1. Introduction
Adsorption of amino acids onto solid surfaces has received much attention because of its scientific importance and applications in the separation and purification processes (Han & Yun, 2007; Hong & Bruening, 2006; Kostova & Bart, 2007; O'Connor et al., 2006; Sánchez-Hernández, Bernal, del Nozal, & Toribio, 2016). Amino acids are biomolecules of great relevance that are widely used in many industries such as food, cosmetic, medicine, biochemistry and others (Bourke & Kohn, 2003; Hartmann, 2005; Infante et al., 2004; Oshima, Saisho, Ohe, Baba, & Ohto, 2009; Palit & Moulik, 2001). They are non-toxic and are used as building blocks for the production of pharmaceutical and agrochemical compounds. In addition, they are interesting molecules as adsorbates because of their molecular size and zwitterionic nature (O'Connor et al., 2006). Similar to many amino acids, L-phenylalanine is essential for animals and the human body. It is extensively used as ingredient in food or feed additive, in infusion fluids, neutraceutical and pharmaceutical (Pimentel, Alves, Costa, Fernandes, et al., 2014; Pimentel, Alves, Costa, Torres, et al., 2014; Zhou, Liao, Wang, Du, & Chen, 2010). Generally, the amino acids have been studied by adsorption on well-ordered surfaces of solids. On the other hand, most of the current methods employed for the removal of L-phenylalanine from protein hydrolysates are based on the adsorption on activated carbon, polymeric resins, zeolites and ion exchangers (Lopes, Delvivo, & Silvestre, 2005; Outinen et al., 1996; Shimamura et al., 2002). These studies give information for practical researches on the purification and separation of amino acids. Over the last years, several studies have been reported for the adsorption of amino acids on porous solids (Casado et al., 2012; El Shafei, 2002; El Shafei & Moussa, 2001; Ghosh, Badruddoza, Uddin, & Hidajat, 2011; Goscianska, Olejnik, & Pietrzak, 2013b, 2013c; Jiao, Fu, Shuai, & Chen, 2012; Long et al., 2009; Mei, Min, & Lü, 2009; Palit & Moulik, 2001; Silvério, Dos Reis, Tronto, & Valim, 2008; Titus, Kalkar, & Gaikar, 2003; Wu, Zhao, Nie, & Jiang, 2009). However, their adsorption capacity is still low because of the small pore volume or wide pores of these adsorbents, which are consequently inappropriate to the molecular size of amino acids. Moreover, the major drawback of the adsorption process is the high cost for the production and regeneration of adsorbents. Such inconvenient resulted in growing research on inexpensive adsorbents (Alves, Franca, & Oliveira, 2013a, 2013b; Clark, Alves, Franca, & Oliveira, 2012; Goscianska, Nowicki, & Pietrzak, 2014; He, Lin, Long, Liang, & Chen, 2015; Sebben & Pendleton, 2015).
In this study, porous activated carbon-based materials obtained from date stones (seeds) are potential adsorbents for L-phenylalanine amino acid. This is mainly due to their physical and chemical characteristics such as highly developed porous structure, good thermal stability, low cost and more accessibility. Date stones are among the most common agricultural by products available in palms growing in the Mediterranean countries like Algeria, which is one of the largest producers in the world. Algeria produces more than 400 different varieties of dates with an annual production of about 400,000 tons (Chandrasekaran & Bahkali, 2013). Date stones constitute roughly 10% of the date weight and this lignocellulosic-based agricultural waste is a good precursor for preparing activated carbon because of its excellent natural structure and low ash content (Bouchenafa-Saib, Grange, Verhasselt, Addoun, & Dubois, 2005; Merzougui & Addoun, 2008). As it is well known, two methods are commonly used for the preparation of activated carbon: physical and chemical activations. Compared with the physical process, the chemical activation presents some advantages like low activation temperature, short activation time, high surface area, well developed microporosity of activated carbon, simple operation and low energy consumption (Deng, Yang, Tao, & Dai, 2009; Pereira et al., 2014). Therefore, the date stones can be activated with chemical agents such as KOH, ZnCl2, H3PO4, K2CO3 and NaOH, to obtain activated carbons with well-developed textural characteristics. To the best of our knowledge, the use of activated carbons for the L-phenylalanine recovery from aqueous solutions by adsorbents based on date stones are not available in the open literature. Thus, the principal objective of this work was to prepare porous activated carbons with high surface areas from date stones by chemical activation with KOH and ZnCl2. The activated carbons proved to be good candidates for the adsorption of L-phenylalanine in an aqueous medium.
2. Materials and methods
2.1. Materials
The date stones used in this study were from Algerian origin. The following reagents were used: L-phenylalanine standard (>98%, Fluka, France), potassium hydroxide (>98%, Sigma Aldrich, USA), zinc chloride (>98%, Sigma Aldrich, USA), KH2PO4 (>99%, Fluka, France), K2HPO4 (>99%, Fluka, France), NaHCO3 (>99%, Sigma Aldrich, USA), Na2CO3 (>99%, Sigma Aldrich, USA), HCl (37%, Sigma Aldrich, USA), KCl (>98%, Fluka, France), NaCl (>99%, Sigma Aldrich, USA), NaOH (>99%, Sigma Aldrich, USA). Ultrapure water was obtained from milli-Q system (Millipore, France).
2.2. Preparation of the activated carbons
The activated carbons were prepared from date stones. At first, the stones were thoroughly washed with distilled water and dried in an air oven at 120 °C; such protocol was effective to facilitate crushing and grinding. A fraction particle size of between 0.5 and 1 mm was used for the preparation of activated carbons by impregnation with ZnCl2 and KOH. The precursor was impregnated with a chemical activating agent in a solid form. The impregnated precursor was carbonized in a horizontal tubular furnace under nitrogen flow with a heating rate of 5 °C min−1, to allow free evolution of volatiles, up to the hold temperature for 1 h. The resulting activated carbon was immersed in HCl solution (0.1 mol L−1) under reflux ebullition (3 h) in order to extract the compound formed and reagent excess. Then, the solution was filtered and the black solid was washed with hot distilled water until the test with AgNO3 became negative. The adsorbent was dried at 120 °C, and kept in tightly closed bottles until use. The activated carbons were named ACZ (1 g ZnCl2: 1 g date stones, activated at 600 °C), ACK (9 mmol KOH: 1 g date stones, activated at 800 °C).
