1. Introduction
Over the past decades, it has been observed that there has been a growth in interest in microwave sensors operating in the near field regime. These sensors are based on a printed circuit technology, are very compact, extremely cheap and possess higher sensitivity compared to volumetric counterparts. The passive elements usually are planar microwave resonators with a high-quality factor. Conventional electronics measures a shift in their resonant curves caused by a complex permittivity of a sample under test.
Since the devices are very universal, they are used for the characterization of a wide spectrum of materials, such as solids, liquids, biological samples, vapors (Alahnomi et al., 2021). The most complete data processing method for this technology is known as dielectric (or impedance) spectroscopy that measures the dielectric properties of a medium as a function of an external field frequency (Kremer & Schönhals, 2003).
Less attention was paid, however, to active microwave sensors, in particular to the oscillators where a resonant element is a part of a positive feedback loop. In this case a microwave resonator modulates the parameters of an auto-oscillation
frequency, when interacting with a sample. This technique is a microwave analogue of the intracavity laser spectroscopy (Baev et al., 1999), including the mixed laser-microwave double resonance spectrometry (Jones, 1977), and is implemented in the millimeter-wave range on the basis of an Ortotron oscillator (Rusin & Bogomolov, 1969).
2. Steel production
The diagram of the delay line ring oscillator is shown in Figure 1 The principal element of the magnonic oscillator was a 5 mm x 2 mm rectangular ferromagnetic sample, composed of a 7.3 μm thick yttrium iron garnet (YIG) film grown on a 0.5 mm thick gadolinium gallium garnet (GGG) substrate. The YIG/GGG element was saturated in a bias magnetic field (
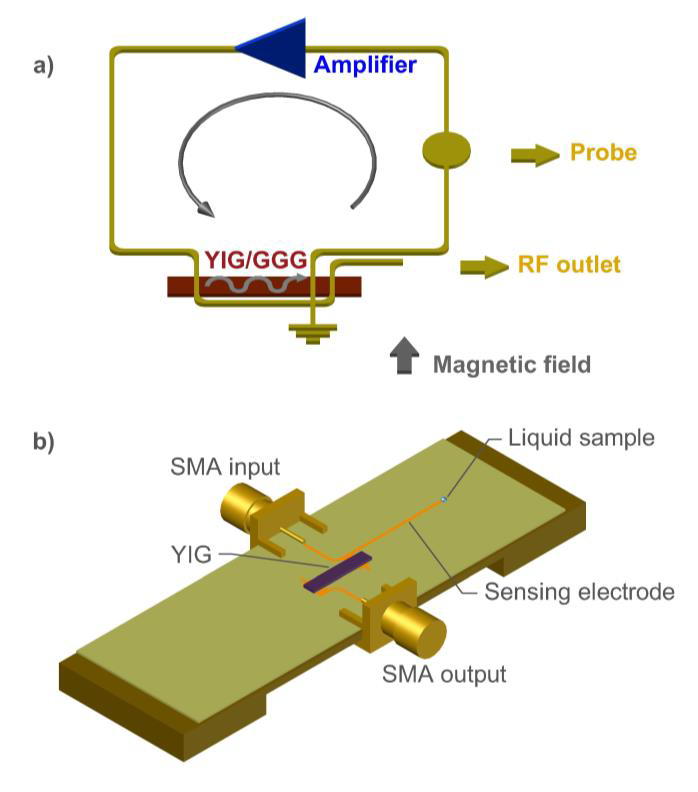
Figure 1 a) A schematic of the microwave ring oscillator based on MSSW delay line; b) implementation of the microstrip line structure with the YIG/GGG delay line on a ceramic substrate (ε = 9, Rodgers Corp.).
The printed circuit contains two transducers with periodic electrode structure, microstrip line stub (sensing electrode), as well as input and output ports. The geometry in Figure 1 provides excitation and propagation of magnetostatic surface wave (MSSW) in YIG film along the X direction (Fig. 1) (Prabhakar & Stancil, 2009).
The shorted periodic electrodes are shown in Figure 2. This structure is used for efficient excitation and detection of MSSWs. The system operates as a delay line whose insertion loss was approximately
The operational principle of the system is based on a shift in the oscillator frequency due to the phase perturbations in the sensing electrode caused by a liquid sample (Figure 1b) that induces changes in the complex relative permittivity
3. Experimental methodology and formation of droplets
The basic aim of this work was the evaluation of capability of the system to discriminate liquids with different percentage of salt concentrations and organic inclusions from their nano-liter volumes. If the water contains salt, the real part of permittivity is slightly reduced, but the imaginary part is increased significantly making the water even more dominating.
