Brief biography of Juan (Joan) Genesca-LLongueras (JGL) and his academic training
JGL received the BSc and EngD degrees in chemical engineering from the Chemical Institute of Sarria (IQS), Ramon Llull University, Barcelona, in 1976 and 1980, respectively.
At the Faculty of Chemistry, UNAM (FQ-UNAM), he has held different positions, highlighting being the head of the Division of Basic Sciences and the Department of Metallurgical Engineering and Coordinator of the master and doctoral program, PMyDI at UNAM, from 2009 to 2011. He is currently a Full Professor of Corrosion Engineering and Electrochemistry at FQ-UNAM. Since 2011 he has held the position of Academic Coordinator of the Polo Universitario de Tecnologia Avanzada, PUNTA-UNAM, located in the Parque de Investigacion e Innovacion Tecnologica, PIIT, UNAM, Apodaca, NL. In September 2022, he kept the position of General Coordinator at Unidad de Investigación y Tecnología Aplicadas, UNITA UNAM at PIIT.
He has received several awards throughout his academic career, highlighting the Teaching UNAM Award in 2004 and the National Electrochemistry Award in 2016. Since its foundation, he has belonged to the National Research System (Sistema Nacional de Investigadores, SNI), reaching the highest level 3 in 2014 and having been a member of the ruling commission of area VII from 2010 to 2013 and president of the same in 2012. In March 2022 he was named Emeritus Investigator by the Mexican Conacyt National Research System (Sistema Nacional de Investigadores, SNI).
He has supervised over one hundred bachelor's, thirty-five master's, and nineteen doctorate theses. Dr. Genesca has published over 150 documents (https://www.scopus.com/authid/detail.uri?authorId=55666986300, https://www.researchgate.net/profile/Juan-Genesca-2/publications), 150 full-length peer-reviewed conference papers, and co-authored five books.
His current research interests include galvanic corrosion in the automotive sector, atmospheric corrosion, cathodic protection, flow accelerated corrosion (FAC), and CO2/H2S corrosion.
Dr. Genesca is on the Editorial Board of the journal Afinidad, Barcelona, a corresponding member in Mexico of the Institut d'Estudis Catalans, IECat, Barcelona, and currently a member of the Societat Catalana de Quimica, Sociedad Mexicana de Electroquímica, SMEQ, National Association of Corrosion Engineers, NACE (now AMPP), and The Electrochemical Society. He has been highly active in scientific societies, participating in different working parties of the NACE (now AMPP) and the European Federation of Corrosion.
Juan Genesca has been active in corrosion for over 45 years, contributing to several areas. Genesca's work appears in top journals, earning him an h-index of 24, which is appropriate for the corrosion area and reflects the impact of his work. He has trained many M.S. and Ph.D. students and postdocs in his career, many of whom are now making significant contributions.
The first years in corrosion and Mexico
During his undergraduate studies in Barcelona, JGL specialized in corrosion studies and worked in the research group led by Professor Lluis Victori. He completed his undergraduate thesis on the corrosion and passivation of titanium in halogenated and alkaline media. [1,2]. Subsequently, after completing his military service, he rejoined Prof. Victori's group as Assistant Professor to do his doctorate on the palladium passivation process from 1976 to 1980 [3-6]. During this period, he combined research with teaching at the IQS and the Universitat Autonoma de Barcelona, UAB, in the Department of Geology. In this Department, he carried out support research through his knowledge of the atomic absorption spectroscopy technique and the equipment he was in charge of installing and operating [7,8].
Upon completing his doctorate in September 1980, he accepted an invitation from the General Directorate of Academic Personnel Affairs, DGAPA, to join the UNAM as a Visiting Professor in the Faculty of Chemistry. The contract, with a validity of two years, was extended to participate in the design of a Semiconductor Electrochemistry Laboratory in the Department of Physicochemistry, headed by Dr. Madeleine Rius de Riepen and with the support of Dr. Carlos Mauricio Castro, who had completed his doctorate on this subject. During this period, its primary function was the development of electrochemical techniques in the laboratory created for this purpose for the study of semiconductors [9].