2.3. Characterization
The specific surface area and pore structure of the activated carbons were characterized by nitrogen adsorption-desorption isotherms at −196 °C using the ASAP 2010 Micromeritics equipment. All the activated carbons were outgassed at 150 °C overnight. The specific surface area was calculated by the Brunauer-Emmett-Teller (BET) equation (Brunauer, Emmett, & Teller, 1938). The external surface area, micropore area and micropore volume were calculated by the t -plot method. The total pore volume was evaluated from the liquid volume of N2 at a high relative pressure near unity 0.99 (Guo & Lua, 2000). The mesopore volume was calculated by subtracting the micropore volume from the total volume. The pore size distribution (PSD) was determined using the density functional theory (DFT) model. The morphology of activated carbons was visualized by scanning electron microscopy (SEM) using a Philips XL 30 equipped with an energy dispersive spectrometer (EDS). The Fourier transform infrared (FT-IR) spectroscopy was used to determine the functional groups of the activated carbons; the spectra were recorded over the range (400-4000 cm−1) on a Perkin-Elmer spectrum two spectrometer using KBr pellets.
2.4. Determination of zero point charge pH PZC
The determination of the point of zero charge (pHPZC) was conducted to investigate how the surface charge of ACK and ACZ adsorbents depends on pH. pHPZC of the activated carbons was determined using the procedure described elsewhere (Prahas, Kartika, Indraswati, & Ismadji, 2008): 0.01 M of NaCl was prepared and the initial pH was adjusted between 2 and 12 using HCl or NaOH solution (0.1 M). 50 mL of NaCl solution was placed in Erlenmeyer flakes with 0.1 g of adsorbent. The flasks were kept under agitation (150 rpm, 48 h), and the final pH of the solution was measured. The intersection point of the curves pHfinal vs. pHinitial and the bisector was taken as pHPZC.
2.5. Adsorption experiments
The batch adsorption experiments were performed in 100 mL Erlenmeyer flasks containing a mass of adsorbent mixed with a known volume of L-phenylalanine solution (200 mg L−1) under agitation (150 rpm). The effect of the contact time was studied to determine the time required for equilibrium at natural pH at 20 °C. For the temperature effect, 50 mg of activated carbon was added to 50 mL of L-phenylalanine solutions with concentrations ranging from 50 to 1000 mg L−1, prepared by dissolving appropriate amounts of L-phenylalanine in ultrapure water (18.2 MΩ cm), the flasks were maintained under constant agitation at various temperatures (20, 25, 35 and 40 °C) for 300 min. The effect of pH on the adsorption was performed by mixing 50 mg of activated carbon into 50 mL of L-phenylalanine solutions in the pH range (2.0-9.4), under constant agitation at 20 °C for 300 min. The pH value of the L-phenylalanine solutions was changed by using different buffer solutions (pH 5.7-7.2 potassium phosphate buffer: pH 2.0 HCl-KCl buffer: pH 9.4 bicarbonate buffer). The concentrations of L-phenylalanine remaining in the supernatant solutions were filtered using a hydrophilic syringe filter with a pore size of 0.45 um. The adsorbed amount was determined using a UV-vis spectrophotometer at 257 nm. (The adsorption capacity) The equilibrium adsorption capacity per unit mass of activated carbons q e (mg g−1) and the removal percentage of the L-phenylalanine η (%) were calculated from the following equation:
where C 0 and Ce are the initial and equilibrium L-phenylalanine amino acid concentrations in the liquid phase (mg L−1), W (g) the weight of adsorbent and V (L) the volume of solution.
2.6. Desorption study
Desorption of L-phenylalanine was investigated in order to explore the regeneration and recycling ability of the two activated carbons. For this, 50 mg of ACK and ACZ were mixed with 50 mL of L-phenylalanine solution at a saturated concentration, and stirred at 150 rpm at optimum adsorption temperature (20 °C) for 300 min. The amount of adsorbed amino acid was determined by the same equation used in the adsorption experiments (see Section 2.5). Thereafter, ACK and ACZ were washed with ultrapure water until the residual concentration of L-phenylalanine becomes negligible. The loaded activated carbons were then allowed to be in contact with 50 mL of two eluent solutions (M NaOH and M HCl, 10−2 M) for 300 min. The desorbed carbons were again subjected to the next batch in order to check desorption and reusability of ACK and ACZ. The amount of desorbed amino acid was calculated from the concentration of desorbed L-phenylalanine in liquid phase using UV-vis spectrophotometer at 257 nm. The percentage of desorbed L-phenylalanine from the activated carbons was calculated according to the following equation:
3. Results and discussion
3.1. Characterization of activated carbons
3.1.1 Scanning electron microscopy (SEM)
The SEM micrographs show the effects of ZnCl2 and KOH on the surface pore structures of the activated carbons (Fig. 1). The external morphology shows more or less homogeneous cavities on the surfaces of ACK and ACZ. These cavities resulted from the evaporation of chemical agents during the activation process, leaving space previously occupied by KOH and ZnCl2. Figure 1 suggests that the large pores on the surface are connected to a whole network of smaller pores inside the activated carbon. Therefore, the ACK and ACZ have great potential as good adsorbents for L-phenylalanine.
3.1.2. Nitrogen sorption
The N2 adsorption-desorption isotherms of activated carbons ACK and ACZ are given in Figure 2. The isotherms are type I, according to the IUPAC classification, assigned to microporous materials, and they present at a very low relative pressure (P /P0 < 0.2) a significant increase of N2 adsorption corresponding to the micropores filling (Fig. 2). However, the amount of adsorbed nitrogen is reduced at higher pressures, suggesting the development of both micro and meso-porosity in ACK and ACZ. The presence of hysteresis loops indicates that some mesoporosity starts to be developed by capillary condensation. The textural parameters of activated carbons determined from nitrogen adsorption-desorption are gathered in Table 1. It can be concluded that the activation of date stones by ZnCl2 and KOH leads to active coals of a well-developed surface area and a high pore volume (Table 1). Among the activated carbons, ACZ exhibits the highest surface area (1235 m2 g−1) and pore volume (0.63 cm3 g−1) while ACK has a smaller surface area (1209 m2 g−1) and pore volume (0.55 cm3 g−1). As shown in Table 1, the activated carbon prepared by KOH is essentially microporous, with 77% of its surface area. Similar trends have been found for the influence of the chemical activating agent on the development of the surface area and pore volume of activated carbons obtained through ZnCl2 and KOH activation of other lignocellulosic materials (Angin, 2014; Bagheri & Abedi, 2009; Foo & Hameed, 2011; Sreńscek-Nazzal, Kamińska, Michalkiewicz, & Koren, 2013; Yorgun, Vural, & Demiral, 2009; Zhu, Wang, Peng, Yang, & Yan, 2014). The pore size distribution is an important property in the adsorption mechanism because the adsorption of molecules of different sizes and shapes is directly related to the pore size of adsorbents. According to the classification adopted by IUPAC, adsorbent pores are classified as micropores (<2 nm), mesopores (2-50 nm) and macropores (>50 nm). Figure 3 shows the pore size distribution for the activated carbons, which clearly indicates that the pore diameter is in the micropore range. It is important to mention that a L-phenylalanine molecule is relatively small with a size of 0.7 nm × 0.5 nm × 0.5 nm (Alves et al., 2013b; Long et al., 2009). Therefore, such micropores with a size of (<2 nm) are accessible for L-phenylalanine molecules.
