and 0.1 μM of LvαATP_R reverse primer (5'-GCCCACTGCACAATCACAAT-3') (Li et al., 2009). Cycling conditions were as follows: an initial denaturation cycle at 95 °C for 10 min, followed by 40 cycles at 95 °C for 20 s and 60 °C for 1 min. Each cDNA sample was analyzed in triplicates. In each run, a non-template control (negative control) was included in triplicates. After the last extension step, a melting curve was run from 60 to 95 °C with an increase of 0.3 °C each 15 s to validate the amplification specificity. The absolute copy number of NKA transcripts in samples was assessed by interpolating the quantification cycle (Cq) values of each sample to a standard curve (R2 ≥ 0.99) constructed with serial dilutions (107-102 copies) of a pCR™ 2.1-TOPO® plasmid (Life Technologies) carrying the 122 bp NKA fragment (Camacho-Jiménez et al., 2018). The copy number of NKA transcripts in each sample was reported per ng of cDNA. The efficiency of primers was calculated using raw fluorescence data from the standard curve with LinRegPCR Software 2016.0 (Ruijter et al., 2009), which was 91.6%.
Statistical analyses
Data were analyzed for normality and homoscedasticity by Shapiro-Wilk and Brown-Forsythe tests, respectively. To determine statistical differences in OC between PBS control and neuropeptide doses, one-way ANOVA with Fisher’s LSD test was performed. Because NKA expression data did not satisfy normality and homoscedasticity criteria, a Kruskal-Wallis test with Dunn’s post hoc test was performed to find differences between hormone treatments and negative control. All the statistical analyzes were done with SigmaPlot 14.0 (Systat Software) with a significance level settled at p < 0.05. The data were plotted as mean ± standard deviation.
Results
Expression and purification of rCHH-B1
The expression and purification of the native recombinant protein were corroborated by Western blot analysis (Fig. 1). The results showed rCHH-B1 as a ~10 kDa band, which is close to the predicted mass of 8.8 kDa. This band was identified as rCHH-B1 by N-terminal sequencing in a previous study (Camacho-Jiménez et al., 2015). No additional bands were detected during the analyses, confirming the elimination of contaminating proteins.
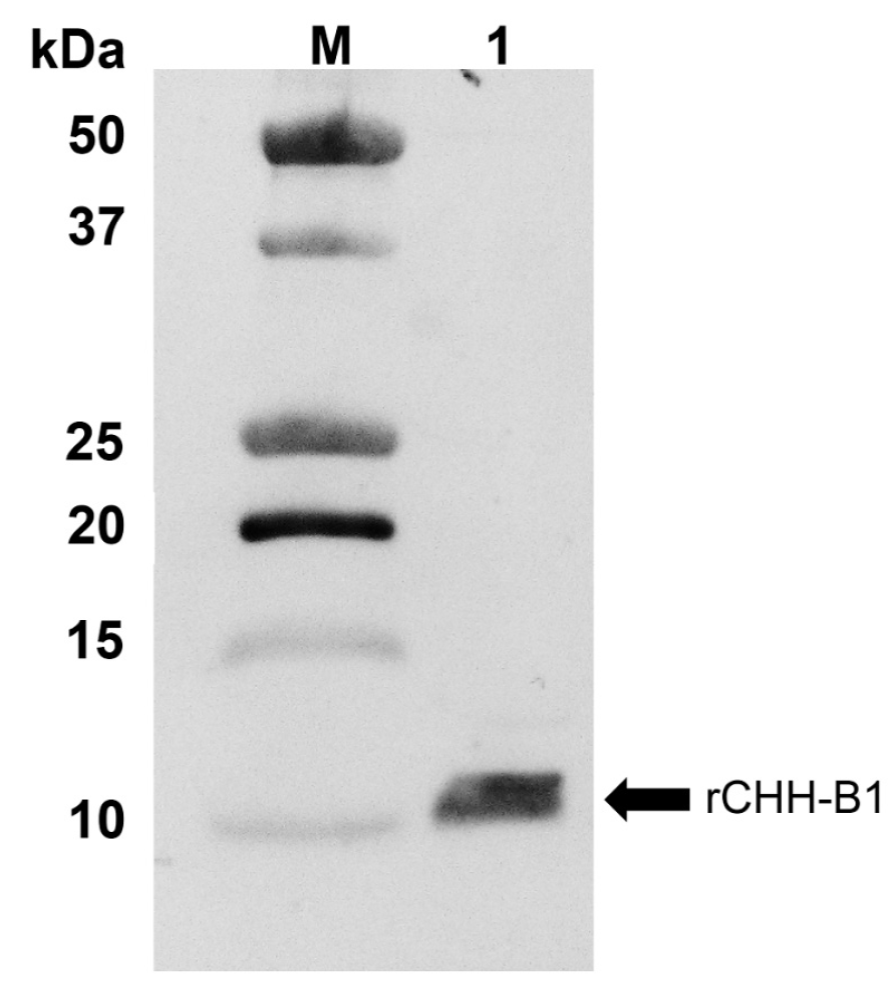
Figure 1 Immunodetection of rCHH-B1 by Western blot. M, molecular weight marker Precision Plus
Protein All Blue (Bio-Rad); 1, recombinant protein purified by
RP-HPLC. The arrow indicates the band corresponding to rCHH-B1.
