1. Introduction
Water quality is one of the most relevant topics in the environmental field. It can range from wastewater treatment processes, restoration of water bodies to conservation of the biota in the area, agricultural and aquaculture applications, as well as for human use and consumption. In this case, one of the aspects that are taken into account in water treatment processes is the content of dissolved oxygen (DO) in water bodies (Blanco-Zúñiga & Rojas-Arias, 2021).
Bearing in mind the above, it is crucial to mention how the lack of DO negatively influences various ecosystems and depends on various factors: physical, biological and climatic, which can reduce the DO content, affecting the biota in the water body (Blanco-Zuñiga et al., 2021; Dien et al., 2019; Kumar et al., 2018). Due to this, several methods have been produced to satisfy the demand for DO in the water body, in addition to allowing the mineralization and elimination of most of the contaminants from the water, among which diffusion systems stand out (Brown et al., 2016; Uby, 2019). This type of system is widely accepted, mainly for its efficiency in large water bodies, where a lower DO content in the water body translates into a higher energy consumption required to achieve the degree of saturation (Blanco-Zuñiga et al., 2021).
The physicochemical composition of the geothermal waters results from the environment from which it is extracted; and these have a wide range in composition, from strongly acidic waters to the more usual neutral-pH or slightly alkaline-pH waters (Petar, 1991). The dissolved oxygen presence in all types of water depends on many factors, such as temperature, atmospheric pressure, and salinity (Tchobanoglous et al., 2003). Because of the high temperature of geothermal water, DO is present in low concentrations in most geothermal waters; however, in low- temperature geothermal systems, waters usually contain DO in concentrations up to a few mg/L (Petar, 1991). The water oxygen loss process can be observed in boiling water. During the boiling process bubbles of water vapor depleted in oxygen, are produced and it is with these that gas exchange takes place. DO is entrained in the bubbles and then liberated to the atmosphere at the liquid surface (Butler et al., 1994). As low- oxygen geothermal water comes in contact with air at the surface level, an oxygen transfer process begins due to contact, turbulence and the high difference between oxygen partial pressures (atmosphere-water) (Abdelrahman & Boyd, 2018; Sander, 2015). The presence of DO in geothermal water is a cause of concern. Atmospheric gases can dissolve in the geothermal water if it is exposed to the atmosphere. As these oxygenated waters are in contact with any metal, a corrosion process takes place (Janik, 1985), and drastically affects the surface of most alloys (Kindle et al., 1984). This situation can be enhanced by pH, water temperature, and the presence of CO2, H2S, and chloride ion (Petar, 1991).
The geothermal waters can represent a serious problem when they are discharged into rivers. The main effect of thermal water discharge is the disturbance of surface water ecosystems. Freshwater pollution can, therefore, result in the poisoning, disease and even death of fish (Zeitoun & Mehana, 2014). The discharge of a large quantity of geothermal water would change the temperature of the local water bodies (Wang & Yu, 2013) as well as their physicochemical properties, heavy metal concentrations and, therefore, water quality (Baysal & Gunduz, 2016; Hills & Viskanta, 1976; Lan et al., 2020). The increase of water temperature in water bodies causes a decrease on the DO concentration and an increase of the biological oxygen demand (BOD) (Lin et al., 1973; Kowalski & Mazierski, 2008). To minimize the effect of geothermal water discharge on the quality of river water, some methods of water treatment can be used, including nanofiltration or reverse osmosis processes (Rajca et al., 2017; Tomaszewska et al., 2016; Tomaszewska & Dendys, 2018).
Furthermore, thermal water bodies present a microbiota that can also be affected by DO content in the water body. The DO content varies from the type and location of the water source to the reservoirs and pools where it is normally used in recreational activities. Nevertheless, one of the main characteristics of thermal water bodies is the presence of radioactivity, as well as the emanation of radon gas, which is considered a carcinogenic element (Thu et al., 2019; WHO, 2009), being the second cause of lung cancer after tobacco with a percentage between 3 - 14% of deaths due to this gas (Matos, 1997; Rojas-Arias et al., 2020).
