Introduction
Nanotechnology has the potential to revolutionize agriculture and play an important role in food and crop production (Parisi et al., 2015). The term “nanotechnology” can be defined as the design, synthesis, manipulation and application of atomic or molecular aggregates between 1 and 100 nm (Hajra and Mondal, 2017). During the last years, a number of patents with nanoparticles (NPs) have been incorporated into agricultural practices, such as nano pesticides, nano fertilizers, and nano sensors, which have been developed with the collective objective of promoting efficiency and sustainability of agricultural practices, which requires less inputs and generate less waste than conventional products and approaches (Liu and Lal, 2015).
Essential microelements play an important role in plant growth, development and yield. Zinc is an essential micronutrient for humans, animals and plants, and the latter generally absorb Zn as a divalent cation (Zn++). Zinc is necessary in protein biosynthesis and carbohydrate metabolism, and plays an important role in gene expression related to environmental stress (Hawkesford and Barraclough, 2011).
NPs can be synthesized from a variety of bulk materials, and their actions depend on their morphology, surface area, size distribution and other parameters, which are the most remarkable key factors that can determine NPs activity (Abramenko et al., 2018; Brunner et al., 2006; Singh et al., 2021). It has been clearly demonstrated that the electromagnetic, optical, catalytic, sensory, thermal and mechanical properties are strongly influenced by their shape and size (Sau and Rogach, 2010). As their size decreases, NPs surface area increases (Khademalrasool et al., 2021). As for morphological change, it is a consequence of complex combinations of molecules, surfaces and crystals; in regards to this, a greater antibacterial activity of spherical NPs has been described as a result of the high surface/volume ratio and its isotropic geometry, compared to the anisotropic geometry (Agnihotri et al., 2014; Mokhtari et al., 2009; Shenashen et al., 2014). ZnO NPs morphology affects their interaction with cell membranes, as well as their cell penetration capacity (Peng et al., 2011). Therefore, nanoparticle shape may influence the way in which the nanomaterial will interact with the environment because of the geometric effect. Thus, the importance to understand how the physicochemical properties of the NPs are related to their biological interactions and functions.
Positive effects related to spherical and hexagonal ZnO nanoparticle use on the growth and quality of agricultural value crops, such as tomato and wheat, have been reported (Faizan et al., 2018; Munir et al., 2018; Pérez-Velasco et al., 2021). NPs can be applied to plants in different ways; however, seed priming is considered an easy method that can bring benefits during the early stages of growth (Mahakham et al., 2017). Seed priming stimulates processes involved in metabolism, prevents seed deterioration, breaks dormancy and induces a systematic resistance against biotic and abiotic stress, besides biochemical and physiological changes that occur when carrying out a seed priming treatment before germination and radicle emergence (Rehman et al., 2011). Priming is an approach that implies seed treatment with different inorganic chemical compounds or with low or high temperatures (Kamithi et al., 2016). Application of ZnO NPs for seed preparation on different crops, such as maize, where a 100 mg L-1 dose improved germination and increased growth parameters has been reported (Itroutwar et al., 2020); whereas in wheat, a significant positive influence in yield, germination and vigor index was reported for a 10 mg L-1 dose (Rai-Kalal and Jajoo, 2021).
Bell pepper (Capsicum annuum L.), which belongs to the Solanaceae family, is a vegetable present in the diet of many populations around the world; and represents an important source of income for farmers and agro-processing sector operators (Fratianni et al., 2020). In this context, this research aimed to study the effect of seed nano priming with two morphology types of ZnO NPs on germination and early growth of bell pepper seedlings.
Material and methods
Geographic location
Experimental testing was carried out in the Ecology Laboratory located in Plant Physiology area of the Botany Department at the Antonio Narro Autonomous Agrarian University, Buenavista, Saltillo, Coahuila, México.
Plant Material
Hybrid bell pepper seeds RZ F1 (35-171) were used, which is a bell pepper of excellent vigor and high production from the Rijk Zwaan seed company.