Figura 1. Análisis por Western blot de
rCHH-B1. M, marcador de pesos moleculares Precision Plus Protein All
Blue (Bio-Rad); 1, proteína recombinante purificada por RP-HPLC. La
flecha indica la banda correspondiente a rCHH-B1.
Effect of rCHH-B1 dosage on the hypo-OC of eyestalk ablated shrimp
The effect of the purified rCHH-B1 on the osmoregulatory capacity was evaluated in bilaterally-eyestalk ablated shrimp acclimated to 35 ppt salinity by a dose-response in vivo assay. Compared to PBS control (-287.27 ± 13.35 mmol kg-1), rCHH-B1 increased the hypo-OC of the shrimp (Fig. 2) due to a decrease in the osmotic pressure of the hemolymph. The effect was significant (p < 0.05) starting from the 100 pmol dose (-311.78 ± 19.15 mmol kg-1) and showed no differences (p > 0.05) with the effect of higher doses of hormone (250-500 pmol). The shrimp injected with SG showed a significant decrease (p < 0.05) in the hypo-OC (-245.00 ± 22.63 mmol kg-1) with respect to PBS due to an increase of the hemolymph OP.
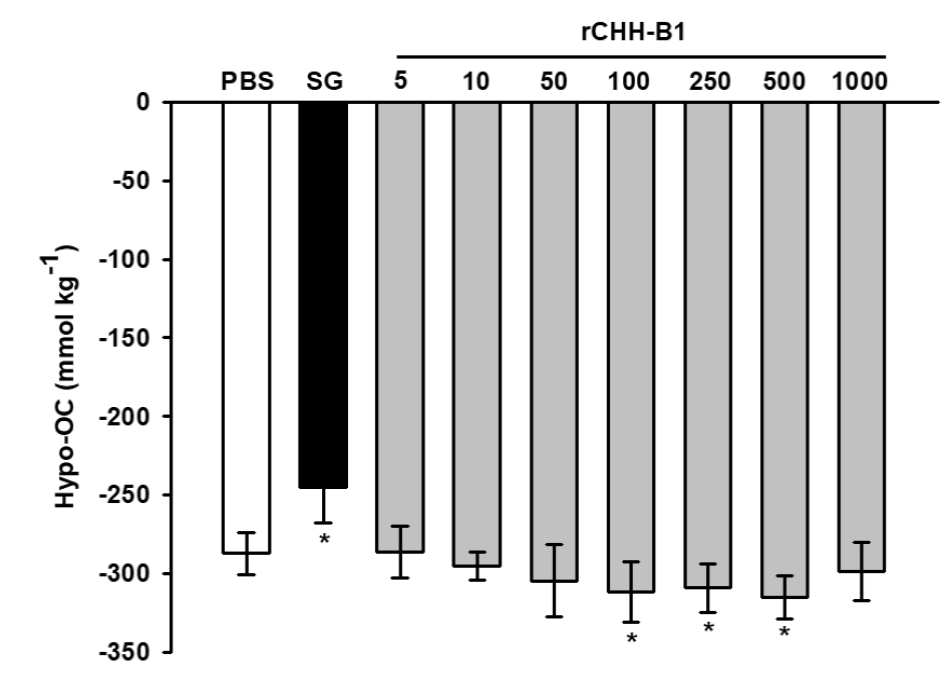
Figure 2 Dose-response effect of rCHH-B1 on the hypo-OC of L. vannamei. PBS,
negative control; SG, sinus gland extract; rCHH-B1, doses of rCHH-B1
(5-1000 pmol). Data are expressed as mean ± standard deviation (n=
6, per dosage or control). Asterisks (*) indicate significant
differences with respect to PBS control (p < 0.05).
