1 Introduction
Around 17,400 metric tons of urban solid wastes are generated daily in Mexico City, from which 50% to 60% correspond to the biodegradable fraction consisting mainly of food waste, fresh wastes from markets, branches, leaves, grass and trees trimmings (INEGI, 2013). The law for urban solids wastes generated in the City stipulates the separation of the organic fraction from the inorganic garbage. Thus, around 2,500 metric tons are transferred daily to the composting plant at Bordo Poniente, one of the garbage dump facilities located east Mexico City, and are stabilized during 30 to 40 days in open windrow piles.
The stabilization through composting produces liquid effluents known as compost leachates that drain from the piles and the amount generated depends on the garbage moisture content and composition. Considering a mean moisture content of 64.8% (Krogmann and Woyczechowski, 2000), we have estimated that around 1000 m3 of these effluents could be produced daily during the dry season, which difficult its proper disposition.
A high organic matter content is characteristic of these effluents and may vary from 47 to 109 g COD/L (Mokhtarani et al., 2012; Rajabi and Vafajoo, 2012) or as high as 116 and up to 120 g COD/L (Gan et al., 2013; Brown et al., 2013). Its nature is acidic because are produced during the acidogenic stabilization of biodegradable garbage (Han et al., 2005; Wagnai et al., 2014) or during composting (Mokhtarani et al., 2012; Rajabi and Vafajoo, 2012; Gan et al., 2013; Brown et al., 2013). Due to the interest in recovering the organic matter contained as a biofuel such as methane, the biological anaerobic treatment has been studied more often than physicochemical treatments.
Nevertheless the instability of single phase methanogenic reactors treating high strength effluents has been reported and associated to organic overloading when HRT is reduced or influent concentration is increased. Because VFA production in excessive amounts may cause acidification, methanogenesis inhibition and methane productivity reduction as well as COD removal efficiency decrease, and eventually, methanogenic reactors performance failure.
During the treatment of fresh raw compost leachate containing 65 g COD/L in an EGSB reactor operated at HRT of 68 hours and OLR of 24 kg COD/m3-d by Liu et al. (2010), attained 91% COD removal efficiency and a methane productivity of 6.0 L/L-d. Nevertheless, when organic loading was increased to 37.2 kg COD/m3-d, removal efficiency dropped to 79% as well as methane production.
Mokhtarani et al. (2012) performed the anaerobicaerobic treatment of fresh raw compost leachate containing 81 g COD/L. The anaerobic reactor was operated at 18 days HRT and 4.5 kg COD/m3-d attaining 91% COD removal efficiency, although 10 g COD/L were detected in the anaerobic effluent. At a loading increase to 10.8 kg COD/m3-d by diminishing the HRT to 7.5 days, a decrease in COD removal efficiency to 77% was observed.
Recently, Wangnai et al. (2014) attained a methane yield of 0.44 L per g of removed COD at an OLR of 6.5 g COD/L-d and HRT of 10 days during the compost leachates treatment that contained 34.5 g COD/L in a hybrid UASB reactor. The increase in loading led to removal efficiency and methane production decrease, as well as VFA accumulation in the effluent.
The acidogenic anaerobic fermentation of complex effluents as a first step in its degradation and treatment and the subsequent methanization of the acidogenic effluent has been reported before for other stringent effluents, such as distillery wastewater (Blonskaja et al., 2003), slaughterhouse waste water, cheese whey and dairy wastewater (Saddoud et al., 2007), pulp and paper waste, olive mill waste (Koutrouli et al., 2009), landfill leachate and urban solid waste organic fraction (Han et al., 2005), with better results than those carried out in a single phase (Demirel and Yenigün, 2002; Ke et al., 2005).