Synthesis and characterization of zinc oxide nanoparticles
Nanoparticle synthesis was done at the Applied Chemistry Research Center (CIQA). Zinc acetate dihydrate (Zn(CH3COO)2 • 2H2O, ZnAc, 99 %), triethylamine (TEA, 99 %), n-propilamine (C3H9N, 99.5 %), industrial use ethanol and triply-distilled deionized water were used. Sigma Aldrich provided all the reagents.
ZnO NPs were prepared according to the methodology described by González et al. (2021). The synthesis was made by mixing two solutions. The first contained 8.928 g of ZnAc dissolved in a water/TEA (300 mL/5.36 mL) mixture, and the second was a n-propilamine/etanol (1.42 mL/ 1700 mL) mixture. Both solutions were mixed and stirred at 80 °C for 6 or 12 h, depending on the desired structure. The precipitate was centrifuged, washed with ethanol to remove excess reagents, and dried overnight at room temperature for 12 h. ZnO NPs with different reaction times were prepared to obtain different spherical and hexagonal morphologies.
ZnO NPs crystalline structure was determined by X-Ray Diffraction (XRD), performed in a Siemens D-5000 diffractometer (CuKα radiation, = 1,5418 Å, SIEMENS, Munich, GER). The size of the crystal was determined according to the Debye-Scherrer equation. ZnO NPs morphology was observed by High-resolution transmission electron microscopy (FEI Titan 80 - 300 kV HRTEM, Hillsboro, OR, USA).
Zinc oxide nanoparticle seed priming (spherical and hexagonal morphology)
Zinc oxide nanoparticle solutions were prepared from a spherical and hexagonal zinc oxide nanoparticle 5000 mg L-1 stock solution by weighing 1 g of NPs; then, 200 mL of distilled water were added. Subsequently, the elements were dispersed with a sonicator (Vevor) for 30 min at 25 °C, in order to form a homogeneous suspension and avoid nanoparticle agglomeration. From this solution, 50 and 100 mg L-1 dilutions were made.
The seeds were placed in 90 x 15 mm Petri dishes, with Whatman 1 (110 mm) filter discs and soaked in different concentrations of ZnO NPs (0, 50 y 100 mg L-1). Fifteen mL of a ZnO NPs suspension from each treatment were taken, and then added to the seeds for the imbibition. The seeds were left in darkness at 28 ± 1 °C for 18 h in an incubator (Model 12-140, Quincy Lab Inc.). Primed seeds were left to rest at room temperature until the original moisture content was reached. Distilled water was used for priming.
Germination stage
ZnO NPs primed seeds were placed in 90 x 15mm Petri dishes under the following treatments: T1: 0 mg L-1, distilled water (control); T2: 50 mg L-1 spherical ZnO NPs; T3: 100 mg L-1 spherical ZnO NPs; T4: 50 mg L-1 hexagonal ZnO NPs; T5: 100 mg L-1 hexagonal ZnO NPs.
Ten seeds per dish per treatment were placed in Whatman 1 (110 mm) filter discs, considering six replicas per treatment. The seeds were germinated in darkness at 28 ± 1 °C in an incubator (Model 12 - 140, Quincy Lab Inc.) (Upadhyaya et al., 2017).
After the radicle length exceeded 2 mm, germination parameters were registered daily. Germination rate was calculated as the relation between the number of sprouted seeds and the total amount of seeds sown, expressed as a percentage (Ng et al., 2012). Fourteen-day seedlings were measured, taking radicle, hypocotyl, and plumule length with a TRUPER LCD Display digital caliper (150 mm). The results were registered in mm (Abou-Zeid and Ismail, 2018).
Greenhouse stage (45 days)
Ten replicas of each treatment were taken from Petri dishes and seedlings were transplanted to Styrofoam cups with a 500 mL capacity, which contained a mixture of peat moss + perlite (1:1 v/v). Plant nutrition was provided since the transplant, with a Steiner solution (Steiner, 1961) at a 25 % concentration. The seedlings were kept for 45 d in a polycarbonate-covered greenhouse during their growth, with a 22 °C average temperature and a relative humidity of 56 %.