Figura 2. Efecto dosis-respuesta de rCHH-B1
sobre la hipo-CO de L. vannamei. PBS, control
negativo; SG, extracto de glándula del seno; rCHH-B1, dosis de
rCHH-B1 (5-1000 pmol). Los datos se expresan como media ± desviación
estandar (n = 6, por dosis o control). Los asteriscos (*) indican
las diferencias con respecto al control PBS (p < 0.05).
Effect of rCHH-B1 dosage on NKA expression
The expression of NKA in the posterior gills decreased in response to rCHH-B1 injection in a dose-dependent manner (Fig. 3). The reduction in NKA mRNAs was significant (p < 0.05) with respect to the PBS control (15.76 ± 4.39 x103 copies of NKA transcript per ng of cDNA) starting from the 50 pmol dose (9.51 ± 2.88 x103 copies of NKA transcript per ng of cDNA), which was lower than the effective dose found for the OC. The expression reached minimum values (p < 0.05) by injecting 1000 pmol of rCHH-B1 (3.68 ± 0.81 x103 copies of NKA transcript per ng of cDNA). Conversely, the SG extract increased the NKA transcripts (41.95 ± 0.92 x103 copies of NKA transcript per ng of cDNA) in comparison to the PBS control (p < 0.05).
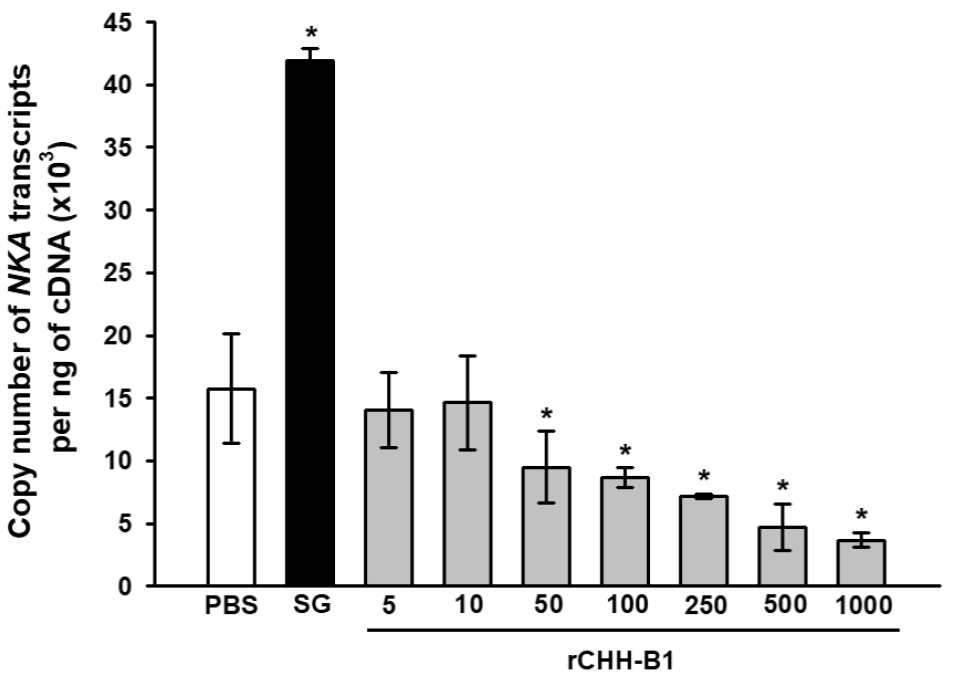
Figure 3 Dose-response effect of rCHH-B1 on NKA expression in posterior gills
of L. vannamei. PBS, negative control; SG, sinus
gland extract; rCHH-B1, doses of rCHH-B1 (5-1000 pmol). Data are
expressed as mean ± standard deviation (n= 3, per dosage or
control). Asterisks (*) indicate significant differences with
respect to PBS control (p < 0.05).