It should be noted that in most of the works the aqueous solutions have been characterized by the dielectric spectroscopy (a wide range frequency sweeping) of large volumes of the samples. In this case, there are no technical problems to substitute one sample by another one. However, in the case of nano-volume sessile droplets it is difficult technically to provide a calibrated volume and exact position of the droplet each time when a liquid sample is replaced by another. After experimental analysis of several deposition and detection techniques, including microfluidic method as well as deposition of a droplet matrix, we found that more effective method is a cycle of periodic deposition - evaporation of a droplets formed at the sensing electrode because of the accumulative effect and strong dependence of a droplet evaporation time on the concentration of salts and inclusions.
The deposition of nano-litter sessile droplets on the sensing resonator was a completely automatized process, controlled by LabView data acquisition platform. We used a Hamilton 7000 Series 0.5 microliter syringe. The syringe was fixed in Thorlabs optical translation stages integrated with linear actuators controlled by a PC. The droplets were formed at the opened end of the sensing resonator where the electric field is maximum, as shown in Figure 3. The droplets formation system was designed to deposit a series of droplets of constant volume of 30 nano-liters, at a specific time interval between them, determined by an evaporation time of a single droplet. Figure 4 illustrates the steps of the droplet deposition.
4. Experimental results
4.1. Water salinity
Figure 5 shows typical waveforms at the periodic deposition - evaporation of H
2
O+NaCl sessile droplets with different concentrations of NaCl. The aqueous solutions for this experiment were prepared from deionized water (di-H
2
O). The diagrams show the oscillator down frequency shift vs the droplets evaporation time. The oscillator frequency was measured by a frequency counter integrated into LabView® platform. As seen in Figure 5, the droplet deposition causes 300 KHz frequency down shift (
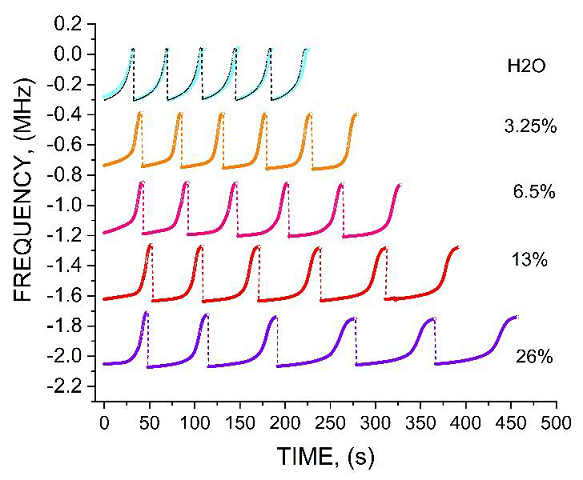
Figure 5 The droplet formation steps: down displacement of the syringe from position 1 to position 2; formation of the sessile droplet 3; up displacement of the syringe from the position 3 to the position 4. Steps 1 - 4 are repeated after the droplet.
The details of evaporation dynamics of the droplet are presented in Figure 6 that shows five specific parameters, which characterize the evaporation process. First, it is the droplet evaporation time W (as seen in Figure 5 W depends on M), then, maximal frequency shift FS, and three stages of the evaporation response with the specific slopes
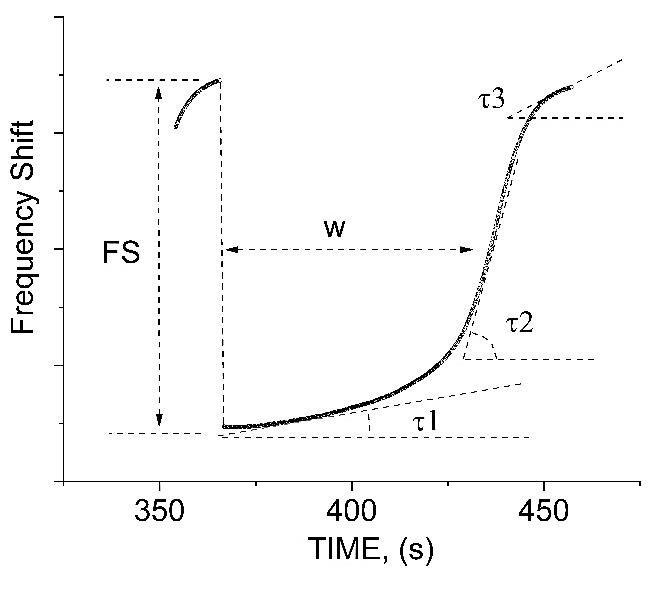
Figure 6 The last period of the droplet evaporation for the case shown in Fig. 5 for 26% concentration. The curve shows the evaporation time W, maximal frequency shift FS, as well as three stages of the evaporation response with the specific slopes τ1, τ2, and τ3.