Table 1 Textural characteristics of ACK and ACZ.
| Adsorbent | Surface area (m2 g−1) | Pore volume (cm3 g−1) | DFT pore size (nm) | ||||
|---|---|---|---|---|---|---|---|
| S BET | S ext | S mic | V T | V mes | V mic | ||
| ACK | 1209 | 276 | 933 | 0.550 | 0.180 | 0.370 | 1.48 |
| ACZ | 1235 | 525 | 710 | 0.630 | 0.341 | 0.289 | 1.59 |
3.1.3. Infrared spectroscopy (FT-IR)
The FT-IR is an important technique to qualitatively determinate the characteristic functional groups of the adsorbents. The spectra of porous activated carbons (Fig. 4) show multiple functions which can also be observed in other carbons activated by KOH and ZnCl2 (Huang, Ma, & Zhao, 2015; Lua & Yang, 2005; Saka, 2012). The FT-IR analysis indicates that ACK and ACZ exhibit a similar shape and the same functional groups. The broad band in the range (3000-3500 cm−1) is ascribed to the O-H stretching mode of hydroxyl groups with hydrogen bending of adsorbed water. Bands (2900-2950 cm−1) are assigned to asymmetric and symmetric stretching vibrations of aliphatic bond -CH, -CH2 and -CH3 while the bands around 1580 cm−1 may be due to the presence of aromatic CC ring stretching vibration. The band at 1400 cm−1 is associated with -COO- asymmetric vibration of carboxylic groups while that at 1384 cm−1 is due to stretching vibration of -CH3 group. Finally, the vibration band centered at 1115 cm−1 is attributed to C-O stretching vibrations, as in alcohols, phenols, acids, ethers or esters. The presence of the functional groups such as carboxyl and hydroxyl are potential adsorption sites for L-phenylalanine amino acid.
3.2. Adsorption studies
3.2.1. Effect of contact time
The contact time is a fundamental parameter in any transfer phenomena such as adsorption. The equilibrium adsorption capacity of L-phenylalanine on activated carbon was investigated to determine the time required to reach the equilibrium between adsorbents (50 mg) and L-phenylalanine solution, (200 mg L−1) (Fig. 5); it can be observed that the adsorption capacities of activated carbons gradually increase with the contact time and does not stop until an equilibrium state is reached (180 min). No obvious variation in the amount of adsorbed L-phenylalanine was observed; the adsorbed mass at equilibrium reflects the maximum adsorption capacity of the activated carbons under the operating conditions; the equilibrium adsorption capacity of L-phenylalanine on ACK (114 mg g−1) is higher than ACZ (68 mg g−1) (Fig. 5). Comparing the results obtained in this work (porous activated carbons) with mesoporous materials, such as the SBA-3 mesoporous silica tested elsewhere (Goscianska, Olejnik, & Pietrzak, 2013a), it can be concluded that the mesoporous materials need a much longer period to reach the equilibrium; in this case the pore size distribution of ACK and ACZ, centered at 1.48 and 1.59 nm, respectively, are beneficial for the adsorption because L-phenylalanine molecules have easy access to the pores.
3.2.2. Temperature effect
The temperature has a direct influence on the adsorption of amino acids. Figure 6 shows the temperature effect on the adsorption of L-phenylalanine by activated carbons. The same behavior is observed for ACK and ACZ and the isotherms plotted at various temperatures show that the equilibrium adsorption capacity decreases with increasing temperature from 20 to 40 °C, indicating that the adsorption of L-phenylalanine is of exothermic nature. These results show that the decrease of adsorption at a high temperature can be ascribed to the greater tendency of L-phenylalanine molecules to form hydrophobic bonds in an aqueous medium, thus hindering their hydrophobic interactions with the adsorbent surface (El Shafei & Moussa, 2001). On the other hand, the decreased adsorption at equilibrium is due to decreased surface activity at higher temperatures. The best uptake of L-phenylalanine was obtained at 20 °C which is selected as an optimal adsorption temperature and will be used for further experiments.
3.2.3. Effect of pH
pH is known to be a crucial parameter that affects the adsorption behavior at water-solid interfaces. Its effect on the amino acid adsorption on ACK and ACZ at different buffer solutions ranging from 2.0 to 9.4 at 20 °C is illustrated in Figure 7. All equilibrium isotherms are of the L-type (Langmuir isotherms) and the amount of adsorbed L-phenylalanine increases with raising the initial concentration. At a low concentration, the amino acid is randomly deposited on the adsorbent, and the fast uptake can be attributed to a large number of empty sites on the surface (Goscianska et al., 2014). In contrast, at high concentrations, the nonpolar groups of amino acids are close to each other until they touch inside their van der Waals radii, leading to a dense packing of molecules on the active sites of the adsorbent. The removal of L-phenylalanine from an aqueous solution is strongly dependent on pH (Fig. 7) and this can be explained by pHpzc. pHPZC (Fig. 8) is found to be 6.18 (ACZ) and 6.80 (ACK). The surface of the activated carbon is positively charged below pHpzc and negatively charged above pHpzc. The maximum adsorption capacity of both activated carbons was obtained at pH 5.7, which is close to the isoelectric point (PI = 5.48) of L-phenylalanine. The latter is known as a Zwitterion containing both amine and carboxylic groups, near to the isoelectric point and presents both negative and positive charges (Jiao et al., 2012). Therefore, the Coulomb repulsive interaction between the L-phenylalanine molecules is almost negligible. Furthermore, the strong hydrophobic interactions amino acid/adsorbents, and intra-molecular interaction between amino acid molecules are responsible of close packing of L-phenylalanine in the micropores of adsorbents, leading to the highest adsorption capacity at this pH. At low pH (<2), the surface charge of the activated carbon is negative and the cationic form of the amino acid is not favorable for the adsorption because of the electrostatic repulsion; this explains the decrease in the adsorption efficiency. Figure 7 also shows that the adsorbed amount of L-phenylalanine decreases with increasing pH from 5.7 to 9.4 and this can be explained by the strong electrostatic repulsion adsorbent/amino acid molecules, negatively charged. This effect is similar to that reported previously (Alves et al., 2013b; Goscianska et al., 2013c); as consequence, the adsorption of L-phenylalanine is inhibited above and below pH 5.7. Based on the experimental results, pH 5.7 was selected as an optimum value.