Measurement of variables
Agronomic variables were registered: plant height was taken from the stem base to the plant apex, with a 5-meter flexometer (Model PRO-5MEC, TRUPER) every five days from the transplant until 45 d after; stem diameter was measured 45 d after the transplant with an LCD Display digital caliper (TRUPER, 150 mm) and registered in mm; number of leaves were counted every five days from the transplant until 45 d after, at the end of the cycle; aerial part dry weight was determined at the end of the cycle, separating leaves and stem; these parts were placed in marked paper bags and dehydrated in a drying oven (Model GO1350C-1 Linderberg/blue) at 80 °C for 24 h. The samples were weighed on an electronic scale (0.1/5000 g); for root dry weight, they were separated manually from the substrate in the cup, removing excess substrate with water. Then, roots were weighed in an electronic scale (0.1/5000 g). To obtain the dry weight, roots were placed in paper bags and dehydrated in a drying oven (Model GO1350C-1 Linderberg/blue) at 75 °C for 17 h. Finally, the samples were weighed on the scale.
Leaf area
Destructive plant testing was carried out and each leaflet from an individual plant was scanned with a leaf area meter (LI-3100C). The leaves were placed precisely and completely extended between the two transparent belts before scanning. This process guaranteed an accurate area representation in each measurement.
Chlorophyll content
Chlorophyll content was determined according to Nagata and Yamashita (1992). For this purpose, 0.1 g of lyophilized leaf tissue was used, mixed with 2 mL of hexane:acetone solution (3:2). An aliquot of the supernatant was taken, and the absorbances were measured at 645 and 663 nm. These results were used to determine chlorophyll content according to the equations:
All data were expressed in mg 100 g-1 dry weight.
Quantification of total phenolic content
Total phenolic content in leaf extract was determined by the Folin-Ciocalteau method, using gallic acid as a standard phenolic compound. The standard curve was obtained from serial dilutions from a 1000-ppm gallic acid stock solution. Phenolic compound quantification was determined according to the methodology of Singleton et al. (1999): 100 mg of extract were weighed in a 2 mL tube, and 1000 μL of water:acetone solution (1:1) were added. The sample was vortexed for 30 seconds, then sonicated (Ultrasonic Cleaner Branson 1510) for five minutes. The samples were centrifuged at 12500 rpm for 10 min at 4 °C in a microcentrifuge (Labnet Prism™ R); the supernatant was extracted and, subsequently, 17 μL of extract and 70 μL of Folin-Ciocalteu reagent were added to an Eppendorf tube, with 174 μL of 20 % sodium carbonate (Na2CO3). Then, 1740 µL of distilled water were added, the tube was vortexed for 30 sec and subsequently the samples were placed into a 45 °C water bath for 30 min. Absorbance was read at a 750 nm wavelength in a spectrophotometer (VE-5600 VELAB). Sample total phenolic content was expressed as milligram of gallic acid equivalents per gram, with the gallic acid (mg g-1) calibration curve.
Statistical analysis
A completely random experimental design was used, with five treatments and six replicas, obtaining 30 experimental units. The experimental unit was a Petri dish with 10 seeds at the germination stage and a seedling with 10 replicas per treatment at the greenhouse stage. For data analysis, Infostat 2020 software was used (InfoStat 2020; Cordoba, Argentina). An analysis of variance (ANOVA) and a comparison of means using LSD Fisher test (P ≤ 0.05) were conducted.
Results and discussion
Characterization of zinc oxide NPs
The ZnO NPs morphology, observed by High-resolution transmission electron microscopy (HRTEM), reveals the presence of spherical NPs at 20.63 nm (Figure 1A). The histogram of the particle size distribution indicates that it ranges from 7.5 to 42.5 nm, with an average of 20 nm (Figure 1B). Moreover, for hexagonal NPs (Figure 2A), individual particles that range from 0.025 µm to 2.5 µm, with an average of 1.7 µm were shown. The morphology and size of the ZnO NPs observed are similar to the results reported by González et al. (2021), who conducted NPs synthesis to obtain spherical, hexagonal and rod NPs.