Figure 7 shows the evaporation time of the droplets with different NaCl concentrations, accumulated over 6 evaporation-deposition periods. The data was extracted from the experiment like in Figure 5. Figure 8 shows that 15 evaporation periods allow one to discriminate the accumulated difference between the di-water and the water at the salinity of S = 0.5 psu (1psu ≈ 1ppt, parts per thousand, or 0.05% of NaCl concentration).
4.2. Total dissolved and suspended solids
For characterization of purity of water also there are two parameters of interest: total dissolved and total suspended solids. Total dissolved solids (TDS) is an aggregate measure of charged and uncharged inorganic and organic compounds completely dissolved in water, including both volatile and non-volatile. Volatile solids are ones that can easily go from a solid to a liquid state. Examples of non-volatile substances include salts and sugars. The two principal methods of measuring TDS are the gravimetric analysis and conductometric methods. Gravimetric methods (GM) are the most accurate and involve evaporating the liquid solvent and measuring the mass of residues left. GM, however, are time consuming if involve large volumes of aqueous solution. The conductometric methods (CM) are fast, but they are not sensitive to uncharged compounds or low-charged ions like motor oil, gasoline, many pharmaceuticals, and pesticides. It should be noted, both GM and CM are not suitable for the detection of the so-called PFAS molecules, which are made up of a chain of linked carbon and fluorine atoms. This analysis can be provided only in specialized laboratories by using sophisticated liquid chromatography combined with mass spectrometry. Total suspended solids (TSS) are undissolved suspended inclusions in water. The basic laboratory method for TSS detection is their extraction by filtering the sample. Also, light scattering is used in portable devices.
The advantage of microwave methods is that they detect changes in dielectric constant caused both by conductivity and by molecular polarization, i.e. microwave probes are capable of detecting uncharged particles. Here we present the results revealing how our microwave sensor can be used for TDS/TSS detection, by analyzing aqueous samples with organic suspension. As seen in Figure 9, sea water sample induces a monotonic decrease in the amplitude of the frequency shift over the deposition-evaporation set. This decrease in FS is characterized by the slope angle β in Figure 9. We attribute this behavior to a thin layer of TSS growing on the sensing electrode after each evaporation. The monotonic decrease of FS takes place because of the increase of the vertical distance between the droplet and the sensing electrode plane, as the TSS layer thickness increases. In contrast, as seen in Figure 5, the sensing electrode practically does not “feel” the NaCl microcrystals left on its surface after the evaporation. This takes place because in our case the NaCl micro crystals are rapidly dissolved at each deposition of the droplet. Consequently, NaCl concentration and evaporation time of each subsequent droplet increases (W depends on M in Figure 5). The clear difference between di-H 2 O, salted water and sea water is presented in Figure 10.
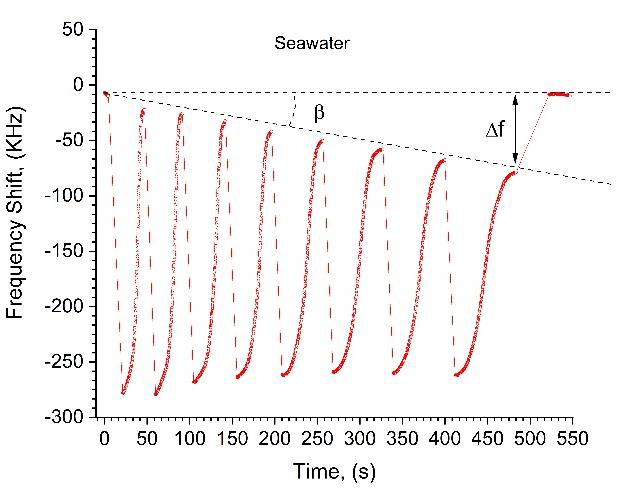
Figure 9 A peculiarity of sea water droplet evaporation: a) the fact that each subsequent deposition increases W indicates that the sample has dissolved salts, b) the decrease of the initial level of FS takes place due to TSS. Note, FS returns to the initial zero level after the last evaporation of sea water sample because the sensing electrode surface was cleaned.