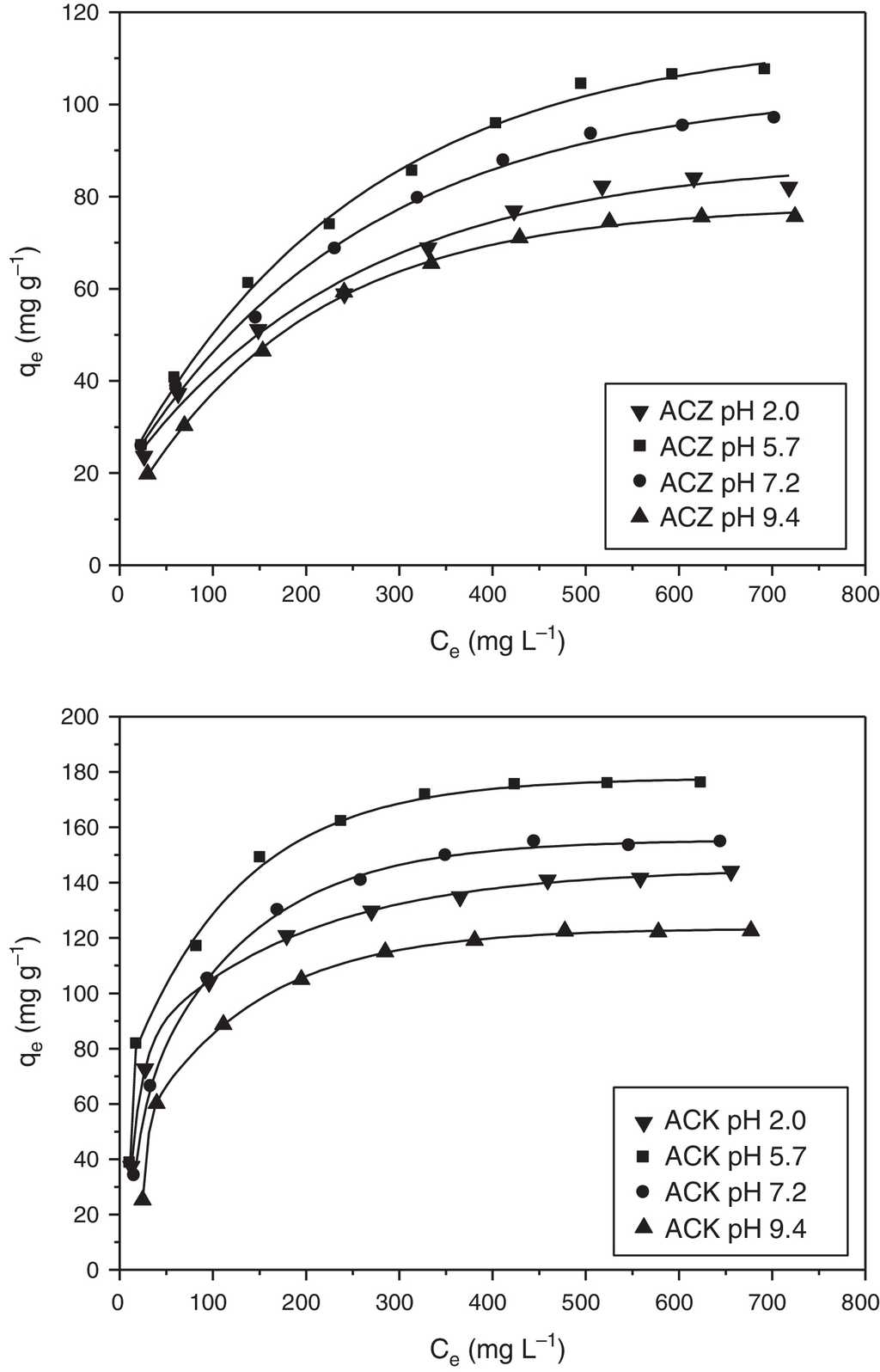
Fig. 7 Effect of pH on l-phenylalanine adsorption onto ACK and ACZ (adsorbent dose=1gL−1, agitation speed=150rpm, contact time=300min, temperature=20°C).
3.2.4. Adsorption kinetic study
Several kinetic models were proposed to understand the behavior of adsorbents and to study the mechanisms controlling the adsorption. In this study, the experimental data of L-phenylalanine adsorption are examined using a pseudo-first and pseudo-second order kinetic model. The pseudo first-order kinetic model is expressed in its linear form by the following equation (He et al., 2015):
While the pseudo second-order kinetic model is based on the assumption of a chemisorption of the adsorbate on the adsorbent (He et al., 2015):
where qt and qe (mg g−1) are the amount of amino acid adsorbed at time t (min) and at equilibrium, respectively, k 1 (min−1) and k2 (g mg−1 min−1) are the rate constant of the pseudo first order and pseudo second order adsorption models.
The best fit was validated on the base of the correlation coefficient (R 2), the difference between the experimental and theoretical adsorption capacities and the normalized standard deviation Δq (%) (Sen Gupta & Bhattacharyya, 2011):
where n is the number of data points, qe,exp and qe, cal are the experimental and calculated equilibrium adsorption capacity values (mg g−1), respectively.