The presence of radon in water is mainly due to the decay of radioactive elements such as uranium and radium, whose concentrations vary depending on the Genesis of the area (Lagos et al., 2019; Rojas-Arias et al., 2020; Thu et al., 2019; UNSCEAR, 2008). Few studies have focused on studying radon gas in water bodies (Baudron et al., 2015; Lagos et al., 2019; Moreno et al., 2014, 2018; Ródenas et al., 2008; Soto et al., 1995). The geology and geological events in an area contribute to the concentration of radon gas in drinking water (Moreno et al., 2014; Talwani et al., 1980). The negative effects that exposure to this gas can generate depends largely on specific environmental factors to the material that emits this gas (Vogiannis & Nikolopoulos, 2015). For that reason, some nations have established a range of permissible radon gas level in water for human consumption, e.g. in European countries these values are established between 0.1-1 kBq·L-1 (European Parliament, 2013), while for Spain, the maximum value is 0.5 kBq·L-1 due to internal laws (Blasco Hedo, 2016).
In the case of Colombia, the Boyacá region has a vast hydrothermal source, which is mainly used in tourist and recreational activities. (Lagos et al., 2019) have studied the radon content that several of these hot springs may present in the region, where it is observed that these bodies of water present a high concentration of gas, mainly from the Paipa- Boyacá area, with values that exceed 100 kBq·m-3 in water, standing above the permissible limit for drinking water set by the European Union (100 kBq·m-3) (Binesh et al., 2010; Europe-Union, 2001). Although there is no limit or restriction on the concentration of radon in hot springs, it can cause problems due to ingestion through the skin, as reported (Sakoda et al., 2016). Considering the above, the objective of this study is focused on analyzing the effect of the application of aeration processes in thermal water bodies on the transfer of DO and the removal of radon gas present in the water. In that way, water samples from Paipa-Boyacá area, were selected and treated by means of diffusion aeration and radon gas measurement through passive detection systems.
2. Experimental
Samples of hot springs were collected from the Paipa-Boyacá area, Colombia, taking as a collection point the source called "Upwelling designed as Pozo Huevos" reported by Lagos et al. (2019) due to this high radon content. The water samples were deposited in hermetically sealed 0.5L and 4L glass containers. In this work, 6 water samples were taken for each measurement test (i.e. 6 water samples of 0.5L and 6 water samples of 4L). The initial radon concentration was obtained from the Pylon AB-6 active detection system in samples of 0.5L (Pylon, 2017). The initial DO content was obtained from a Hach-flexi HQ30d oximeter, Measurements were made at a height of 2800 m.a.s.l., pressure of 740hPa and temperature of 17°C (Blanco-Zuñiga et al., 2021). The initial DO concentration for the collected samples was 3.21 g‧L-1.
The oxygen transfer process was carried out by means of a bubbling device interconnected to the system, applying an air volume of 2.5 L·min-1 in the 4L samples. Three measurements were carried out simultaneously, using a bubble aeration system for every two water samples, for a total of 6 water samples. The application of a bubble aeration system allows an optimal removal of radon gas in the water (Jalili-Majareshin et al., 2012), allowing a greater contact relationship between radon gas and the air that is being injected into the body of water (Battino, 1984). Measurements of DO concentration were performed in 10 minutes intervals, inserting the oximeter at a depth of 10cm. The calculations performed for the determination of DO were based on previous studies (Blanco-Zuñiga et al., 2021; Blanco-Zúñiga & Rojas-Arias, 2021). The radon gas measurement in the tests carried out was carried out using passive detectors LR-115 arranged in adjacent chambers interconnected to the water container as shown in Figure 1. Each of the experiments was carried out until reaching the degree of DO saturation in the water sample. The data obtained were averaged. The 0.5Lsampleswere processed for radon gas measurement. A small volume is used as the calibration of the Pylon system is directed at this volume. Measurements of DO transfer in water show better performance in larger volume water samples. The extrapolation of the rate of gas supplied in both types of samples was taken into account.
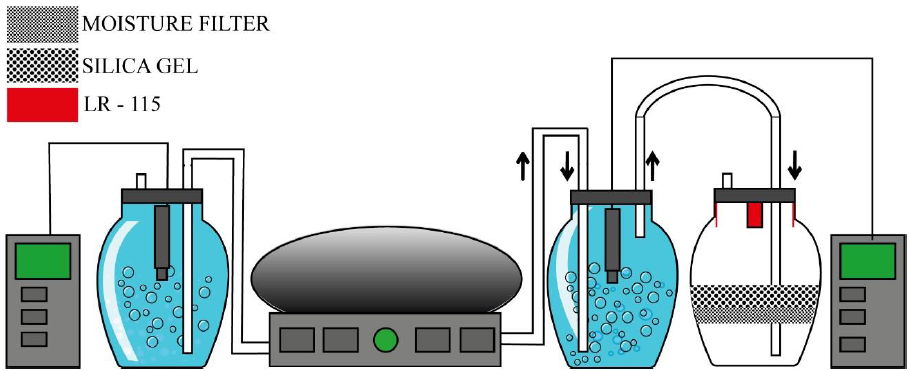
Figure 1 Bubble aeration system used in this work. The system was equipped by two degassing chambers with built-in oximeters and a diffusion chamber (right). Four LR-115 (red) detectors for radon measurement are arranged in the upper part of the degassing chamber, protected by two layers of silica-gel and a humidity filter.