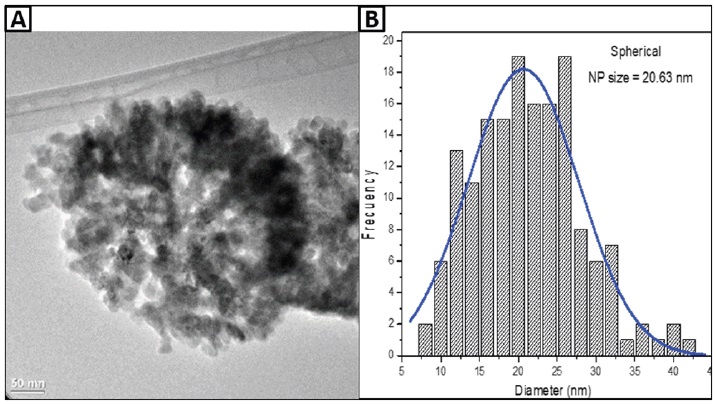
Figure 1 Transmission electron microscopy micrograph showing the spherical morphology of the ZnO NPs (A) and the particle size distribution histogram (B).
Effect of the ZnO NPs on germination
The results showed that germination percentage had statistically significant differences: the spherical nanoparticle treatment at 100 mg L-1 had a 13 % increase compared to the control (Figure 3D). These results differ from those reported by García-López et al. (2018), where no statistical differences in germination percentage of pepper seeds were found for 100, 200, and 500 mg L-1 12-20 nm spherical zinc oxide nanoparticle concentrations. On the other hand, Mahakham et al. (2017) calculated seed germination index (GI) and germination rate (GR) and found that the nano priming treatment with spherical silver NPs at 10 and 20 mg L-1 concentrations significantly increased the GI and GR values, compared to non-primed seeds. Nounjan et al. (2016), noted that priming treatment might improve seed water absorption since primed seeds showed faster imbibition than non-primed ones.
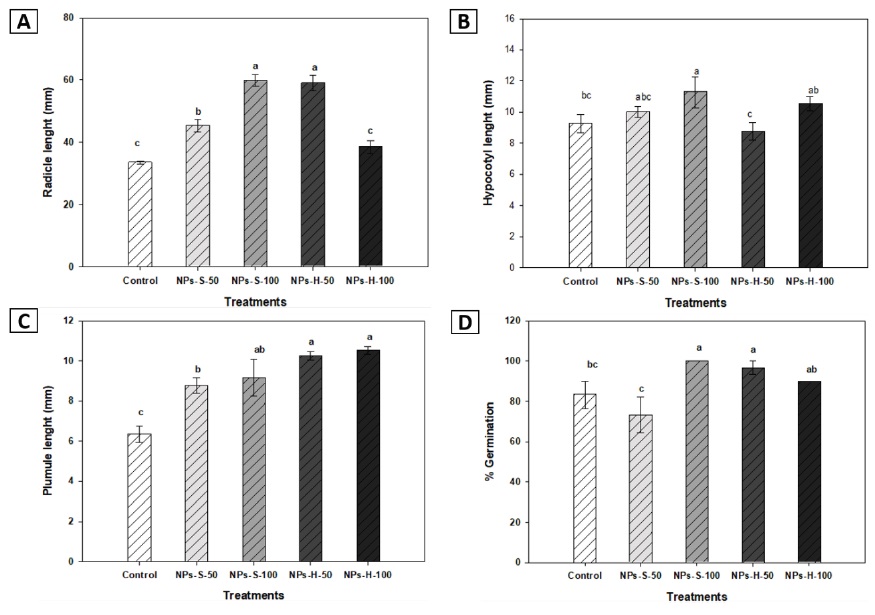
Figure 3 Radicle length (A), Hypocotyl length (B), Plumule length (C) and Germination percentage (D), of bell pepper seedlings at 14 DAS. NPs-S-50: spherical nanoparticles 50 mg L-1, NPs-S-100: spherical nanoparticles 100 mg L-1, NPs-H-50: hexagonal nanoparticles 50 mg L-1, NPs-H-100: hexagonal nanoparticles 100 mg L-1 Different letters indicate significant difference (LSD, ≤ 0.05). Lines over the bars indicate mean standard error.