The aim of this kinetic study was to find the appropriate model that better describes the experimental data and that determines the kinetic parameters of the mass transfer of L-phenylalanine. The data were examined by using Eqs. (4)-(6). The calculated kinetic parameters for L-phenylalanine adsorption on activated carbons are gathered in Table 2. The adsorption capacities calculated from the pseudo second-order model are very close to the experimental ones as evidenced from the low values Δq (%) and high correlation coefficient (R2 > 0.99). Although the R2 values for the pseudo first-order model are all above 0.98 (Table 2), the eminent variances (the relative error of L-phenylalanine onto ACK and ACZ are 45.35% and 33.84%, respectively) between the experimental and calculated adsorption capacities reflect the poor fitting of the pseudo first-order model. Hence, L-phenylalanine adsorption kinetics on both ACZ and ACK is well described by the pseudo second-order kinetics.
Table 2 Kinetic parameters for l-phenylalanine adsorption on ACK and ACZ.
| Model | Parameters | Adsorbent | |
|---|---|---|---|
| ACK | ACZ | ||
| q e,exp (mg g−1) | 114.36 | 68.03 | |
| η (%) | 56.89 | 33.85 | |
| Pseudo-first order | q e, cal (mg g−1) | 62.49 | 45.01 |
| k 1 (min−1) | 0.0194 | 0.0205 | |
| R 2 | 0.982 | 0.995 | |
| Δq (%) | 30.63 | 23.00 | |
| Pseudo-second order | q e, cal (mg g−1) | 119.05 | 72.46 |
| k 2 (g mg−1min−1) | 0.0007 | 0.0009 | |
| R 2 | 0.999 | 0.999 | |
| Δq (%) | 2.65 | 4.00 | |
3.2.5. Adsorption isotherms
The adsorption isotherm is generally applied to analyze the experimental data at equilibrium. The isotherms were further investigated by performing batch adsorption experiments over the pH range (2-9.4) at the optimal temperature of 20 °C. The curves were fitted by the most used models namely the Langmuir and Freundlich ones. The Langmuir model is based on the assumption that the maximum adsorption corresponds to a saturated monolayer of adsorbate molecules on homogeneous sites of adsorbent with a constant energy, and no interaction between adsorbed species. The linear form is expressed as follows (Yang, Yu, & Chen, 2015):
where qmax and qe are the equilibrium and maximum adsorption capacities (mg g−1), respectively; KL , the Langmuir constant related to the affinity of the binding sites (L mg−1); and Ce , the equilibrium concentration of adsorbate in an aqueous phases (mg L−1). The values of qmax and KL are calculated from the slopes (1/q max ) and intercept (1/q max KL) of the linear plots of (Ce /qe ) vs. Ce (Fig. 9).
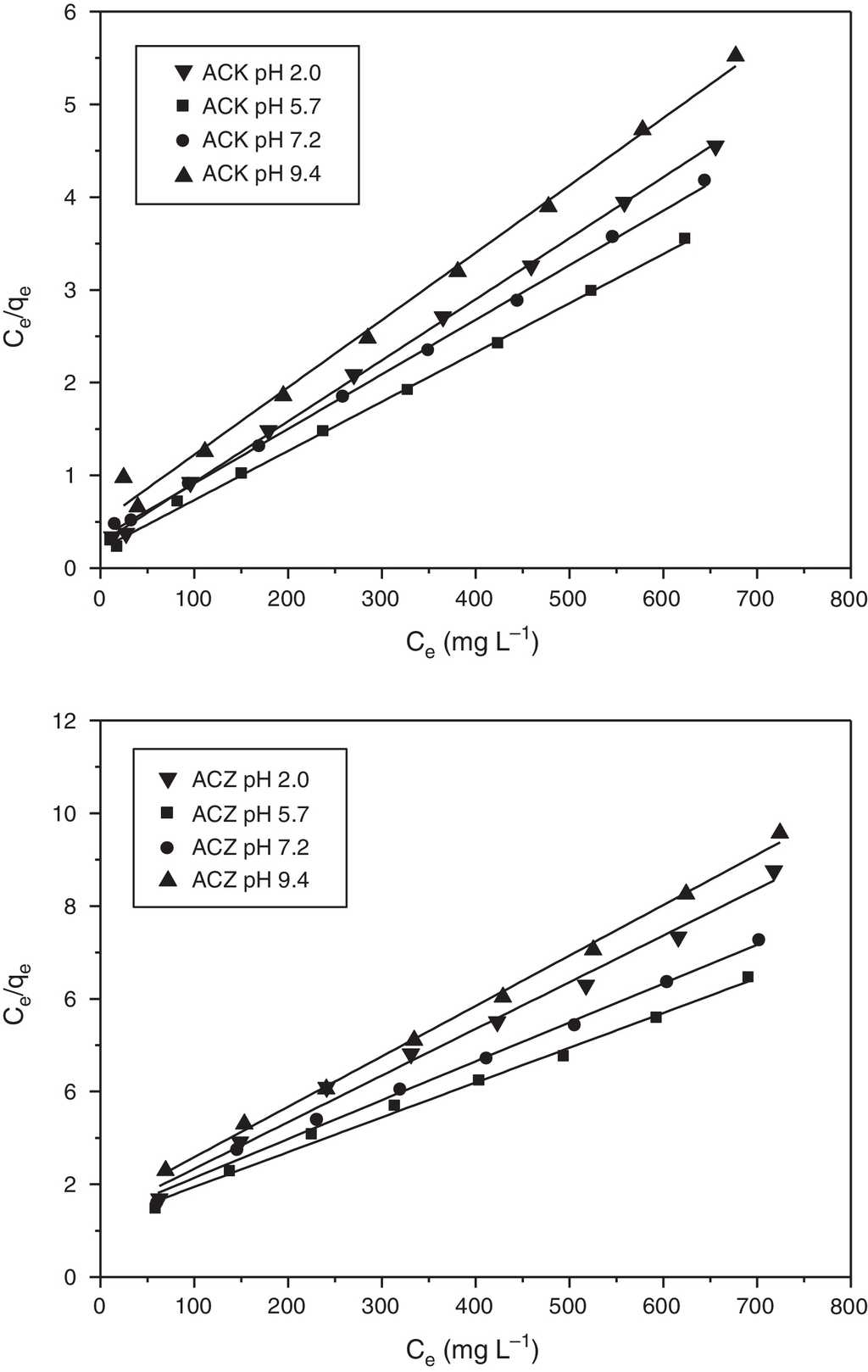
Fig. 9 Langmuir isotherm for adsorption of L-phenylalanine on ACK and ACZ at different values of pH (adsorbent dose=1gL−1, agitation speed=150rpm, contact time=300min, temperature=20°C).
