1. Introduction
Current established cancer treatments that exist, such as chemotherapy and radiotherapy, still have considerable side effects. The mitigation of these effects demands new adjuvant therapies that mitigate the consequences of traditional therapies. Differences between healthy and tumor cell behavior under temperature have been the basis for the treatment of tumors by hyperthermia; generally, healthy cells show better resistance to heat than the tumor ones (Wust et al., 2002). Because of this, it is imperative to engineer new compounds and composite materials to control unique thermal, chemical, and electric properties. One of the most promising directions of magnetic particles investigations is the opportunity to use them in engineering, medicine, and biology, particularly for the creation of new magnetic recording systems, biological fluid purification, drug and gene delivery, and hyperthermia (Bubnovskaya et al., 2014; Kalita et al., 2015; Laurent et al., 2008; Vatta et al., 2006). Magnetic hyperthermia is a medical treatment that consists in injecting magnetic particles into cancerous tissue which are in turn heated under the influence of external alternating magnetic fields (Mehdaoui et al., 2010). Also, they have to demonstrate high heating efficiency under an alternating magnetic field to be able to heat the cancerous area with temperatures ranging from 42°C to 45 °C (optimal for destroying the tumors) (Solopan et al., 2011).
Magnetite particles (Fe3O4) with spinel structure have already found some practical application in medicine (Thiesen & Jordan, 2008). However, Mössbauer effect investigations show that Fe3O4nanoparticles are non-stable: Fe2+partially oxidizes to Fe3+, and this leads to the creation of maghemite (γ-Fe2O3) phase. Also, it has the characteristic that the transition temperature from magnetically ordered to paramagnetic state (Curie temperature) is relatively high:TC = 585 °C (Nikiforov et al., 2013). Since magnetic-field-induced heating is only operative in a magnetically ordered state, high Curie temperature may give rise to uncontrolled and non-uniform heating of tumors to high temperatures, which, in turn, may lead to destroying healthy tissues. These problems can be solved using new materials with tunable Curie temperature by different chemical concentrations on atoms that force magnetic effects (one of them could be Sr as reorder magnetic domains) in the required range of temperature (Shlapa et al., 2016).
Recently, manganese oxides perovskites with general chemical formula R1-xAxMnO3 (where R3+= La and A2+= Ca, Sr) have been considered as promising materials capable of meeting these requirements. Curie temperature depends on the chemical composition and can be adjusted by partial La substitution by Sr and Ca according to needs. Lanthanum manganite doping has been studied as La0.7Sr0.3MnO3 (LSMO) nanoparticles (NPs) stoichiometric in various biomedical applications such as magnetic fluid hyperthermia (MFH), a contrast agent for magnetic resonance, and drug release due to their remarkable thermal and magnetic properties (Epherre et al., 2011; Kačenka et al., 2011; Louguet et al., 2012). The interest in LSMO nanoparticles is mainly due to tuned Curie temperature (by Sr2+ doping) between 283 K and 380 K and a large magnetic moment at room temperature. The gradual replacement of Sr by Ca in La0.75(Ca0.25-xSrx)MnO3 decreases in Tc from ~340 K for x=0.25 to ~225 K for x=0 (Guo et al., 1998). These nanoparticles have been synthesized by co-precipitation, solid state reaction, microemulsion, thermal decomposition, sol-gel, and hydrothermal synthesis (Jadhav et al., 2013; Thorat et al., 2012).
In the present work, we used the co-precipitation method to synthesize calcium lanthanum manganite with strontium as a dopant. From the study of the structure and magnetic properties of La0.7Ca0.3-xSrxMnO3, the influence of cation concentration (Sr, Ca) on the Curie temperature was investigated. It claims tunable control with chemical composition. This study can be useful for applying lanthanum manganite particles doped with strontium and calcium in hyperthermia treatments.
2. Materials and methods
Strontium doped calcium lanthanum manganite particles were synthesized by the co-precipitation method. Appropriate amounts of La2O3, SrCO3, CaCO3, and MnO2 were weighted to reach desired stoichiometry, and each compound was dissolved separately in 25 ml of a hydrochloric acid aqueous solution for about 30 min. In all precursor solutions, were used a molar relationship of 6:1 between salt-acid or oxide-acid. After that, all solutions were mixed, and the mixture was maintained under magnetic stirring for 2 h at room temperature; after this time, the aqueous solution was evaporated at 70 °C. Finally, the manganese particles were dried and calcinated in air at 900 °C for 24 h. All samples of La0.7Ca0.3-xSrxMnO3 with nominal composition between 0≤x≤0.2 were obtained by changing the precursor amount stoichiometrically.