Figura 3.
Efecto dosis-respuesta de rCHH-B1 sobre la expresión de
NKA en branquias posteriores de L.
vannamei. PBS, control negativo; SG, extracto de
glándula del seno; rCHH-B1, dosis de rCHH-B1 (5-1000 pmol). Los
datos se expresan como media ± desviación estandar (n = 3, por dosis
o control). Los asteriscos (*) indican las diferencias con respecto
al control PBS (p < 0.05).
Discussion
The CHHs are neuropeptides of crustaceans with a well-established role in carbohydrate metabolism (Fanjul-Moles, 2006). Experiments injecting rCHH-B1 into eyestalk-ablated L. vannamei have demonstrated its participation in controlling the hemolymph glucose levels (Camacho-Jiménez et al., 2015). The CHH-mediated hyperglycemia has been proposed as an adaptive response to cope with increases in the energy needs of tissues during stressful situations (Chang, 2005). Nonetheless, the CHHs are recognized as pleiotropic hormones with multiple functions proposed in the physiology of crustaceans, including osmo-ionic regulation (Chung et al., 2010; Webster et al., 2012). Interestingly, CHH-B1 mRNA levels were up-regulated in L. vannamei eyestalks during exposure to extreme salinities, pointing to a role in osmoregulation under osmotic stress (Lago-Lestón et al., 2007). Additionally, the relative expression of the chh transcripts showed to be sensitive to salinity, being higher at salinities far from iso-osmotic point (26 ppt). Recent studies have shown that rCHH-B1 injection into L. vannamei shrimp acutely exposed to iso-osmotic (26 ppt) and hyper-osmotic salinity (45 ppt) increased NKA α-subunit mRNA expression in posterior gills (Camacho-Jiménez et al., 2018). However, since this study was done with non-ablated shrimp, the effect of other endocrine molecules present in the eyestalks could not be ruled out. Diverse biogenic amines that are produced in the eyestalks may affect metabolism and osmoregulation in a way that can be or not dependant on CHH action (Liu et al., 2008; 2009; Lorenzon et al., 2005). Moreover, according to experiments in Penaeus monodon, other eyestalk peptides, like the red concentrating hormone, can elicit changes in NKA activity in gills, suggesting that it may exert an overlapping endocrine function to CHHs in osmoregulation (Sathapondecha et al. 2014). Eyestalk ablation is used to eliminate the primary source of neuropeptides in crustaceans to test their individual effects. Although this surgical procedure causes impairments in metabolism and osmoregulation by itself (Charmantier-Daures et al., 1994; Sainz-Hernández et al., 2008), it is still a classic approach for the functional characterization of CHHs, as it completely eliminates XO-SG, which is the main tissue secreting CHH (Chang et al., 2010; Liu et al., 2014; Mosco et al., 2015).
In this study, the experiments were performed with bilaterally eyestalk-ablated sub-adult shrimp acclimated to hyper-osmotic conditions (35 ppt), in which organisms are hypo-osmotic with respect to the external medium. Interestingly, rCHH-B1 treatment increased the hypo-OC of eyestalk-ablated shrimp after 1 h within a dose range of 100-500 pmol due to a reduction in the hemolymph OP. These results agree with a previous study in which the injection of 226 pmol (2 µg) of rCHH-B1 increased hypo-OC of non-ablated shrimp after 1 h (Camacho et al., 2018). Thus, the evidence herein confirms that CHH-B1 acts on the osmoregulatory performance of shrimp during hypo-osmoregulation at high salinities besides its metabolic effects. Considering that in L. vannamei, the Na+ and Cl- ions comprise ~80% of the hemolymph OP at 35 ppt (Castille and Lawrence, 1981), the effect of rCHH-B1 on the hypo-OC could be related to changes in their concentrations, in part by modifying branchial ion transport. The rCHH-B1 peptide suppressed NKA expression in gills of eyestalk-ablated shrimp in a dose-dependent way, starting at a dosage that is a half lower than the effective dosage for the OC (100 pmol). These results indicate that CHH-B1 might regulate the OC of shrimp during hypo-regulation by modulating NKA activity. NKA modulation could occur through a signal transduction pathway that triggers changes at a transcriptional level.