The mechanism behind high seed germination in nano priming is the higher penetration through the seed coat, which improves the efficiency of water and nutrient absorption (Dutta, 2018). As previously mentioned, morphology will have an impact on the interactions with cell membranes and, as for spherical NPs, will be due to their isotropic geometry that might provide higher reactivity (Agnihotri et al., 2014; Peng et al., 2011).
Germination increase with spherical ZnO NPs treatment at 100 mg L-1 in our research could be due to the NPs size, which is within a 20.63 nm range, approximately smaller size compared to the hexagonal NPs. The difference may be interpreted from the surface/volume correlation point of view (Méndez-López et al., 2022), since reducing nanoparticle size increases surface area, which may show a better reaction and smaller NPs can release more ions than larger NPs (Xiu et al., 2012).
It has been reported that water absorption during the seed germination process is essential because ripe seeds are in the latency phase and need enough amount of water to start the metabolism and cell growth (Bewley et al., 2013). Therefore, an increase in germination percentage may be due to an increase on the level of aquaporin transcription after the nanoparticle seed priming treatment. Aquaporins (water channels) are transmembrane proteins that not only facilitate water diffusion through biological membranes and regulate water homeostasis, but also transport gases such as CO2 and nutrients (Maurel et al., 2015).
In commercial agriculture, fast and uniform germination and seedling emergence are important determinants for good crop establishment (Chen and Arora, 2013; Méndez-López et al., 2022). The germination starts with water uptake by the dry ripe seed and ends with the embryonic axis elongation, usually the radicle, through the seed coat. The consequence is root protrusion, followed by shoot protrusion (Rajjou et al., 2012).
The quantified results of radicle, hypocotyl and plumule length showed that spherical NPs at 100 mg L-1 treatment caused a 79 % increase for radicle length and 22 % for hypocotyl, while hexagonal nanoparticle at 100 mg L-1 treatment increased plumule length by 66 % compared to the control (Figures 3A, 3B, 3C). Li et al. (2021) reported similar results, indicating that root length improved substantially with 36 nm ZnO NPs at 50 mg L-1 treatment. However, Singh et al. (2018) showed that growth increased at its maximum in 50 mg L-1 treated seedlings; while above 100 mg L-1, namely, 500 and 1000 mg L-1, ± 36 nm ZnO NPs had phytotoxic effects on rice seedling growth.
Prasad et al. (2012) also reported an improvement in the growth, development, and yield of peanut plants with low concentrations of 25 nm spherical ZnO NPs, since the plants absorb them at a higher degree than bulk ZnSO4. Moreover, it was reported that 18 nm ZnO NPs (20 and 30 mg L-1) treated onion plants (Allium cepa L.) registered better and earlier growth than the control (Lawre et al., 2014). Positive effects of zinc oxide NPs in early growth parameters on bell pepper seedlings may be attributed to the action of zinc as an essential micronutrient for a variety of important activities in the cell membrane, such as cell elongation and protein biosynthesis in plant growth (Boonchuay et al., 2013; Cakmak, 2000; Welch, 1982).
On the other hand, morphology has been described as an important factor in NPs, potentially affecting their interaction with cell membranes, as well as their cell penetration capacity (Peng et al., 2011). Therefore, nanoparticle shape also affects their cell absorption. In this regard, it has been mentioned that spherical NPs show higher absorption rates compared to rod-shaped and cylindrical NPs (Chithrani and Chan, 2007; Zhao and Stenzel, 2018); although conflicting evidence has been reported, which suggests the complexity of the nano-bio interactions (Park et al., 2008).
Nanoparticle physicochemical properties interact with biological systems. This means that morphology will have an impact by interacting with the plant; its interaction with cell membrane proteins and receptors is determined by the NP design, which in fact, affects cell uptake, gene expression and toxicity. NPs can interact with the cell surface membrane in multiple scenarios (Albanese et al., 2012). Nanoparticle shape affects cellular uptake directly: stick-shaped NPs show greater uptake, followed by spherical, cylindrical and cubic NPs (Gratton et al., 2008).
