Introduction
Proteins are compounds which are of special interest for food industry due to their bioactive, nutritional and functional properties (Lafarga et al., 2019a). Proteins from animal sources have been traditionally used in the food industry to obtain edible foams and emulsions; however, it is known that 6 kg of vegetable protein are required to produce 1 kg of animal protein, which has led to large-scale animal protein production being considered one of the main causes of environmental problems (Aiking, 2014; Lafarga et al., 2019a). Among animal proteins, egg white proteins are extensively used due to their excellent functional properties such as foam formation, emulsification and stabilization (Herald et al., 2008; Lin et al., 2017; Aslan and Ertaş, 2020); however, these proteins are strongly associated with food allergies, especially in young children and infants (Caubet and Wang, 2011; Park et al., 2017; Meurer et al., 2020; Alsalman et al., 2020a; Włodarczyk et al., 2022). In addition to egg allergy, the increased awareness of healthiness and sustainability of the modern consumer and food industry, together with an increase in the proportion of vegan people (Arozarena et al., 2001; Boye et al., 2010; Janssen et al., 2016; Asioli et al., 2017; Lin et al., 2017; McClements et al., 2017; Sharif et al., 2018; Aschemann-Witzel and Peschel, 2019; Buhl et al., 2019;), have motivated a growing interest for plant-based proteins, mainly of soy, peas and chickpeas, as possible candidates to replace animal-based proteins (Sharif et al., 2018; He et al., 2019; Kim et al., 2022; Silva et al., 2022), because they have functional properties such as water holding capacity, fat binding, solubility as well as foaming, gelling, and emulsifying capacities, which are comparable with proteins from animal and dairy sources (Boye et al., 2010; Ma et al., 2016; Sharif et al., 2018; Sharima-Abdullah et al., 2018; Bessada et al., 2019), but with the advantages of low allergenicity, sustainable production, high production volumes and low price (Papalamprou et al., 2010; Gumus et al., 2017; Buhl et al., 2019; Lafarga et al., 2019a).
Recently, it was discovered that aquafaba, the viscous liquid resulting from cooking chickpea seed or other legumes in water, or that found in canned products of the same origin (Mustafa and Reaney, 2020; Aslan and Ertaş, 2021), is a valuable food resource due to its high content in protein and health-promoting compounds such as saponins and polyphenols (Damian et al., 2018; Huang et al., 2018; Lafarga et al., 2019a; Lafarga et al., 2019b). Aquafaba from chickpeas has been gaining popularity since 2014 due to its having showed to be a useful thickener, emulsifier and foaming agent in various formulations such as mayonnaise, meringues, cheeses and cakes (He et al., 2019; Alsalman et al., 2020a; Meurer et al., 2020; Raikos et al., 2020; He et al., 2021b; Muhialdin et al., 2021; Nguyen and Tran, 2021).
It has been reported that aquafaba contains approximately 94 % of water, 1.5 % of protein, 0.5 % of ash and 4 % of carbohydrates (Mustafa et al., 2018; Serventi et al., 2018; Shim et al., 2018; Stantiall et al., 2018; Alsalman et al., 2020b); however, the chemical composition and functional properties of aquafaba, can vary depending on factors such as chickpea composition and genotype, processing methods, processing auxiliary agents (Mustafa and Reaney, 2020) and operational conditions.
Based on the aforementioned, the objective of this paper was to study the effect of operational conditions (cooking temperature, cooking time and solid to liquid ratio) on functional (foam capacity and stability) properties of aquafaba from natural chickpea. In addition, the functional properties of the aquafaba obtained were compared against egg white proteins and canned aquafaba properties.
Materials and methods
Materials
Dried Mexican Kabuli chickpeas were purchased from a local supermarket located in the city of Tuxtla Gutiérrez, Chiapas (México). Chickpeas were stored at room temperature until their use. Canned Kabuli chickpeas and fresh egg whites were also purchased from the same store and were used for comparison.
Raw material pretreatment
Chickpeas were subjected to a manual cleaning and washing in order to remove some impurities such as stones, insects and rotten grains. After that, clean chickpeas were soaked in tap water for 24 h at a chickpeas to water ratio of 1:2 (w/v). Then, soaking water was drained and discarded, and the chickpeas obtained were used in the next experiments.