The spatial separation of acidogenesis from acetogenesis/methanogenesis in two operating in series reactors provides the optimal environmental conditions for each group of microorganisms, improving the whole treatment efficiency, hydrogen and methane productivity, as well as the control of the methanogenic reactor organic loading rate fed and pH, thus avoiding overloading and acidification (Demirel and Yenigün, 2002; Ke et al., 2005; Valdez and Poggi 2009). Because acidogenic bacteria have higher growing rates than acetogenic bacteria and methanogenic archea and tolerate higher organic loading rates, thus being able to prevail at acidic pH. During the acidogenic fermentation, short chain VFA and alcohols are produced that are suitable substrates for acetogens and methanogens (Demirel and Yenigün, 2002; Ke et al., 2005). Also are able to produce hydrogen as reported in several acidogenic pretreatments (Valdez and Poggi 2009).
The aim of this work was the treatment of compost leachates diluted with municipal wastewater in a two phase anaerobic acidogenic-methanogenic system of reactors operated in series, to recover the organic matter contained in the leachates as biofuels, such as hydrogen and methane. VFA composition, COD, alkalinity, ammonium and dissolved sulfide content of the reactors influents and effluents was determined periodically to assess the organic matter conversion to VFA, hydrogen and methane production in each stage of the in series system. The leachates used were collected at a composting plant during 2012 and 2013 and were physicochemically characterized previously to being fed to the reactors system.
2 Materials and methods
2.1 Sampling of compost leachates generated during the stabilization of the organic solid waste fraction
Leachates batch samples of 20 L were collected in the composting plant facility of Bordo Poniente located east of Mexico City, after 24 to 36 hours of being generated. The dry season sampling was carried out in January, April and December 2012 and in January, February and March 2013. The rainy season sampling was made in June, August and September 2012. The collected batches were kept at 4 °C until analysis.
2.2 Acidogenic Reactor (AR)
A 3 L UASB reactor (see Figure 1) was inoculated with 450 mL of anaerobic sludge from a pilot plant UASB reactor treating municipal wastewater that contained 23 g VS/L. The feeding was made up with mixtures of leachate and municipal wastewater containing around 500 mg TCOD/L (Cervantes et al., 2011; Gan et al., 2013), in proportions from 5% to 20% to attain an increasing organic matter concentration for the feeding from 4.9 g TCOD/L during start-up to a maximum of 26.5 g TCOD/L. A HRT of 0.5 day was set to attain OLR from 9.8 to 53.0 g TCOD/L-d. The feeding pH was kept at 4.1±0.2 and at 25±2 °C. VFA formation and COD conversion efficiency to VFA were calculated using equations 1 and 2. To calculate conversion efficiency, TCOD concentration determined in the feeding and in the effluent, as well as VFA in COD basis equivalence (VFA COD ), were considered.
2.3 Methanogenic Reactor (MR)
A zeolite packed filter reactor of 14 L volume similar to that reported by Gan et al. (2013) (see Figure 1), was inoculated with 3 L of granular sludge that contained 43 g VS/L obtained from a UASB reactor treating food industry wastewater.
The MR was fed with the effluent produced in the acidogenic reactor, neutralized at pH 6.0 with alkali during start up and with methanogenic effluent afterwards in a proportion 5:2, due to the high alkalinity produced during methanogenesis. The organic matter concentration in the feeding was of 5.0 g TCOD/L during startup and increased to 23.5 g TCOD/L during operation time. It was operated at a HRT of 1.5 days for OLR ranging from 3.3 to 15.7 g TCOD/L-d at 25±2 °C.
Both reactors were operated in parallel during startup for 20 days at a constant feeding concentration, afterwards were operated in series for 180 days, increasing the feeding concentration in several stages as presented in Table 1, at a system HRT of 2 days.
2.4 Analytical techniques
The composition analysis of the leachates samples collected included total and soluble COD content, total, fixed and volatile solids, total organic carbon (TOC method HACH 10173) and total nitrogen (TN method HACH 10072), ammonium, dissolved sulfide, pH and VFA content. The anaerobic reactors performance was followed up through the influent and effluent content of total and soluble COD, VFA, pH, produced alkalinity (as CaCO3), alkalinity ratio (produced alkalinity to produced VFA ratio), ammonium and dissolved sulfide, according to standard methods (APHA-AWWA-WEF, 2005). The biogas produced in each reactor was measured by displacement of a saline solution in a Mariotte column, while hydrogen, methane and carbon dioxide contained in the biogas, were determined through gas chromatography using a thermal conductivity detector and a Carbosphere 80/100 stainless steel packed column (Alltech) with helium as carrier gas. The VFA content and composition (acetic, propionic, butyric and valeric acids) were determined by gas chromatography using a flame ionization detector and an AT-1000 capillary column (Alltech) with nitrogen as carrier gas.