In 1982 he was hired by the FQ-UNAM to join the Department of Metallurgical Engineering for the start-up of a Corrosion Laboratory, LabCorr, together with Dr. Javier Avila, who had recently graduated from his doctoral studies at the University of Oxford in the group of Prof. John Sykes. A fruitful and friendly collaboration began, allowing LabCorr to position itself as the leader in this field of knowledge at UNAM and Mexico for many years. Avila and Genesca made the substantive work at the UNAM, teaching, research, and diffusion, coupled with professional training in corrosion with the implementation of the "Diplomature in Corrosion and Protection Engineering" for a large group of engineers from PEMEX, CFE, Peñoles, STC Metro Mexico City and a very long, etc. Thus, he contributed to disseminating specialized knowledge on corrosion in the Mexican productive sector per the proposal outlined in the Hoar Report to reduce corrosion costs. [10-20].
A first look at electrochemistry in Mexico in the 80s of the last century [21,22]
By the end of the 1970s, the number of academics researching topics related to electrochemistry in Mexico was limited. Only a few scattered laboratories in the country made up the critical mass for this branch of chemistry and operated independently, with little interaction between them. One of the laboratories that began to generate research in electrochemistry during this time was that of the FQ-UNAM, led by Dr. Miguel Saloma Terrazas, who had recently earned his doctorate at the University of Trondheim in Norway. Dr. Saloma's main goal was to promote his specialty, so he sought funding to invite renowned scientists to increase interest and development in electrochemistry in Mexico. His efforts were successful in 1979 when could organize the first scientific event in electrochemistry at UNAM, the "Symposium on Modern Electrochemistry and its Applications."
As far as I remember, and to my knowledge, electrochemical research in Mexico began to gain traction with the arrival of French scientific collaborators such as Gerard Poillerat at CINVESTAV and Yunny Meas at UNAM. The Mexican electrochemistry community began to meet annually in 1979 at CINVESTAV-IPN. These meetings were informal with a mini-symposium format. The Mexican Society of Electrochemistry was established in 1980 to bring together the participants of the conferences and courses
Years before, Latin American Electrochemical and Corrosion Meetings (RELEC) began to be held in South America, whose central participating countries were Argentina, Chile, Venezuela, and Brazil. At the V RELEC, Dr. Alejandro Arvía, president of the then Latin American Electrochemical Society, proposed to the participants from Mexican institutions that the VI RELEC taken place in Mexico. The participants, including Dr. Poillerat and his group, accepted the invitation, returning to Mexico to organize this international congress. The regular participants in those Electrochemistry Academic Meetings of the late seventies and early eighties, among whom were the now renowned academic researchers Omar Solorza, Juan Genescá, Alvaro García, Yunny Meas, Guadalupe Alonso, Miguel Saloma, Mauricio Castro, Elsa Arce, Javier Ávila and Silvia Tejada, among others (21,22).
Once the Society was established, its first major accomplishment was successfully organizing the VI RELEC in May 1983 in Oaxtepec, Morelos (Fig. 1) [22]. This congress had the participation of a large group of researchers from different countries of Latin America, as well as from some European countries. The above drew the attention of the directors of the Latin American Electrochemical Society, leading to the formation of the current Ibero-American Society of Electrochemistry (SIBAE) in the future [22].

Fig. 1 Front cover of the VI Latin American Meeting on Electrochemistry and Corrosion, Oaxtepec, Morelos, Mexico, 1983 [22].
The Sociedad Mexicana de Electroquímica (SMEQ) was founded in 1983 to support the administrative organization if the VI RELEC. The founding members of the SMEQ were Yunny Meas Vong (President), Miguel Saloma Terrazas (Vice President), Omar Solorza Feria (Secretary), and Guadalupe Alonso Viveros (Treasurer).
In 2001, the XVI SMEQ Congress was held in Santiago de Querétaro, Qro; the first electronic database of SMEQ members was created. A follow-up was given to the different work areas of the electrochemical community. And the first Seminar on Electrochemical Techniques for Corrosion Control, edited by Juan Genescá, was also published.
Teaching Corrosion at UNAM
A statement of principles: I am not a researcher in the traditional term. I am a teacher who researches to ensure that the information I provide to my students is accurate and verified through experimentation, simulation, and mathematical modeling.