Aquafaba production
One hundred grams of cleaned and soaked chickpeas were placed in a sealed glass jar, then mixed with distilled water at different chickpeas to water ratios (CWR) and cooked for different times and temperatures according to the monofactor test experiments. After cooking, the aquafaba obtained was drained from cooked chickpea grains using a stainless-steel strainer, and then stored under refrigeration at 4 °C for 24 h. Prior to analysis the aquafaba was allowed to cool down to room temperature. Aquafaba was analyzed in terms of its foaming capacity and foam stability, and compared with canned samples.
Monofactor test
In order to study the effect of operational conditions on functional properties of aquafaba, cooking temperature, cooking time and CWR were evaluated by monofactor test. In this sense, two variables were kept constant at their respective central test range values and the other variable varied within its experimental ranges. The variables studied were cooking temperature (60 to 98 ± 2 °C), cooking time (20-100 min) and CWR (1:1 to 1:5). All experiments were performed in triplicate.
Foaming capacity and foam stability
Foaming capacity (FC) and foam stability (FS) were determined according to Shim et al. (2018); briefly, 50 mL of sample (natural aquafaba, canned aquafaba or egg white) was placed in a 14 cm diameter bowl. The sample was shaken at maximum speed with a Hamilton Beach hand mixer (model 62647), for 2 min. After that, the obtained foam was placed in a 500 mL graduate cylinder. Measurements of the foam volumes of the whipped samples were made at time 0 (VF0) and after 30 min (VF30), and the FC and FS were calculated according to the equations (1) and (2), respectively (Mustafa et al., 2018).
Analysis of the aquafaba physicochemical properties
Aquafaba was analyzed in terms of the following physicochemical properties. pH (981.12) and density (962.37) were determined according to AOAC standard methods (AOAC, 1990). Protein concentration was determined by the Bradford dye binding method, using bovine serum albumin as the reference and recording the absorbance at 595 nm (Bradford, 1976). Starch content was measured qualitatively by Starch-Iodine Complex method reported by Street (1974); the sample was allowed to act on an amylose solution for 15 min, and then the blue color formed by adding iodine-iodide solution was compared with the color of an amylase free control, using a spectrophotometer at 578 nm (Street, 1974).
Determination of total polyphenol content
Total polyphenol content was determined by the Folin-Ciocalteu method, performed as described by Lu et al. (2018). One mL of Folin-Ciocalteu reagent was added into 0.3 mL of polyphenol solution and mixed for 5 min. Then, 5 mL of sodium carbonate (10 %) was added and oscillated for 3 min. After that, 20 mL of distilled water were added, and the mixture was incubated at room temperature for 2 h. Finally, absorbance of the solution was measured at 765 nm using an ultraviolet-visible spectrophotometer (VE-5100UV, Científica Vela Quin, México). Gallic acid was used as standard.
Antioxidant activity measured by ABTS method
Antioxidant activity was determined by ABTS [(2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)] method according to the reported by Cai et al. (2015). Briefly, potassium persulphate (2.45 mM) and ABTS stock solution (7 mM) were mixed and left in the dark at room temperature for 16 h, to produce the ABTS radical cation. Prior to the analysis, the ABTS radical solution was diluted in 10 mM phosphate buffered saline (pH 7.4) to an absorbance of 0.8 ± 0.1 at 734 nm. After that, 1 mL of diluted ABTS radical solution and 1mL of sample were mixed, and ten minutes later the absorbance was measured at 734 nm against the corresponding blank, and using TROLOX (Sigma Aldrich) as standard. The ABTS scavenging activity of samples was calculated using equation 3 (Cai et al., 2015).
where A1 is the absorbance of the control and A2 is the absorbance of the sample.
Statistical analysis
All statistical analyses were performed using Minitab® statistical software version 16.0 for windows. Mean comparisons were made by analysis of variance (ANOVA) with a significance level of p < 0.05. All experiments were performed in triplicate and data are presented as mean ± standard deviation.