2.5 Statistical analysis
To assess the statistical significance of the differences between mean values of leachate samples collected, Student's t-distribution test was applied to all parameters measured, considering 6 samples for the dry season and 3 samples for the rainy season. Influent and effluent samples from the reactors system were analyzed statistically calculating the mean and standard deviation values.
3 Results and discussion
3.1 Compost leachates physicochemical characteristics
The leachates samples collected from the composting plant were characterized and all data were statistically analyzed. No significant statistical differences were found between parameters mean values measured in relation to the season of the year in which samples were collected, with the exception being the ammonium content. As can be seen in Table 2, TCOD and SCOD contents are similar to those reported in leachates generated in other composting plants (Mokhtarani et al., 2012; Rajabi and Vafajoo, 2012; Gan et al., 2013; Brown et al., 2013).
Table 2 Physicochemical parameters determined in compost leachates during 2012 and 2013 dry and rainy seasons.

6s, ds: six samples, data corresponding to samples taken during the dry season.
3s, rs: three samples, data corresponding to samples taken during the rainy season.
TOC represented 33.1% and 35.1% of TCOD and SCOD content for samples taken in rainy and dry seasons, respectively. VFA represented 20.1% for the dry season and 16.1% for the rainy season of TCOD. Also the predominance of acetic acid and high concentration of VFA indicated that leachates had been produced in the first hours of the composting process, which is in agreement with Brinton (1998) who found that these compounds are produced during the first 20 hours of the composting process.
Low ammonium content showed that protein hydrolysis began also with the acidification of the biodegradable wastes and represented 52% of TN for the dry season samples and 30% for the rainy season samples, being the only significant difference found between batches collected in both seasons, indicating that rain percolated through the composting piles probably diluted the amount of ammonium found in the leachate. Dissolved sulfide content found in leachates for both seasons could be also due to the proteolysis process. VS content for both seasons indicated the biodegradable nature of the organic matter contained in this effluent and although the high organic matter content found, leachates are constituted by 90% water.
3.2 COD Conversion efficiency to VFA and VFA production in the AR
The reactor was operated at organic loading rates that varied from 9.8±0.4 to 53.0±4.7 g COD/L-d due to the short HRT of 0.5 day. VFA concentration found in the AR effluent increased during operation time from 2.8±1.8 g VFA/L after start up to 10.0±1.1 g VFA/L accompanied of a maximum VFA formation rate of 0.918 g VFA COD /d (0.306 g VFACOD/L-d), as feeding COD concentration and OLR were increased during stages I to IV. Organic matter conversion efficiency to VFA of 73.5±12.2 % was attained during stage I which remained in a maximum of 73.6±7.7% during stage III, when the feeding concentration was of 10.0±0.5 g TCOD/L and OLR of 20.0±1.0 g COD/L-d (Figure 2).
During stage IV, OLR was increased to 25.6±1.4 g COD/L-d by increasing feeding concentration to 12.8±0.7 g TCOD/L, and instability and a decrease in conversion efficiency to 65.7±20.1% was observed. During stage V, at an OLR of 31.0±1.6 g COD/L-d and a feeding concentration of 15.5±0.8 g COD/L, an acute diminution to 13.3±9.7% in conversion efficiency was observed and VFA formation dropped from 6.4 to 1.1 g VFA/L. This failure was attributed to the intense gas production that dragged out solids from the sludge bed, reducing the initial sludge volume to 150 mL. An important amount of biogas was going out13from the reactor and small bubbles in the effluent were observed, consequently, a very low amount of produced biogas was measured, only 2 L/d, composed of 22% hydrogen and 78% carbon dioxide. The energy contribution of hydrogen would be of 0.05 W.