The main characteristics of the Langmuir isotherm can be expressed by a dimensionless separation factor, RL , which is defined by the following formula (Liu, Zheng, Wang, Jiang, & Li, 2010):
The RL value indicates the possibility of the adsorption process being favorable (0 < RL < 1), unfavorable (R1 > 1), linear (RL = 1), or irreversible (RL = 0).
The Freundlich isotherm model is based on heterogeneous adsorbent surface (Yang et al., 2015):
The linear plot of lnqe vs. lnCe (Fig. 10) enables the determination of the Freundlich constants KF and n from the intercept and slope, respectively.
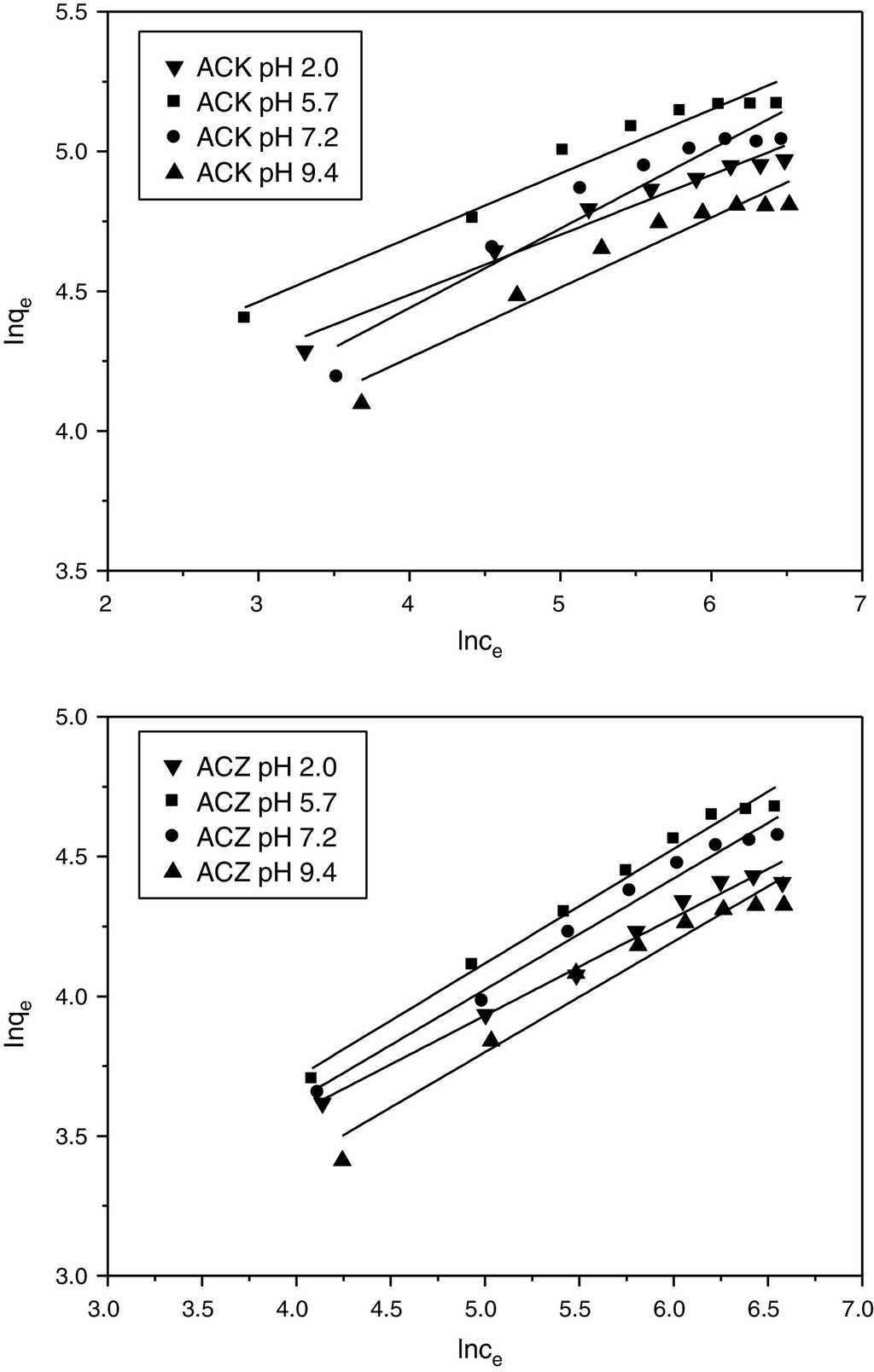
Fig. 10 Freundlich isotherm for adsorption of l-phenylalanine on ACK and ACZ at different values of pH (adsorbent dose=1gL−1, agitation speed=150rpm, contact time=300min, temperature=20°C).
The isotherm parameters of L-phenylalanine are calculated from the Langmuir and Freundlich models (Table 3), all the R 2 values of the Langmuir model are greater than 0.99. These values are much higher than those of the Freundlich model, whatever the pH, suggesting the applicability of the Langmuir model which reveals a monolayer coverage of L-phenylalanine on homogeneous sites for both adsorbents. The RL values are between 0 and 1, indicating that the L-phenylalanine adsorption on ACK and ACZ is favorable under the operating conditions. As the pH increases from 2.0 to 9.4, qmax shows a significant decrease, reflecting that the adsorption is more favorable at pH 5.7, which is close to the isoelectric point (PI = 5.48). ACK exhibits a maximum monolayer adsorption of L-phenylalanine (188.3 mg g−1) compared to ACZ (133.3 mg g−1) due to its largest microporous surface area. As the pore size of ACK is 1.48 nm, most pores including micropores are easily accessible to L-phenylalanine with a size of 0.7 × 0.5 × 0.5 nm3. Consequently, the microporous surface area plays an important role for determining the adsorption capacity of the small biomolecule L-phenylalanine. The maximum adsorption capacities of ACK and ACZ for L-phenylalanine are compared to those values reported in the literature for other adsorbents (Table 4). In comparison with various adsorbents, our activated carbons ACK and ACZ have high adsorption capacities and can be considered as effective adsorbents for the recovery of L-phenylalanine from aqueous solutions.