Results and discussion
Effect of processing conditions on functional properties of aquafaba
The effect of the variables cooking temperature, cooking time and CWR on functional properties of aquafaba (foaming capacity and foam stability) from natural chickpeas were studied by monofactor test. Chickpeas to water ratio (w/v) was studied in a range from 1:1 to 1:5; in this case, the variables cooking temperature and cooking time were maintained at 98 ± 2 °C and 60 min, respectively. As it can be seen in Figure 1(a), the best CWR for both foaming capacity and foam stability was 1:2, while an increase in the amount of water negatively affected the functional properties of the aquafaba. Similar results were obtained by Serventi et al. (2018), who boiled chickpeas seed in water with 1:1.75 of chickpeas to water ratio for 90 min (Serventi et al., 2018). This can be due to that an excessive amount of water in mixture from higher ratios (1:4 and 1:5, mainly) which would inevitably lower the concentration of proteins and carbohydrates, that are the main responsibles for the aquafaba functional properties (Mustafa et al., 2018; Shim et al., 2018). In fact, a negative correlation between CWR and protein concentration was reported, indicating that the protein content of the aquafaba obtained boiling the chickpeas at a lower CWR had a higher protein concentration (Lafarga et al., 2019a). The foaming ability of most plant proteins increases with low degrees of hydrolysis and high concentrations of proteins in solution. As demonstrated by Patino et al. (2008), this ability tends to a maximum when the air-water interface is saturated by the protein (Patino et al., 2008).
Figure 1 Effect of variables a) chickpeas to water ratio, b) cooking temperature and c) cooking time on foaming capacity and stability of aquafaba. Results were expressed as the mean value ± standard deviation. Different letters between treatments indicate statistical significant differences (p < 0.05).
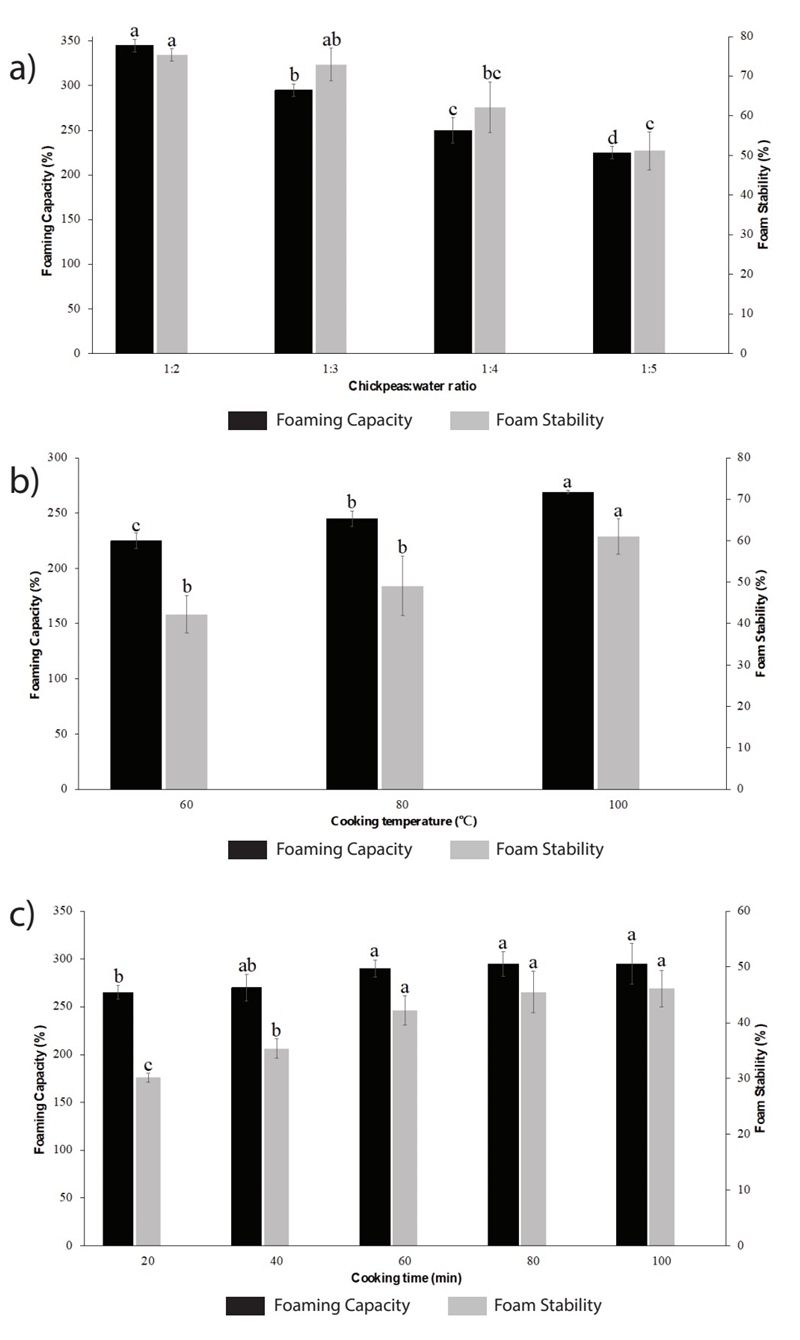
Figura 1: Efecto de las variables a) relación garbanzos:agua, b) temperatura de cocción y c) tiempo de cocción sobre la capacidad y estabilidad de espuma de aquafaba. Los resultados se expresaron como promedio ± desviación estándar. Letras diferentes entre tratamientos indican diferencias estadísticas significativas (p < 0.05).