I have taught corrosion since 1975 when I began my Engineer Doctorate studies. I have many years of experience and am satisfied with my progress. Beyond the good students, many of whom are excellent, the challenge that Javier Ávila and I set for ourselves in 1982 in the newly opened Corrosion Laboratory in building D of the Faculty of Chemistry at UNAM is still valid. We have made significant progress in research, as evidenced by the increasing number of articles by Mexican authors, as shown in a search on Scopus. However, the problem of basic knowledge of electrochemical aspects of corrosion remains unresolved, as it is not yet included in the curriculum of engineering programs.
Recent reviews of various technological projects and pipeline failure analysis have allowed me to verify that shortcomings in terms of, for example, the correct application of cathodic protection criteria continue to be a challenge. The interpretation of corrosion potential, including the recommended practice of -0.85 V vs. Cu/CuSO4, whether it should be measured with the current on or off, and the effect of cathodic shielding on delamination and other issues, are ongoing challenges that engineers often face daily. This is why studies on the cost of corrosion are still very high and why the U.S. Department of Defense has commissioned the University of Akron to offer a degree in corrosion engineering, the first generation of which graduated in 2015. Far behind is the Hoar Report [23], but yet fully valid, which pointed out as one of the leading causes of the high cost of corrosion in Great Britain, 3.5 % of gross domestic product (GDP), the following: The need for better education in corrosion and protection with the introduction in the curriculum of engineering careers of a corrosion subject.
As one of the initial responses to the Hoar Report, the European Federation of Corrosion, EFC, drafted a reference textbook for engineering institutions. Among other actions, this led to the publication of the book "Introduction to corrosion prevention and control for engineers" by Prof. P.J. Gellings, which the EFC sponsored and whose first edition was published in 1976 [24], and the last one is from 2005.
Corrosion cost studies
In the past 60 years, numerous studies have been conducted to estimate the losses caused by corrosion (Table 1). Uhlig calculated the cost to the United States in 1950 to be around 2.1 percent of GDP. Similar findings were made by the Hoar report in the U.K., which revealed that the cost of corrosion was roughly 3.5 % of GDP. This study founded the University of Manchester Institute of Science and Technology Corrosion Centre. On the other hand, a 1974 study in Japan revealed that the cost of corrosion was 1.2 % of its GNP. The National Bureau of Standards and the Battelle Research Institute estimated the cost of corrosion in 1975 for the United States to be 4.5 percent of the Gross National Product (GNP). The most current research, conducted in 2002 by the U.S. Federal Highway Administration (FHWA), indicated that the cost of corrosion in 1998 was $276 billion, or 3.1 percent of the country's GDP. Corrosion and environmental degradation undeniably impact developed countries' economies, although there may be some variations in the numbers. The 2002 GNP will likely be more heavily weighted toward the service economy and less towards materials, assets, and maintenance costs than the 1975 GNP [25].

Fig. 2 Front cover of the two books "Beyond Rust" and "Beyond Rust. The Fight against Corrosion". Fondo de Cultura Economica, FCE (Economic Culture Fund), Mexico City.
Table 1 Studies on the Cost of Corrosion (25)
| Publication Year | Author | Country | Portion of Country's GDP (%) |
| 1950 | H.H. Uhlig | United States | 2.1 |
| 1970 | T.P. Hoar | United Kingdom | 3.5 |
| 1974 | Japan | 1.2a | |
| 1975 | Battelle/NBS | United States | 4.5a |
| 2000 | DTI | United Kingdom | 2.5-3.5 |
| 2002 | NACE/FHWA | United States | 3.1 |
aPortion of GNP, https://nap.nationalacademies.org/read/12560/chapter/3 From: National Research Council. 2009. Assessment of Corrosion Education. Washington, DC: The National Academies Press [25]. https://doi.org/10.17226/12560
To refer to some of those directly related to the teaching of corrosion. One of the recent studies in the USA, Cost of Corrosion and Preventive Strategies in the United States , points to 276 billion dollars. One of the leading causes, referred to as the teaching of corrosion, is still valid. I point out here, in their original language, the two conclusions referring to corrosion teaching and research:
Improve education and training of staff, and
Advance corrosion technology through research, development, and implementation.