In euryhaline hyper-osmoregulator crustaceans, the catalytic α-subunit of NKA located in the basolateral membrane of epithelial cells from gills provides the primary driving force for the uptake of Na+ from diluted media by exchanging K+ (Lucu and Towle, 2003). The NKA mRNA expression was stimulated in L. vannamei gills in response to salinity stress (Sun et al., 2011; Wang et al., 2012). A high NKA gene expression level has also been observed in the gill tissue of black tiger shrimp (P. monodon) exposed to high salinity conditions (55 ppt) (Shekhar et al., 2014). Additionally, a transient induction of the mRNA levels for the α-subunit of NKA has been reported during acclimation of the euryhaline blue crabs Callinectes sapidus from 35 ppt to 10 ppt salinity (Lovett et al., 2006). NKA expression in the euryhaline crab Eriocheir sinensis gills showed adaptive up-regulated expression response to the salinity changes (Zhang et al., 2018). In contrast, NKA expression in the E. sinensis gills was significantly downregulated in organisms acclimated to seawater (25 ppt) compared with the freshwater group (Yang et al., 2019). Nonetheless, in animals capable of hypo-osmoregulation, like L. vannamei, branchial NKA could also drive the movement of ions through the Na+/K+/2Cl- cotransporter and other transport proteins in cell membranes, as has been suggested in some marine teleosts (Evans et al., 2005). These results suggest the importance of tight regulation of NKA activity for the survival of crustacea to osmotic stress caused by both high and low salinities. The effect of rCHH-B1 on NKA expression found in this work, in which organisms were acclimated at 35 ppt, contrast with those reported in a previous study in which a transitory up-regulation in transcript copy number occurred in shrimp treated with rCHH-B1 (226 pmol) and acutely transferred to hyper-osmotic (45 ppt) conditions (Camacho-Jiménez et al., 2018). Since the optimum salinity range for L. vannamei growth is 33-40 ppt and survival is compromised at salinities above 40 ppt (Ponce-Palafox et al., 1997), the level of stress experienced by animals at 35 ppt probably was not the same as at 45 ppt. In agreement, shrimp acclimated to iso-osmotic salinity (26 ppt) and injected with rCHH-B1 or rCHH-B2, displayed a weaker effect on NKA induction than shrimp acutely transferred to 45 ppt. Moreover, the transference of animals injected with the recombinant peptides to low salinity (8 ppt) for 1 h did not induced NKA expression. However, the authors also reported a less-magnitude increase in shrimp under the same salinity conditions that not received hormone injection, which suggests that other endocrine factors involved in osmoregulation could be acting under salinity stress (Camacho-Jiménez et al., 2018).
Results presented in this study showed that eyestalk-ablated shrimp injected with the SG extract had an strong and opposite response to that of rCHH-B1 treatments. SG extract caused a reduction in hypo-OC (increase in OP) and an increase in NKA transcripts, suggesting an increase in ion uptake dependant on NKA activity. The SG extract contains a mixture of endocrine molecules (i.e., biogenic amines and neuropeptides) that potentially have different effects on the osmo-ionic regulation of L. vannamei (Liu et al., 2008; 2009). Thus, differences among intact and eyestalk-ablated animals can be expected, as well as between the effects of SG extract and single CHH neuropeptides. In this sense, recent studies revealed that the rCHH-B2 variant decrease the hypo-OC in the hemolymph of bilaterally eyestalk-ablated L. vannamei exposed to 35 ppt (Camacho-Jiménez et al., 2017b). CHH-B2 has also been shown to increase the hemolymph ion concentrations and NKA expression in gills of non-ablated shrimp transferred to high salinity (45 ppt) (Camacho-Jiménez et al., 2018). Liu et al. (2014) reported that recombinant CHH peptide of L. vannamei (rLvCHH) increased the NKA activity in intact shrimp acclimated to 31 ppt.