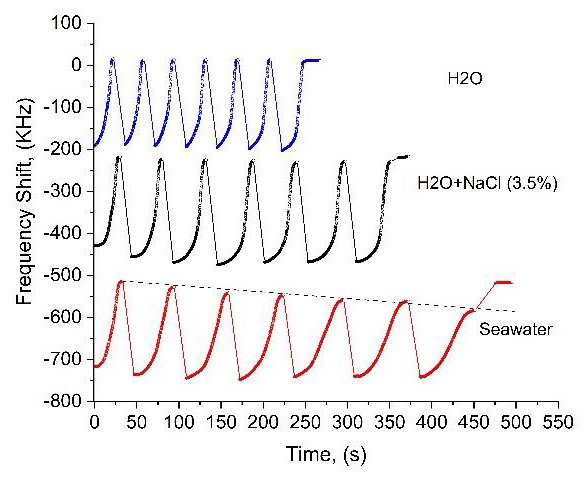
Figure 10 The effects of the salinity (the curve H2O+NaCl), and the salinity combined with TSS (seawater). The data indicate that sea water contains more types of dissolved salts compared to NaCl solution, because, as seen in the figure, the accumulated evaporation time of sea water exceeds the accumulated evaporation time of NaCl solution.
Figure 11 shows similarity between sea water and urine sample that includes organic suspension. It is clearly seen in Figure 10, and Figure 11 that the width W of each subsequent evaporation profile in the curves with organic compounds increases, i.e. W is a function of M, like in Figure 5. It means that the samples contain dissolved salts. Their concentration can be evaluated from the dependence W(M), and the difference M (WH2O - WH2O+NaCl). Therefore, we expect that the sensor is capable of discriminating TSS from dissolved salts.
5. Discussion
For the comparison, in Figure 12 we present the measurement of FS for different liquids by using a thin-wall Teflon capillary tube, at 0.2 mm inner diameter, attached along the Y direction to the substrate shown in Figure 1b, close to the sensing electrode end. This configuration provides a fixed volume of aqueous solution. Figure 12 shows the oscillator response, as a liquid sample was periodically pumped in and out of the capillary tube. The difference in the frequency shift (
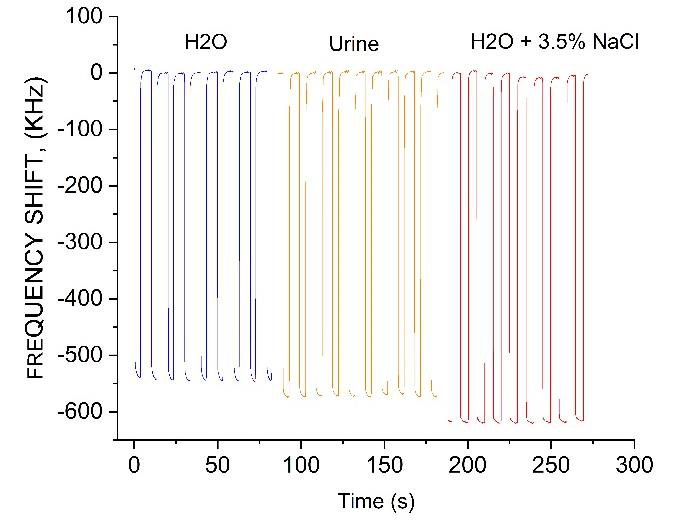
Figure 12 Frequency shift of the oscillator due to microfluidic flow of di-water, urine, and 3.5% NaCl solution through a thin-wall Teflon capillary tube attached to the sensing electrode end.
On the other hand the difference in the accumulated evaporation time (W), due to
Here we do not discuss the accuracy required for the determination of the absolute values since the device has to be carefully calibrated. It is especially important when measuring TSS. Also, we suppose that the device can be calibrated to measure TDS. For the experiments shown in Figure 11 we used NaCl solution at the concentration that approaches to the salinity of the sea water sample. In addition to the discussed TSS effect, the observed difference in the accumulated evaporation time between di-H2O, NaCl solution, and sea water allows one to extract the information about TDD in sea water, by deducting the accumulated evaporation time from H2O+NaCl and sea water samples.
6. Conclusions
In this work we described the microwave intracavity sensor for the characterization of aqueous solutions of nano-liter sessile droplets, deposited on the microstrip line electrode. It operates at GHz frequency and is based on tunable magnonic delay line oscillator. The samples were characterized by using their evaporation dynamics, by measuring their dielectric constants. The presented (proof of concept) experiments have shown that the sensor can detect the salinity of water with the required precision and resolution. The advantage of the sensor is the possibility of measuring the parameters such as S, TSS and TDS, simultaneously.











 nueva página del texto (beta)
nueva página del texto (beta)
