After 115 days of continuous operation and at the end of stage V, the reactor was reinoculated with the same sludge used initially that led to some recovering in conversion capacity during stages VI and VII, which increased to a mean value of 23.4% while the amount of VFA formed increased to 4.0 g VFA/L for a VFA formation rate of 0.525 g VFACOD/d (0.175 g VFACOD/L-d).
In contrast and although COD to VFA conversion dropped, a mean organic matter removal efficiency of 10.3% was determined for this reactor throughout operation time, indicating that the main function performed was organic matter conversion to VFA. Comparatively, Blonskaja et al. (2003) attained a removal efficiency lower than 54% that diminished to 10% as OLR was increased for distillery waste acidogenic treatment.
VFA composition fed to the AR is shown in Figure 3a, where the predominance of acetic acid can be appreciated indicating also the freshness of the collected leachate according to Brinton (1998). The proportion of formed VFA in the AR is shown in the exterior circle of Figure 3b, where butyric and valeric acids represented 93% of all VFA formed. During acidogenesis at all loading rates, a very small amount of propionic acid was formed and no acetic acid produced was detected. The interior pie in Figure 3b shows the VFA proportion obtained in the effluent of the acidogenic reactor, where the amount of acetic acid found corresponds to the amount fed.
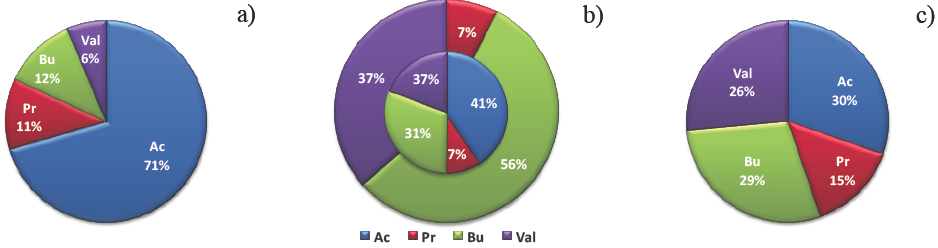
Fig. 3 VFA proportion in the AR and in the MR. (a) AR influent. (b) AR effluent: formed VFA shown in the exterior circle; VFA composition in the effluent shown in the interior pie. (c) VFA composition in the MR effluent.
The AR effluent pH remained at 5.08±0.4 due to the high amount of butyric and valeric acids formed. Probably the influent pH of 4.1±0.2 inhibited the formation of acetic acid because it has an acidic pK of 4.76. Similar results were reported by Horiuchi et al. (1999), who found that at pH 6.0 only butyric acid was produced, while at neutral pH acetic and propionic acids were mainly formed. Acetic and propionic acids were the main products in an acidogenic filter bed reactor fed with slaughterhouse wastewater at pH above 7.0 and also in a acidogenic stirred tank reactor fed with cheese whey at pH 6.5 (Saddoud and Sayadi, 2007; Saddoud et al. 2007).
VFA composition found in the MR effluent is shown in Figure 3c, in which similar proportions of acetic, butyric and valeric acids indicated that acetogenesis of butyric and valeric acids and in a lesser proportion of propionic acid, was taking place in the methanogenic reactor. VFA residual concentration in the MR effluent was of 207±200 mg VFA/L and VFA a) removal attained was of 92.9±17.4%.
3.3 Organic matter removal efficiency and conversion to methane in the MR
This reactor operated at OLR that increased from 3.3±0.2 to 15.7±1.5 g TCOD/L-d. At these conditions the organic matter consumption rate in the methanogenic reactor increased from 22.0 to 122.3 g TCOD/d, and maintained a removal efficiency of 94.7±1.4% for TCOD and of 96.0±1.3% for SCOD (Figure 4a) at all OLR applied during operation time. Organic matter concentration in the effluent increased Figure 5. from 0.3 g/L after start up to 1.3 g/L for the highest OLR applied in stage VII.