Interestingly, CHH variants of L. vannamei are differentially distributed among tissues. LvCHH peptide is highly expressed in eyestalks, heart, nervous systems, muscle, and hepatopancreas (Liu et al., 2014), while CHH-B2 has been only detected in eyestalks (Lago-Lestón et al., 2007). Moreover, L. vannamei ion transport peptide (LvITP), a peptide with high sequence identity with CHH-B1, is expressed in gills, suggesting an osmoregulatory role in this tissue (Tiu et al., 2007). These dissimilarities between tissue distribution patterns of CHHs have been related to their structural variability, as well as to their functional diversity (Liu et al., 2015).
The involvement of CHHs in osmotic adaptation has been mostly studied in crustaceans upon transfer to hypo-osmotic environments. In lobster and crayfish species, the injection of purified CHH reverted the reduction in the ability to regulate the internal OP and/or Na+ in eyestalk ablated animals kept in diluted media (Charmantier-Daures et al., 1994; Serrano et al., 2003), probably by promoting changes in the transepithelial potential and Na+ influx across the posterior gills, as has been shown in Pachygrapsus marmoratus (Spanings-Pierrot et al., 2000). In agreement with our findings, Spanings-Pierrot et al. (2000) suggested that CHH could indirectly modulate the NKA activity in gills by controlling the metabolic energy available for this mechanism. rCHH-B1 has been demonstrated to cause hyperglycemia in eyestalk-ablated shrimp at 35 ppt (Camacho-Jiménez et al., 2015). Moreover, rCHH-B1 has proven to elicit triglycerides and phospholipids mobilization into hemolymph, making lipids available for uptake by tissues (Montiel-Arzate et al., 2020). In this sense, unsaturated phospholipid content in posterior gills tissue has been positively related to the level of NKA activity (Chapelle and Zwingelstein, 1984). Thus, the metabolic effects of CHH-B1 in hemolymph and gill tissue can aid shrimp to osmoregulate in changing environmental conditions. Alternatively, CHH may be directly involved in the activation of signal transduction pathways that control NKA activity. Specific binding sites for CHH have been detected in the gills tissue from Carcinus maenas and C. sapidus, where the hormone rises the cGMP concentration (Chung and Webster 2006; Katayama and Chung, 2009). Moreover, it has been predicted that the NKA α-subunit of the euryhaline crab P. marmoratus has a cAMP- and cGMP-dependent protein kinase phosphorylation site, while in the promoter region of the α-subunit gene potential recognition sites have been located for activating transcription factor/cAMP response element-binding protein (ATF/CREB) (Jayasundara et al., 2007). This family of transcription factors is involved in the cellular stress response in mammalian cell lines (Fawcett et al., 1999). In this sense, the existence of a signal transduction pathway dependant on CHH and cyclic nucleotides (as second messengers) that controls NKA expression must be elucidated.
Conclusions
Based on our results, CHH-B1 is involved in the osmotic regulation of L. vannamei, which is correlated with the transcriptional regulation of NKA activity in gills. The rCHH-B1 peptide had a dose-dependent suppressive effect on NKA expression in gills of eyestalk-ablated shrimp acclimated to 35 ppt. Even though these results support a regulatory role for CHH in osmo-ionic regulation in crustaceans, they contrast with previous evidence on CHH-dependant stimulation of Na+ uptake and NKA activity in gills (Spanings-Pierrot et al., 2000; Liu et al., 2014; Camacho-Jiménez et al., 2018). However, salinity conditions seem to have a significant influence on gene expression and activities of CHH variants of L. vannamei (Lago-Lestón et al., 2007; Camacho-Jiménez et al., 2018). Moreover, osmoregulation appears to be a highly complex physiological process involving diverse endocrine molecules with unknown interactions among each other (Charmantier et al., 2009). Further research is needed to clarify the signal transduction pathways and the effects of the different CHH peptides of L. vannamei under diverse environmental and physiological conditions. The study of their effects in response to stressors, such as salinity fluctuations, could be interesting for aquaculture production since they impact the growth, health, and even survival of shrimp.











 nueva página del texto (beta)
nueva página del texto (beta)