Early growth parameters of bell pepper seedlings
Plant early growth parameters, such as height, stem diameter, leaf number, aerial part dry weight, root dry weight and leaf area were affected by the zinc oxide NPs used in the research (Figure 4). The best results were obtained with spherical NP treatment at a 100 mg L-1 concentration, with a 25 % increase in plant height, and a 15 % increase in stem diameter, compared to the control. As for leaf number, hexagonal NP treatment at 50 mg L-1 showed the best results, with a 13 % increase (Figure 4). Regarding aerial part dry weight, spherical NP treatment at 100 mg L-1 and hexagonal NP treatment at 50 mg L-1 were proved to be statistically equal, with a 78 % and 85 % increase, respectively, compared to the control (Figure 4E). Meanwhile, for root dry weight, a 61 % increase was observed due to hexagonal NP treatment at 100 mg L-1 (Figure 3D). Regarding leaf area, spherical NP treatment at a 100 mg L-1 concentration stimulated a 13 % increase in comparison with the control (Figure 4F).
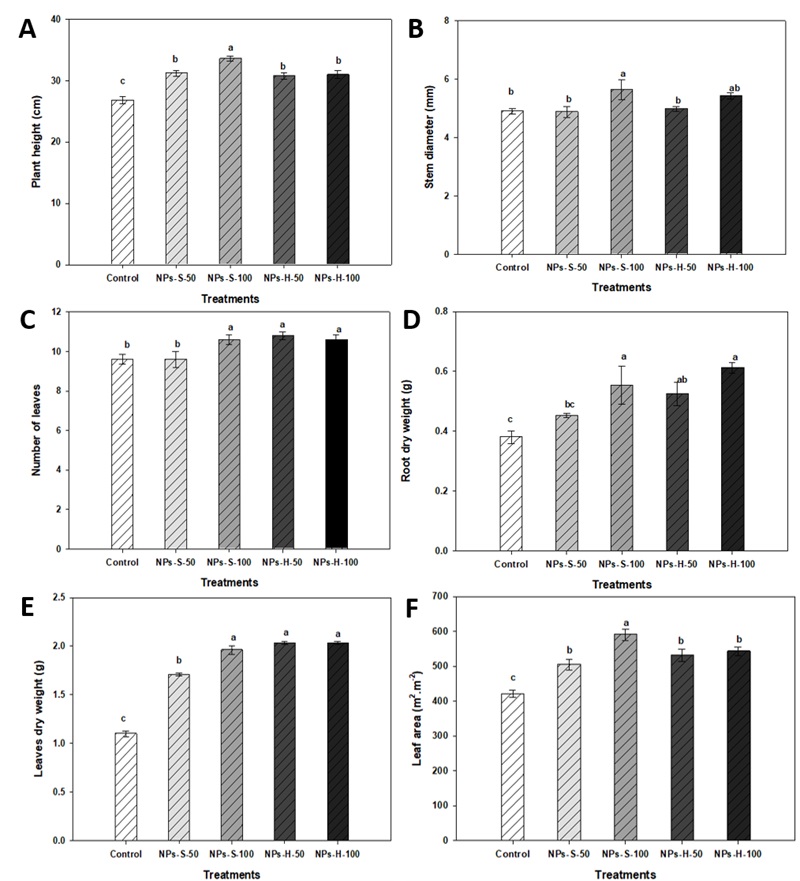
Figure 4 Plant height (A), stem diameter (B), leaf number (C), root dry weight (D), leaf dry weight (E), leaf area (F) of bell pepper seedlings at 45 days. NPs-S-50: spherical nanoparticles 50 mg L-1, NPs-S-100: spherical nanoparticles 100 mg L-1, NPs-H-50: hexagonal nanoparticles 50 mg L-1, NPs-H-100: hexagonal nanoparticles 100 mg L-1. Different letters in bars indicate a significant difference (LSD, ≤ 0.05). Lines over the bars indicate mean standard error.