The results of the effect of the cooking temperature on the functional properties of aquafaba are presented in Figure 1(b). In this case, the CWR and the cooking time were kept at 1:2 and 60 min, respectively. As can be seen in Figure 1(b), temperature plays an important role in the foam capacity and foam stability of the aquafaba. An increase in the value of this variable from 60 °C to 98 ± 2 °C, improves the functional properties of the product. This behavior can be explained taking into account that high temperature treatment during cooking leads to hydration and denaturation of proteins, gelatinization of starch, solubilization, depolymerization and/or loss of pectic polysaccharides from the cell wall. Therefore, during cooking, the outer cell layers of the seed coat become a selective membrane that controls the diffusion of molecules from the seed to the cooking water; thus, exposure to higher temperatures can cause disruption of the seed coat and greater transfer of undissolved materials to the cooking water, giving it better functional properties (He et al., 2021a).
Figure 1(c) shows the results for cooking time; this variable was studied in a range from 20 to 100 min, where CWR and cooking temperature were maintained at 1:2 and 98 ± 2 °C, respectively. As it can be observed, an increase in FC and FS was found when the cooking time increased from 20 to 60 min; however, after this time functional property values were not significantly improved. This may be due to the fact that in 60 min the greatest possible quantity (under the evaluated conditions) of compounds of interest that confer its functional properties to aquafaba have been leached from the chickpea to the cooking water, so prolonging cooking time does not promote the release of more compounds. In addition, it has been reported that, in general, long cooking times can cause protein denaturation, and thereby affect the functional properties of aquafaba (He et al., 2021a).
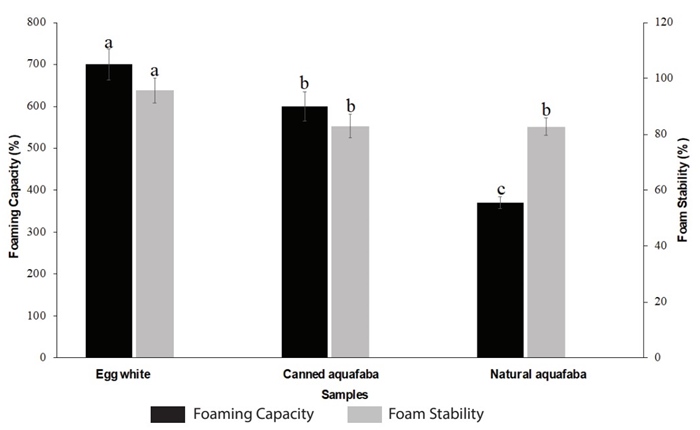
Based on the results of monofactor tests, the conditions selected for the production of aquafaba were CWR of 1:2, cooking temperature of 98 ± 2 °C and cooking time of 60 min. The aquafaba produced under these conditions presented a foaming capacity and stability of 370 ± 14.14 % and 82.78 ± 3.1 %, respectively, which is very close to the reported by Mustafa et al. (2018), who found a foaming capacity and stability ranged between 182 to 476 % and 77 to 92 %, respectively (Mustafa et al., 2018). Functional properties of natural aquafaba were compared with the functional properties of canned aquafaba and egg white, as it can be seen in Figure 2. As expected, the egg white presents the best functional properties (foam capacity and stability) due to its proteins, such as ovoalbumin, ovotransferrin, lysozym, ovomucoid, and ovomucin, and their interactions with each other that are particularly capable of keeping the foam formed (Bovšková and Míková, 2011). These results are in accordance with Buhl et al. (2022) and Stantiall et al. (2018), who find that foam prepared by fresh egg has significant higher foam capacity than natural aquafaba of chickpeas.