Crystallographic phase indexing was obtained by using a PANalytical X´pert PRO powder X-ray diffractometer (Cu Kα radiation, λ=1.5406 Å) at 35 kV and 25 mA. The magnetic measurements were carried out employing a vibrating sample magnetometer (VSM) Versalab in the temperature range from 50-350 K. Magnetization curves were analyzed to obtain the Curie temperature, Tc, for different compositions. Particle size and morphology were determined by scanning electron microscopy (SEM) using a HITACHI SU 5000 at 20 kV.
3. Results and discussion
3.1. Structural properties
XRD patterns for La0.7Ca0.3-xSrxMnO3 powders with 0≤x≤0.2 compositions are shown in Figure 1. According to X-ray patterns, all compositions annealed at 900 °C do not present secondary phases. X’pert High Score Plus software was used to analyze the XRD patterns. For sample La0.7Ca0.3MnO3 the diffraction peaks were compared with standard pattern PDF 01-089-8084 and indexed on the basis of an orthorhombic phase with spatial group Pnma.
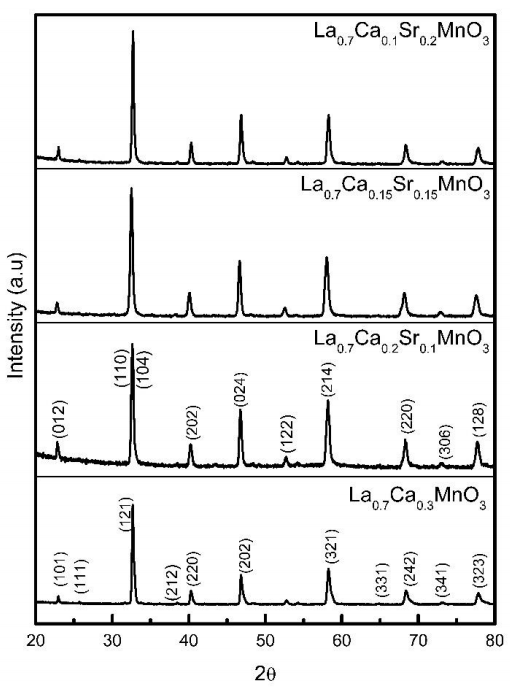
Figure 1 XRD patterns for La0.7Ca0.3-xSrxMnO3 samples. The patterns were indexed according to orthorhombic structure for La0.7Ca0.3MnO3 and rhombohedral structure for La0.7Ca0.3-xSrxMnO3, respectively.
La0.7Ca0.3-xSrxMnO3 samples with x=0.1, 0.15 and 0.2 doped with strontium also present a single phase. The diffraction peaks were indexed to the standard pattern PDF 01-089-5612, corresponding to rhombohedral phase with spatial group R-3c. All indexed peaks correspond accurately to the crystallographic file used.
3.2. Microstructure analysis
Figure 2 shows the morphology and particle size of the samples observed with a scanning electron microscope (SEM). It is interesting to note that all samples calcinated at 900 °C for 24 h show cube-shaped particles, which could be associated with the preparation method since wet chemical methods favor this geometry (Spooren et al., 2005). This morphology is similar to that reported by Spooren et al. 2005, which was obtained by hydrothermal synthesis in La0.5M0.5MnO3 (M=Ca, Sr, Ba) compounds. We observed a similar morphology in strontium doped calcium lanthanum manganites.
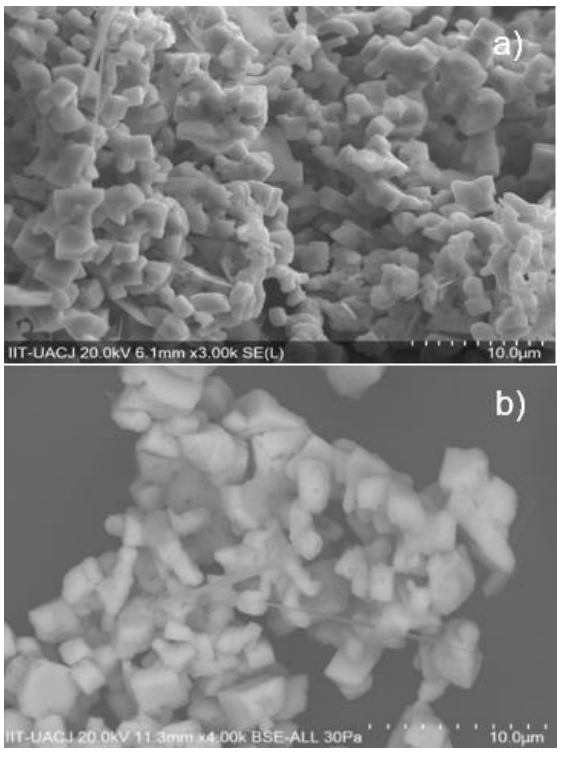
Figure 2 Micrographs of La0.7Ca0.3-xSrxMnO3 particles. a) La0.7Ca0.1Sr0.2MnO3, b) La0.7Ca0.2Sr0.1MnO3, c) La0.7Ca0.3MnO3.