These results are similar to those of Munir et al. (2018), who reported that at a 100 mg L-1 concentration of 34 nm hexagonal ZnO NPs, an increase in plant height, tiller number, spike length, shoot dry weight and root dry weight was observed. Likewise, an increase in plant growth on mung bean (Vigna radiata) was registered, by using 20 nm spherical NPs at 20 mg L-1, in chickpea (Cicer arietinum) and cucumber (Cucumis sativus), with 10 nm spherical NPs, at 400 and 800 mg kg-1. In addition, it has been mentioned that dry biomass significantly increases with the ZnO NPs application at low concentrations (Liu and Lal, 2015; Mahajan et al., 2011; Zhao et al., 2013). Faizan et al. (2018) reported that an 8 mg L-1 concentration of 35 nm spherical ZnO NPs increased leaf area by 29.9 % compared to the control on tomato plants. Leaf area is an important plant parameter, so both farmers and scientists can monitor or model plant growth and well-being (Pokovai and Fodor, 2019). Leaves represent most of the canopy surface of field crops and are the main interface for energy and mass exchange between the atmosphere and plants. The important processes, such as the canopy light interception, deposition of nutrients, transpiration, respiration and assimilation, are directly proportional to the leaf surface (Rundquist et al., 2014).
ZnO NPs application through seed nano priming treatment improved vegetative growth on bell pepper (Capsicum annuum), including seedling early growth variables. This improvement may be attributed to the Zn role in tryptophan synthesis, a prerequisite for the phytohormone indole-3-acetic acid (IAA). Zinc oxide NPs can also modulate the biosynthesis of the phytohormones cytokinins and gibberellins, which may lead to an increase in the number of internodes per plant (Sturikova et al., 2018). Moreover, improved cell elongation may lead to an increase in plant height in the early stages of plant development (Sharifi, 2016). Mahdieh et al. (2018) reported maximum improvement in plant height on pinto bean (Phaseolus vulgaris L.) with ZnO NPs seed treatment.
Similarly, the effects can be attributed to the interaction of NPs with cell surface membranes; the interaction between the NP-bound ligands and cellular receptors depends on the designed geometry; thus, the NPs acts as a scaffold whose design dictates the number of ligands that will interact with the receptor target (Albanese et al., 2012).
Non-enzymatic antioxidant compounds
The application of spherical nanoparticles induced an increase in chlorophyll content in leaves of bell pepper plants, obtaining the best results with a 100 mg L-1 concentration. Chlorophyll a content in leaves was higher with spherical NP treatment at 100 mg L-1, showing a 78 % increase compared to the control (Figure 5A). Meanwhile, chlorophyll b content showed a 92 % increase with spherical ZnO NP priming at 50 mg L-1 (Figure 5B). Total chlorophyll content was higher with spherical nanoparticles at 50 and 100 mg L-1, increasing by 59 and 56 %, respectively, compared to the control (Figure 5C). Regarding phenolic content, it increased with spherical NP treatment at 100 mg L-1 and hexagonal NPs treatment at 50 and 100 mg L-1, which were statistically equal between them but significantly superior to the control treatment (Figure 4). Our results differ from those reported by Chen et al. (2018), who evaluated 50 nm spherical ZnO NP treatments at 25, 50 and 100 mg L-1, and registered a negative effect in chlorophyll synthesis and rice seedling growth in hydroponic culture. Munir et al. (2018), reported that total chlorophyll content increased with hexagonal NPs at 100 mg L-1. In contrast, Faizan et al. (2018) state that photosynthetic pigments improved with ZnO NPs treatments, obtaining a maximum chlorophyll content observed in plants treated with 35 nm spherical NPs at 8 mg L-1. Govorov and Carmeli (2007) reported that metallic NPs may induce the efficiency of chemical energy production in photosynthetic systems.