The LR-115 detectors were subjected to an etching process in Na(OH) 2.5N solution and cleaning in distilled water for 10 minutes (Rojas-Arias et al., 2020). The reading of the traces found in each of the detectors was carried out by optical microscopy at 10X (Leica-Instruments, 2019). Trace density was performed using ImageJ software (ImageJ, 2020). Radon concentration was calculated as reported in (Faisca et al., 1992; Rojas-Arias et al., 2020).
3. Results and discussion
The effect of DO transfer on radon gas removal was studied in thermal water samples from the central region of Colombia. The tests were carried out by applying passive detectors LR- 115 and a diffusion system. The collected samples have a pH of 7.8, that is way this can be classified as an alkaline water source (Komatina, 2004). Its conductivity measured in situ was 2350 S·cm-1. The data obtained in this study are presented in the form of graphs, allowing the behavior during DO transfer to be observed more easily, which are presented in Figure 2. The variation in the percentage of DO, until reaching the degree of saturation is presented in Figure 2(a), while the transfer percentage for each of the time intervals is presented in Figure 2(b).
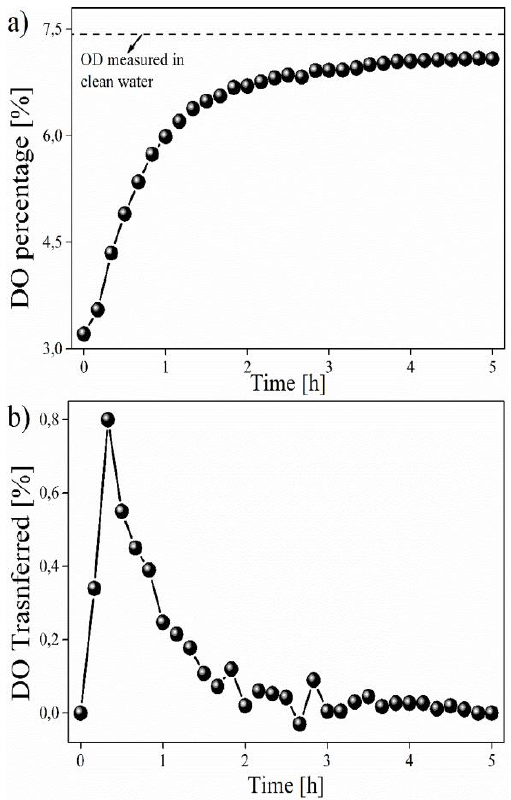
Figure 2 Behavior on the DO transference in thermal water obtained in this work. In a) the variation in DO concentration (in %), and b) the DO percentage transferred at different measurement intervals.
A higher DO transfer is observed in the water samples during the first 30 minutes after starting the experiment, reaching a maximum transfer peak of 0.8 g·L-1 (Figure 1(b)). From this point on, a decline in DO supply in the body of water begins to be observed until reaching the saturation point (Figure 1(a)). This reduction in the DO transfer rate is explained due to an Arrhenius-type behavior where, through the DO diffusion and transfer process, a greater contribution is observed for bodies of water that present a greater difference in concentration in relation to the DO saturation point (7.18 in this study). As concentrations close to this saturation value are obtained, the contribution of DO to the water body is drastically reduced until reaching a point of equilibrium due to the difference in pressures between DO and atmospheric oxygen (Abdelrahman & Boyd, 2018; Bahadori & Vuthaluru, 2010; Sander, 2015). This reduction in the DO transfer rate is translated into an increase in the energy consumption required to provide DO to the system (Bahadori & Vuthaluru, 2010). Previous studies have shown that oxygen-deficient water samples, i.e. in a state of anoxia or hypoxia, allow a higher rate of DO transfer in the first minutes of the process, compared to what was reported in this study (Blanco-Zúñiga & Rojas-Arias, 2021).