Figure 2 Comparison of the functional properties of aquafaba obtained from natural chickpeas, canned aquafaba and egg white. Results were expressed as the mean value ± standard deviation. Different letters between treatments indicate statistical significant differences (p<0.05).
Figurea 2: Comparación de las propiedades funcionales de aquafaba obtenida a partir de garbanzos naturales, aquafaba enlatada y clara de huevo. Los resultados se expresaron como promedio ± desviación estándar. Letras diferentes entre tratamientos indican diferencias estadísticas significativas (p < 0.05).
In the case of the aquafaba produced in this work (natural aquafaba) compared to the canned aquafaba, it was found that the latter has a better foam capacity; this can be explained considering the differences in industrial canning procedures (cooking conditions such as pressure, temperature, time, etc.) (He et al., 2021c; Alsalman and Ramaswamy, 2021; Alsalman et al., 2022), the use of additives, such as salt and preservatives, and genetic differences among cultivars used by manufacturers which can result in changes in aquafaba composition and its functional properties (Mustafa et al., 2018). Interestingly, no statistically significant differences (p>0.05) were found between foam stability of natural aquafaba and canned aquafaba. This may be due to the fact that both natural aquafaba and canned aquafaba have a similar content of protein (albumins, mainly) (Mustafa et al., 2018; Buhl et al., 2019) (as it can be seen in Table 1), which are surface-active agent (Shim et al., 2018) and that they were whipped for the same time. Whipping of this protein solution promotes the incorporation of air into the solution, which leads to bubble formation and adsorption of proteins at the gas-liquid interface to form protein-encapsulated bubbles. The shear force involved in whipping causes denaturation and coagulation of proteins on the air cell surfaces, increasing foam rigidity and stability (Mustafa et al., 2018). Similar results were reported by Mustafa et al. (2018), who studied aquafaba from different brands of canned chickpeas. Among their results, they found that, for example, brands A and B presented a foam capacity of 182.22 and 288.89 %, respectively, with a statistically significant difference between them; however, for foam stability, these aquafaba presented values of 77.2 and 77.5 %, for A and B, respectively, without significant statistical differences.
Table 1 Physicochemical properties of natural aquafaba produced in this study in comparison with canned aquafaba.
Table 1: Propiedades fisicoquímicas de la aquafaba natural producida en este estudio en comparación con la aquafaba enlatada.
| Property | Natural Aquafaba | Canned Aquafaba |
| Density (g/mL) | 1.28 ± 0.29a | 1.33 ± 0.13a |
| Starch | Yes | ND* |
| pH | 5.17 ± 0.25b | 5.85 ± 0.11a |
| Protein content (mg/mL) | 0.93 ± 0.19a | 1.11 ± 0.16a |
| Antioxidant activity (%) | 27.57 ± 0.64a | 23.78 ± 0.42b |
| Total polyphenols content (mg GA/g) | 1.68 ± 0.0007a | 0.84 ± 0.0005b |
*Not detectable Different letters in the same row indicate statistical significant differences (p<0.05).