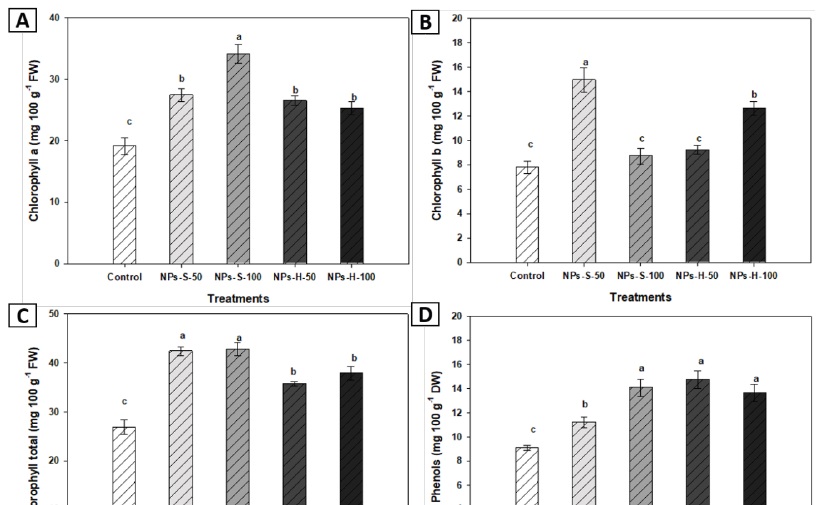
Figure 5 Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and total phenols (D) of bell pepper seedlings at 45 DAS. NPs-S-50: spherical nanoparticles 50 mg L-1, NPs-S-100: spherical nanoparticles 100 mg L-1, NPs-H-50: hexagonal nanoparticles 50 mg L-1, NPs-H-100: hexagonal nanoparticles 100 mg L-1. Different letters in the bars indicate significant difference (LSD, ≤ 0.05). The lines over the bars indicate mean standard error.
A positive effect of the NPs in chlorophyll synthesis may be associated with the essential role of Zn biosynthesis in this compound (Sturikova et al., 2018) through the participation in LHC (light-harvesting complex) protein synthesis (Wang and Grimm, 2021), a family of proteins involved in chlorophyll synthesis regulation. Zinc also takes part in chloroplast development, as well as in the photosystem II repair process by recycling damaged D1 protein (Hänsch and Mendel, 2009). Besides, spherical NPs have been reported to regulate protein build-up, such as kinase 2 from the cell division cycle and protochlorophyllide oxidoreductase (Syu et al., 2014). These results suggest that spherical morphology may have higher reactivity in the production of this enzyme, which catalyzes a vital step in chlorophyll biosynthesis, acting as a key regulator. Furthermore, the reduction of spherical NPs size leads to a surface area increase, which may be related with a higher photocatalytic activity (Nguyen et al., 2021).
According to our results, ZnO NPs application in seed nano priming increased total phenols in bell pepper leaves. Phenylpropanoid pathway produces different secondary metabolites as defense mechanisms (Nazerieh et al., 2018). Garza et al. (2021) studied the effect of moringa (Moringa oleifera) seed treatment with 16.49 nm spherical ZnO NPs at 7.5 and 10 mg L-1 concentrations, reporting a significant increasing effect in phenolic content, of 13 and 11 % respectively, compared to the control. The increase in these compounds can be attributed to the interaction of nanomaterials with plant cells (Juárez-Maldonado et al., 2019). Likewise, Zafar et al. (2016) carried out a study about the effect of ZnO NPs (1, 5,10, 20 mg L-1) (100 nm) in black mustard (Brassica nigra) under in vitro conditions and a concentration-dependent increase in total phenol content was found. Phenolic compounds play an important role in the non-enzymatic system. When the plant undergoes heavy metal stress, in this case, due to the ZnO NPs, phenolic compounds may act as metal-chelating agents or suppress reactive oxygen species (ROS) (Michalak, 2006).
NPs properties depend mainly on size and shape. For instance, nanospheres have been reported to have high biological activity (Liu et al., 2004). Besides, a nanoparticle of the same composition but a different nanostructure, in this case, nanosphere, may lead to a higher bio-availability of the elemental composition of NPs (Aguilar-Tapia and Zanella, 2018; Nel et al., 2006).
Conclusions
The application of zinc oxide NPs as seed nanopriming improved germination and early growth of bell pepper seedlings. Besides, ZnO NPs treatments proved to be better to increase total chlorophyll and phenolic content in leaf, generally with the application of spherical nanoparticles at 100 mg L-1, therefore, nanoparticle use in seed treatment may help homogenize germination and improve bell pepper plant growth and development, acting as a stimulant.











 nueva página del texto (beta)
nueva página del texto (beta)