Table 3 Isotherm parameters for l-phenylalanine adsorption on ACK and ACZ.
| Model | Adsorbent | Parameters | pH | |||
|---|---|---|---|---|---|---|
| 2.0 | 5.7 | 7.2 | 9.4 | |||
| Langmuir | ACK | q e,exp (mg g−1) | 144.07 | 176.01 | 154.68 | 122.66 |
| q max (mg g−1) | 151.97 | 188.32 | 170.06 | 137.74 | ||
| k L (L mg−1) | 0.0246 | 0.0262 | 0.0181 | 0.0146 | ||
| R L | 0.0483 | 0.0455 | 0.0646 | 0.0789 | ||
| R 2 | 0.999 | 0.998 | 0.998 | 0.993 | ||
| ACZ | q e,exp (mg g−1) | 84.01 | 107.45 | 96.94 | 75.65 | |
| q max (mg g−1) | 99.40 | 133.33 | 119.33 | 91.99 | ||
| k L (L mg−1) | 0.0076 | 0.0063 | 0.0065 | 0.0073 | ||
| R L | 0.1412 | 0.1655 | 0.1613 | 0.1462 | ||
| R 2 | 0.988 | 0.993 | 0.993 | 0.997 | ||
| Freundlich | ACK | k F | 37.738 | 43.613 | 27.222 | 26.016 |
| n | 4.667 | 4.364 | 3.520 | 3.986 | ||
| R 2 | 0.963 | 0.952 | 0.911 | 0.921 | ||
| ACZ | k F | 8.874 | 7.927 | 7.667 | 6.191 | |
| n | 2.860 | 2.443 | 2.516 | 2.529 | ||
| R 2 | 0.978 | 0.985 | 0.980 | 0.944 | ||
Table 4 Comparison of the maximum adsorption capacities (Qm) of various adsorbents for l-phenylalanine.
| Adsorbent | Q m (mg g−1) | Reference |
|---|---|---|
| Polymeric adsorbent | 115.6 | (Grzegorczyk and Carta, 1996) |
| Polymeric resins | 65.9-100.8 | (Díez et al., 1998) |
| Carbonated calcium phosphates | 44.0 | (Bihi et al., 2002) |
| NAZSM-5 zeolite | 41.3 | (Titus et al., 2003) |
| Commercial activated carbon | 100.0 | (Garnier et al., 2007) |
| Organic-inorganic hybrid membranes | 1.2 | (Wu et al., 2009) |
| Spherical carbon aerogels | 66.1 | (Long et al., 2009) |
| Macroporous resins | 12.8-84.0 | (Mei et al., 2009) |
| Activated defective coffee beans | 69.5 | (Clark et al., 2012) |
| Calcined CuZnAl-CO3 layered double hydroxides | 46.4 | (Jiao et al., 2012) |
| Activated corn cobs | 109.2 | (Alves et al., 2013a, 2013b) |
| Mesoporous materials CSBA-15, CSBA-16 and CKIT-6 | 0.27-0.30 | (Goscianska et al., 2013b) |
| Mesoporous silica | 36.0-69.0 | (Goscianska et al., 2013a) |
| Mesoporous carbon CMK-3 | 273.0 | (Goscianska et al., 2014) |
| Multi-walled carbon nanotubes CNTs | 233.0 | (Goscianska et al., 2014) |
| Activated carbon ACK | 188.3 | This study |
| Activated carbon ACZ | 133.3 | This study |
3.2.6. Adsorption thermodynamics
A thermodynamic study was performed for the determination of the free energy change (ΔG °), entropy (ΔS °) and enthalpy (ΔH °). A previous study of the temperature effect on L-phenylalanine adsorption over ACZ and ACK enabled us to determine the thermodynamic parameters at 20-40 °C and 800 mg L−1. They were calculated from the following equations (Bouguettoucha, Reffas, Chebli, Mekhalif, & Amrane, 2016; Milonjic, 2007):
where K C is the equilibrium constant (L g−1), T the absolute temperature (K), R the universal gas constant (8.314 J mol−1 K−1) and ρ the density of water (g L−1). ΔH ° and ΔS ° are calculated from the slope and intercept of the plots of ln (ρK C ) vs. 1/T , respectively (Fig. 11 and Table 5). The negative enthalpies (ΔH °) indicate the exothermic nature of the adsorption, in agreement with the temperature effect study (see Section 3.2.2). It has been reported that ΔH ° is in the range (2.1-20.9 kJ mol−1), indicating a physisorption (Liu, 2009). ΔH ° (−6.947 and −7.153 kJ mol−1 for ACK and ACZ, respectively) showed a physisorption of L-phenylalanine, with weak interactions while the negative free enthalpy (ΔG °) indicates the spontaneous nature of L-phenylalanine uptake over the studied temperature range. The variation of ΔG ° for physisorption is in the range (0-20.9 kJ mol−1), whereas this energy ranges from 80 to 200 kJ mol−1 for a chemisorption (Liu, 2009). In our case, ΔG ° (Table 5) is characteristic of a physical adsorption. The entropy (ΔS °) is used to describe the randomness at the solid-solution interface during the recovery process. The positive values of ΔS ° demonstrate an increase in randomness during the adsorption of L-phenylalanine on ACK and ACZ.