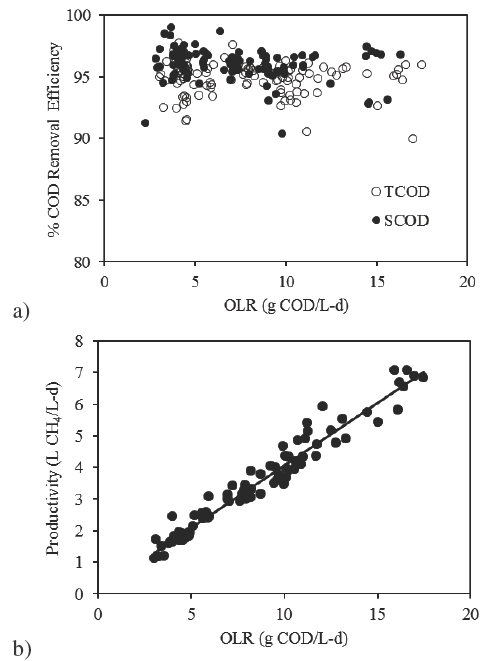
Fig. 4 (a) Organic matter removal efficiency profile. (b) Methane productivity profile related to OLR fed to the MR.
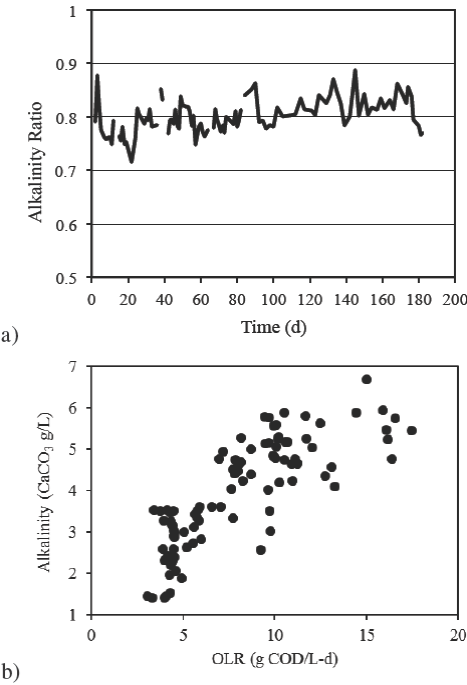
Fig. 5 (a) Alkalinity ratio profile and (b) alkalinity produced in the MR related to the OLR applied.
The performance of the MR was optimum and was not affected by the drop in organic matter conversion efficiency to VFA observed in the AR when 31.0±1.6 g COD/L-d was applied. As a consequence, methane and carbon dioxide ratio in the biogas was of 70:30 and methane production rate increased from 9.5 to 47.8 L CH4/d to render a methane productivity of 6.4±0.4 L CH4 /L-d (Figure 4b) at the highest OLR in stage VII.
The methane production related to the organic matter consumption was of 0.385 L CH4 per g of consumed COD, which corresponds to the slope of the methane productivity versus OLR shown in Figure 4b. With the methane productivity attained, an energy supplement of 390 kW-h per m3 of treated raw leachate or 3.2 kW-h per kg treated COD could be obtained.
Similar results were achieved by Liu et al. (2010), whom observed that the optimal OLR was 24 kg COD/m3-d and a HRT of 68 hours at which an EGSB reactor used for compost leachates treatment was operated, to avoid loss of removal efficiency and methane productivity.
The MR effluent pH remained in 7.80±0.16 and alkalinity ratio around 0.8±0.03 (Figure 5a), because produced alkalinity due to methanogenesis increased from 1.2 to 5.5 g CaCO3/L, as shown in Figure 5b. It is probably that the carbon dioxide formed in the AR contributed to the high amount of carbonate detected in the MR effluent, since this gas tends to remain dissolved in water as carbonic acid, particularly at acid pH.