Analysis of the aquafaba
The natural aquafaba prepared in this work and the aquafaba from canned chickpeas were compared in terms of some of their physicochemical properties. As it can be seen in Table 1, no statistically significant differences (p>0.05) were found in protein content between natural aquafaba and canned aquafaba, and these results are consistent with the findings of Włodarczyk et al. (2022), Buhl et al. (2019), Mustafa et al. (2018) and Stantiall et al. (2018), who reported a protein concentration of 1.26, 1.3, 1.5 and 0.95 % of aquafaba, respectively. As mentioned above, the similar protein content between natural aquafaba and canned aquafaba could explain the similar behavior in the stability of the foam formed by both samples of aquafaba. Similarly, no significant statistical differences (p > 0.05) were found between the density values of the two samples studied, and such values are similar to those reported by Mustafa et al. (2018).
On the other hand, concerning starch, no presence of this compound was found in canned aquafaba, a result that is consistent with other reports (Damian et al., 2018; Stantiall et al., 2018). However, in natural aquafaba the presence of starch was found, and this may be due to less drastic processing conditions than those used in other studies, such as the use of high pressures, which together with a high temperature, can lead to degradation of this polysaccharide (Guha and Zakiuddin, 2002). Regarding the pH, it was found that the canned aquafaba presented a higher pH (5.85) than natural aquafaba (5.15) obtained in this work, which may be due, as previously mentioned, to the various differences in cooking time, cooking temperature, addition of salts and preservatives, pressure during cooking, chickpea cultivar and genotype, chickpea to water ratio (He et al., 2021a; Erem et al., 2021). It is important to mention that, the differences in pH between both samples can explain the different foam capacities found in them, which, as already shown in the corresponding section, was higher in canned aquafaba than in natural aquafaba, and this is due to the fact that pH had a negative effect on foaming capacity, that is, an increase in this parameter will cause a decrease on foaming capacity. This is because the pH modifies the net charge of the protein, which affects foam formation and, in general, its viscoelastic properties (Lafarga et al., 2019a).
Finally, the antioxidant activity and total phenol content of natural aquafaba was statistically higher than that of canned aquafaba (p > 0.05), and this may be due to the conditions and processing steps used in the canned aquafaba affecting these properties to a greater extent. It has been reported that the canning procedure includes soaking (25 °C 12 h), bleaching (85 °C 30 min), canning in salt water (1.3 % salt and 1.6 % sugar) and final heating (121 °C 14 min) (Erem et al., 2021), which is different from the process used in this work. In addition, it is important to note that, although there are about twice as many polyphenolic compounds in natural aquafaba compared to canned aquafaba, in both cases the levels are low. This can be favorable for the physicochemical properties of the material. The presence of phenolic compounds solubilized in aquafaba, although it can add bio-functional properties, can reduce the foaming properties of the proteins present. This is because the ability of proteins to interact with the aqueous interface can be reduced when blocked by phenolic compounds that preferentially integrate with them, as observed by Fernando and Manthey (2002) in an assay with black bean soluble components (Fernando and Manthey, 2022).
Conclusion
The functional properties of the aquafaba produced from natural chickpeas are influenced by the process variables, in such a way that the increase in temperature and cooking time and the decrease in the CWR increase both the capacity and the foam stability. In this way, it is possible to find operating conditions that generate aquafaba with functional properties similar to that of canned aquafaba, and with the potential, through optimization of operating conditions, to be compared with the functional properties of egg white. In addition, regardless of whether it is aquafaba produced from natural chickpeas or canned aquafaba, this product presents antioxidant activity derived from the presence of bioactive compounds, such as phenolic compounds, which are extremely valuable in various industries.
This study reinforced the need to add value to grain cooking water, previously seen as a waste material, but which may contain bio-functional value and be of great interest in the technological aspect of its application. The production conditions of natural aquafaba, that is, obtained by boiling the grains and not draining the can, allowed us to conclude that this material can be obtained in an accessible and applicable way, both industrially and at home. Its production, as demonstrated here, is simple and does not require the incorporation of other ingredients. While it may have a slight foaming disadvantage compared to egg whites, the data indicate that aquafaba as a vegan food option holds real promise.











 nueva página del texto (beta)
nueva página del texto (beta)