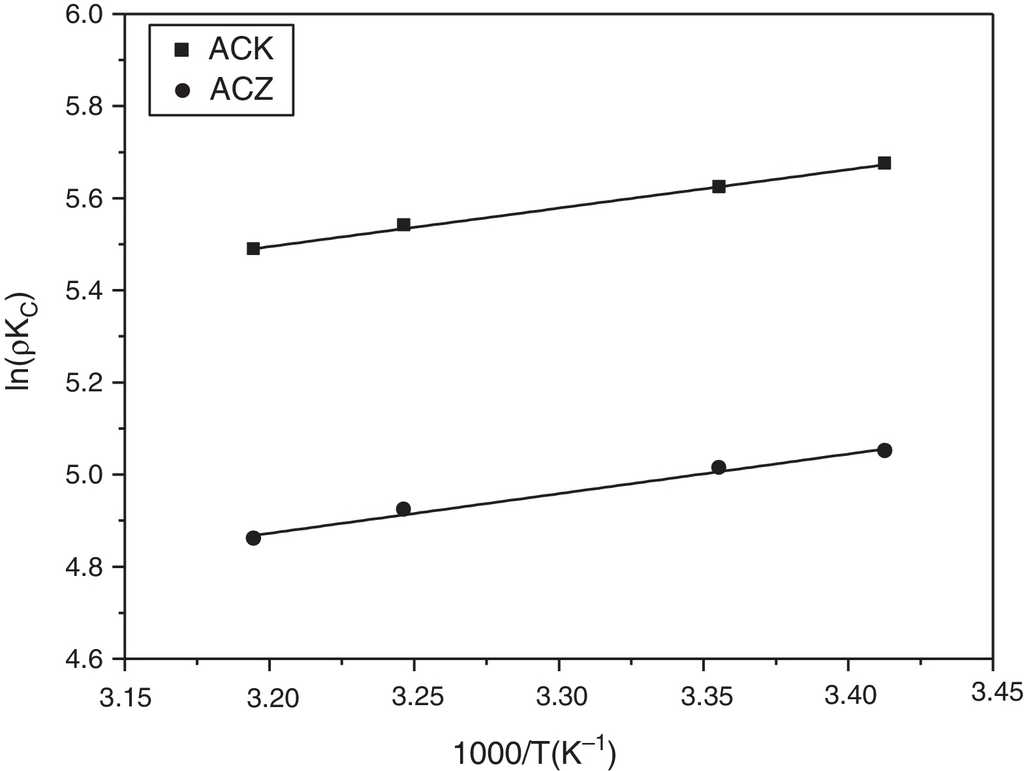
Fig. 11 Regressions of Van't Hoff for thermodynamic parameters of l-phenylalanine adsorption on ACK and ACZ.
3.3. Proposed mechanism of adsorption
To further understand the adsorption behavior and select a desorption approach, the adsorption mechanism of L-phenylalanine amino acid was discussed. The adsorption mechanisms occurred mainly because of the hydrogen bonding formation, hydrophobic and electrostatic interactions of amino acid molecules with the activated carbons surface. The main active sites for binding of L-phenylalanine by the activated carbons are the hydroxyl and carboxyl groups on the surface of ACK and ACZ, which react with polar molecules and various functional groups. The surface of porous activated carbon can include electrically charged groups (ACZ/ACK surface -OH2 +: below pHpzc) and (ACZ/ACK surface -O−: above pHpzc), electrically neutral groups (ACZ/ACK surface -OH: near pHpzc). L-phenylalanine amino acid has dissociation constants (pK1 = 1.83 and pK2 = 9.13) and isoelectric point (PI = 5.48) (Jiao et al., 2012). The molecule is positively charged (+NH3-R-COOH) for pH < PI and negatively charged (NH2-R-COO−) for pH > PI, and behaves in an aqueous medium as a dipolar zwitterion (+NH3-R-COO−) at pH-PI. In acid solution, the presence of H3O+ ions in the surface of ACZ and ACK causes repulsion of protonated amino groups with the surface functional groups, and thus lower the adsorption efficiency. In a basic solution, OH− ions present on the adsorbent surface compete with anionic carboxylic groups for L-phenylalanine molecules (repulsion effect) and inhibit the adsorption. The highest uptake of L-phenylalanine at pH 5.7 indicates a dominant hydrophobic interaction with π-π type between the phenyl rings of amino acid molecules and graphene rings of the activated carbons surface (Doulia, Rigas, & Gimouhopoulos, 2001; Rajesh, Majumder, Mizuseki, & Kawazoe, 2009). Electrostatic attraction between anionic carboxylic groups of L-phenylalanine molecules and OH− ions in the surface of activated carbons also accounts for the increased adsorption. Additionally, such strong bindings between L-phenylalanine and the adsorbent surface can be explained by the formation of hydrogen binding between oxygenated groups at the activated carbons surface and amino groups of L-phenylalanine. According to these results, the adsorption mechanism is proposed in Figure 12.
3.4. Desorption behavior of L-phenylalanine from activated carbons
Regeneration and reuse of adsorbents for further cycles is important from the economic perspective. Desorption of L-phenylalanine from the activated carbons was evaluated using two different eluents: NaOH and HCl (0.01 M). The highest desorption was achieved in the NaOH solution with almost 95.7% for ACZ and 88.8% for CAK against 21 and 6.5% in the HCl solution. This may be due to the enhancement of the number of negatively charged sites at high pH which increases the electrostatic repulsion, which liberates L-phenylalanine from ACK and ACZ. To check the adsorption efficiency, the desorbed ACK and ACZ were dried overnight and subjected to a new adsorption/desorption cycle. During the second cycle, the adsorption capacities obtained were 29.4 (ACK) and 23.5 mg g−1 (ACZ). A significant decay in the adsorption capacity of both activated carbons was observed and may be attributed to the depletion of active sites of the adsorbents being occupied by the amino acid. With the increase of the repeated cycle, the rate of desorption was also greatly decreased (Fig. 13).
4. Conclusions
Porous activated carbons, namely ACK and ACZ, were successfully synthetized using the chemical activation method from agricultural wastes (date stones), which were later characterized using various analytical techniques such as SEM, FT-IR and N2 adsorption-desorption isotherms. The prepared activated carbons showed well-developed textural characteristics, with high BET surface areas, large pore volumes and tight pore size distribution. The pseudo second-order model was more suitable to describe the adsorption kinetics. The results showed that the temperature range (20-40 °C) and pH (2-9.4) exhibited remarkable influences on the adsorption of L-phenylalanine on activated carbons. The adsorption equilibrium data were well described by the Langmuir model, suggesting homogeneous adsorption. The maximum adsorption capacity was obtained at pH 5.7, which can be ascribed to the hydrogen bonding formation, hydrophobic and electrostatic interactions. The thermodynamic analysis indicated that L-phenylalanine adsorption was spontaneous, exothermic in nature and followed a physisorption mechanism. For desorption of the L-phenylalanine from the activated carbons, NaOH and HCl solutions with same concentration of 0.01 M were used. The desorption of the amino acid was better in the NaOH solution. Because of the high adsorption efficiency and desorption possibility, the present activated carbons from date palm seed wastes could be successfully applied as low-cost adsorbents in amino acid purification and separation.











 nueva página del texto (beta)
nueva página del texto (beta)