The amount of alkalinity produced due to the extent of organic matter conversion to methane led to the deposition of carbonates into the reactor and in the sludge, in such a way that during the last five operation days a diminution in removal efficiency to 90.4% for TCOD and of 93.1% for SCOD was observed. The effect in performance diminution can be seen in the alkalinity ratio as well, that slightly diminished around day 180.
3.4 Performance of the two phase acidogenic-methanogenic reactors system
The reactors system maintained 94.9±1.1% of removal efficiency, and the failure in organic matter conversion observed in the AR did not affect the performance of the MR. This result is in agreement with removal efficiency in a similar two phase system treating distillery waste, although at longer HRT. Also, 98.7% and 98.5% COD removal efficiencies for slaughterhouse wastewater and cheese whey, respectively, in a two phase reactors system have been reported that operated at HRT of 1.5 and 2 days (Saddoud and Sayadi, 2007; Saddoud et al. 2007).
Although the organic matter concentration fed to the two phase system is lower than that used by other authors, no acidification produced by the high OLR fed or by the amount of VFA present in the feeding was detected in the MR.
Nevertheless, dissolved sulfide was determined in raw leachate and consequently in the feeding of the AR. Its concentration increased during acidogenesis from 65.2±10.0 to 263.1±53.6 mg/L and diminished in 75% in the MR, probably due to polysulfides formation (S n2− ) that might have been favored by the alkalinity produced during methanogenesis, forming a complex and precipitating along with carbonates and ultimately affecting removal efficiency. Wangnai et al. (2014) found a diminution in methane content in the biogas to 60% when dissolved sulfide increased from 100 to 2500 ppm, although the combined effect of sulfides and alkalinity on methanogenesis was not pointed out.
During the two phase system operation, ammonium was also formed and increased in the MR from 0.21±0.07 to 0.913±0.14 mg/L in relation to the amount fed to the AR of 0.06±0.01 mg/L after star up to 0.4±0.05 mg/L during stage VII. Formed ammonium profile is shown in Figure 6, from which 54.5% was produced in the MR. Nevertheless, no effect of ammonium on methane productivity was observed and ammonium removal by zeolite was not detected as reported before by Gan et al. (2013).
Moreover, the ratio g COD/g NH 4 + in the methanogenic effluent ranged from 1.4 to 2.2, indicating that a nitrification-denitrification postreatment should be suitable for polishing the methanogenic effluent to recover the water contained in this effluent (Chiu et al., 2007).
Conclusions
The physicochemical characterization of leachates sampled in the composting plant showed that the season of the year in which were collected had no significant influence in the organic matter content, and the only significant difference was found for ammonium content. The two phase acidogenicmethanogenic reactor system used for the diluted compost leachates treatment represented a feasible strategy for diminishing the environmental impact of these effluents due to the organic matter conversion extent (above 90%), providing an aggregated value through methane production (3.2 kW-h per kg COD treated), and a small amount of hydrogen in the acidogenic phase. Ammonium formed in the system did not affect the performance of the acidogenic and methanogenic reactors and its postreatment may allow the recovery of the water contained in this effluent. Carbonates and dissolved sulfides probably affected the methanogenic reactor performance.
Abbreviations
AR |
acidogenic reactor |
COD (g/L) |
chemical oxygen demand |
EGSB |
expanded granular sludge bed reactor |
HRT (days) |
hydraulic retention time |
MR |
methanogenic reactor |
TN (g/L) |
total nitrogen |
OLR (g COD/L-d) |
organic loading rate |
SCOD (g/L) |
soluble chemical oxygen demand |
TS (g/L) |
total solids |
VS (g/L) |
volatile solids |
TCOD (g/L) |
total chemical oxygen demand |
TOC (g/L) |
total organic carbon |
UASB |
upflow anaerobic sludge bed reactor |
VFA (g/L) |
volatile fatty acids |
Ac |
acetic acid |
Pr |
propionic acid |
Bu |
butyric acid |
Va |
valeric acid |
VFA COD (g/L) |
volatile fatty acids expressed as COD equivalent |











 nueva página del texto (beta)
nueva página del texto (beta)