A decrement in particle size with increasing Sr content was observed. It seems that incorporation of Sr into the Ca sites enhances particle size reduction, since the particle size decreases from 3 µm for x=0 to 1.5 µm for x=0.2. Particle size decrement helps for chemical and physical interaction during oncoming particles to cancerous area on hyperthermia applications.
3.3. Magnetic properties
Figure 3 shows the temperature magnetization dependence in Field Cooling (FC) curves for strontium-calcium doped lanthanum manganites samples with different Ca-Sr ratio. These measurements were used to obtain the Tc from magnetization and its temperature derivative (dM/dT). This relation suggests a dependence with the stoichiometric values. It can be observed in Figure 3 according to slope changes. The FC curves show a slight reduction in magnetization as Sr content increase, this behavior is opposite to La0.7Sr0.3MnO3 compounds with calcium substitution obtained by Salili et al. (2015) whose magnetization is higher than in the case of undoped lanthanum manganite. However, we observe a slightly smaller magnetization value in x=0.10 (blue line) with respect to x=0.15 (pink line). The above is due to different amounts used in magnetic measurements.
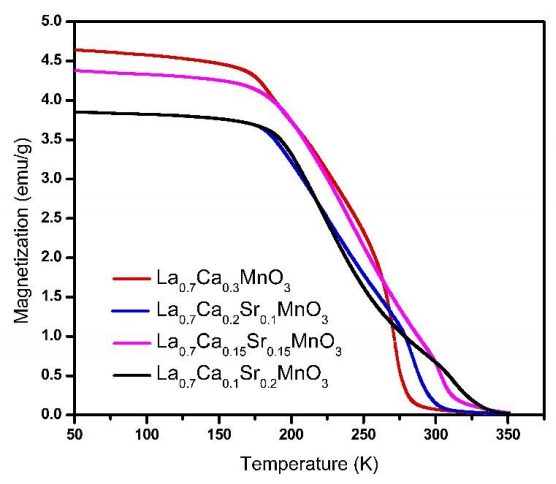
Figure 3 Temperature versus magnetization dependence for samples from x=0 to x=0.2 as composition in La0.7Ca0.3-xSrxMnO3 (100 Oe field applied).
Figure 4 shows Tc as a function of Sr content. As we can observe, Tc raises from 272 K for x=0 to 315 K for x=0.20. These results favor Sr as a good candidate for self-controlled (tunable) hyperthermia applications.
The rising of Tc is associated with the increment of Sr2+ content since a change in ionic radius takes place. As Sr decrease, the average ionic radius site for the A-site ˂rA˃ diminishes, therefore the Mn-O bond distance and Mn-O-Mn bond angle of the perovskite structure. This change causes a weakening in double magnetic exchange interaction between ions Mn3+ and Mn4+, formed by Ca and Sr doping, bringing along an increase in Tc (Venkatesh et al., 2012).
4. Conclusions
In summary, we obtained La0.7Ca0.3-xSrxMnO3 series compounds from x=0 to x=0.2 composition by co-precipitation method to study the effect of Ca:Sr ratio on Curie temperature. This study has demonstrated that Sr plays an essential role in controlling Tc, which can be tuned by varying the cation ratio as evidenced by magnetic measurements. The rhombohedral structure was obtained when strontium amount increase. This structure suggests better magnetic properties and it is evidence that strontium enter to La0.7Ca0.3-xSrxMnO3 particles. The SEM microstructure analysis was favored with increasing strontium since we observed a reduction in particle size, representing one more advantage in hyperthermia applications.











 nueva página del texto (beta)
nueva página del texto (beta)