The KLa coefficient value obtained in this study was 1.46 ± 0.03 a.u. finding a N value of 0.37 ± 0.05 kgO2‧kW-1‧h-1 for each of the samples treated in this study. It is observed a lower efficiency than other studies and within the range obtained for this type of systems worked in the field (Blanco-Zuñiga et al., 2021; Tchobanoglous et al., 2003). This is mainly due to the reduced volume worked in this study, in addition to a representative initial DO content in the water samples, as mentioned above, which further hinders the efficiency of the process. Within the study, the influence and interaction that can be caused between the ions from the dissolved salts in the water samples and the DO injected through a diffusion system was not taken into account, because no formation of precipitates was observed in the worked samples.
Various devices have been built and patented for the removal of radon gas in underground water bodies (Espinal, 2000; Lamarre, 1989; Lee et al., 2010). These devices work by generating disturbances in bodies of water in contact with the ambient air, presenting an optimal removal of radon gas. In that sense, the hypothesis of radon gas removal from a diffuse aeration system used for DO transfer is favored.
The results obtained in this study allowed to observe the degree of radon gas removal in the water samples, which is transferred through the air bubbles to the detector, i.e. like the Pylon detection system, measurements were taken in the gas and not directly on the liquid sample. The data obtained in Table 1 shows the data on radon gas concentration before and after the aeration process of the water samples. The removal of radon gas presented in the water favors a lower exposure and intake of this gas through the skin in people who are exposed to contact with these waters in recreational activities such as swimming pools (Sakoda et al., 2016).
Table 1 Radon gas values obtained with Pylon system and LR-115 detector.
| System | Rn concentration | Ref. |
|---|---|---|
| LR-115 | 1.50 kBq·m-3 | This work |
| Pylon | 2.45 kBq·m-3 | This work |
| Pylon | 0-88 kBq·m-3 | (Lagos et al., 2019) |
Indeed, there is a removal of radon gas that is transferred to the environment through the bubbles produced by the injection of air by the diffuser system. The results obtained show an average initial concentration of 2.45 kBq·m-3, this being the reference value to determine the degree of removal by the aeration process. These types of hydrothermal vents are typical of the Colombian Andean region, which is also subject to areas with high risk due to the presence of seismic events (Jaramillo et al., 2017; Moreno-Sánchez et al., 2016). The concentrations of the six samples analyzed by the Pylon active detection system present close values, thus ensuring that all the 0.5L and 4L samples present the same initial concentration. This is because all the samples were collected under the same conditions, on the same day during the same time, reducing the variation in radon concentration between the water samples as reported by Jalili-Majareshin et al. (2012).
The radon gas concentration values obtained in the oxygenated samples show an average removal value of 1.5 kBq·m-3 measured in air with an efficiency of 63%. The data gathered present a standard variation of 12% due to the application of passive detection systems presents a greater efficiency for the analysis of samples with a high concentration or in prolonged measurement periods, as shown (Rojas-Arias et al., 2020). Temperature, atmospheric conditions and measurement station have an effect on radon concentration, which explains the variation in the data obtained in this work and those presented by (Lagos et al., 2019). However, the application of this type of detectors favors the implementation of mass measurement processes, allowing monitoring of large bodies of water, located at different measurement points. Simultaneously, it reduces the probability of measurement errors due to environmental effects such as temperature, environment, relative humidity, measurement period, so on.
The interaction of water with air injected into the system in the form of bubbles is lower compared to the application of mechanical aeration processes (Blanco-Zuñiga et al., 2021). The application of this type of system can also favor the removal of radon during the oxygenation process of water bodies (Rojas Romero, 2010). The authors reflect their interest in studying the influence that the application of mechanical aeration mechanisms can generate in subsequent studies.
4. Conclusions
In this work, the effect of the application of diffuse aeration processes on the removal of radon gas in thermal water samples was studied. Although, the concentration of dissolved oxygen in the worked samples is minimal, they are not low enough to samples of anoxic and hypoxic waters. This hinders the transfer and contribution of DO to the water samples, reducing the efficiency of the process. However, values observed are still within the range reported in the literature. In turn, the application of a diffuse aeration system allows optimal removal of radon gas present in water samples. The data obtained show a radon removal of around 63% around the initial concentration of the samples. The application of passive detectors allows the mass measurement of radon gas and its removal in several water samples simultaneously, ignoring measurement errors that can be generated by physical variations in the environment, such as changes in climate, temperature and seasons.











 nueva página del texto (beta)
nueva página del texto (beta)