From the most recent studies available, one particular reference is the Proceedings of the Materials Forum 2007: Corrosion Education for the 21st Century, Michael H. Moloney, Editor, Corrosion Education, Workshop Organizing Panel, National Research Council, Washington [26].
A verbatim copy of the second paragraph of the preface: "Better education for the nation's engineers is essential to improving corrosion control and management practices throughout the national infrastructure." In this regard, assessing the corrosion curricula of undergraduate engineering schools is an unfinished task in many universities.
The above and the papers presented indicate that they have detected the same problem. Two other essential references are [27,28]:
J. R. Scully and R. G. Kelly, "Corrosion Education in the 21st Century," 16th International Corrosion Congress: Corrosion Tech. in the High Technology Era, Sept. 19-24, Beijing, China (2005).
National Research Council. 2009. Assessment of Corrosion Education. Washington, DC: The National Academies Press. https://doi.org/10.17226/12560.
Prof. John Scully has grouped, in a pyramidal way, the different actors, the workforce after all, who participate in education and research in corrosion. A copy is presented in Fig. 3. It is structured based on the diversity of corrosion professionals and the level of education and knowledge they require. It can serve as a starting point and, simultaneously, as a summary, regarding strategic and tactical recommendations necessary to improve corrosion education at the levels it should be given. The study carried out by the National Research Council cited above suggests that one of the keys for all groups involved, from undergraduate to doctorate, is to adopt the model of knowledge-based teaching. Given the multidisciplinary nature of corrosion phenomena, an expert or specialist must possess much knowledge. In addition to the fundamental skills required for an engineering career, expertise in materials science and engineering, organic and inorganic chemistry, and applied electrochemistry (with a focus on corrosion) is necessary. Knowledge of fracture mechanics is also crucial if you plan to work with forms of corrosion involving mechanical stress, such as stress corrosion cracking and fatigue.
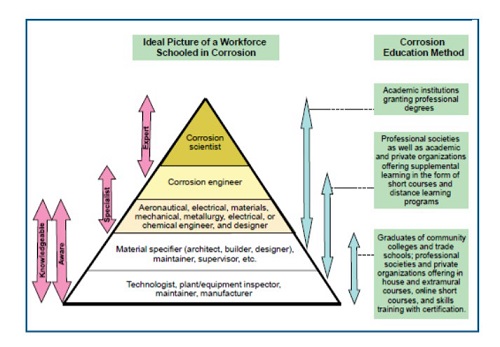
Fig. 3 The corrosion workforce pyramid. The pyramid shows the various categories of corrosion professionals and the education they need. The green boxes on the right indicate the educational paths they follow. Adapted from J. R. Scully, presented at the 16th International Corrosion Conference, Beijing, China, September 2005 (29) From National Research Council. 2011. Research Opportunities in Corrosion Science and Engineering. Washington, DC: The National Academies Press. https://doi.org/10.17226/13032.
Other topics, such as colloidal chemistry, fluid mechanics, surface physicochemistry, polymers, and electrical engineering, would also be helpful. After all, the proposed model is that of knowledge-based education. It must be considered that corrosion events and processes must be studied and understood in space-time coordinates. Corrosion processes are influenced by dimensional scales that range from the atomic level (0.1 nm) to the micrometric level (the beginning of a pit, approximately 1 (m), and up to the macroscopic level (the propagation of a pit, 1mm). Understanding these processes at each scale is essential in controlling the properties and behavior of corrosion at the macroscopic level. Additionally, the time scale must be considered since many of these corrosion processes can occur in as short as picoseconds, and others need several years (atmospheric corrosion, for example).
Use of standards in corrosion education
Studies conducted in the United States and Europe have identified three levels of corrosion expertise: corrosion science, corrosion engineering, and corrosion management (30-32). The progression from one level to the next should be smooth, intuitive, and systematic (Fig. 4(A)). However, in practice, there is often a gap between each group (Fig. 4(B)). Additionally, research has not emphasized the importance of standards in early education and skill development, specifically at the corrosion science level. Integrating existing corrosion standards into the education system at this level is one way to close the gap. These standards are minimum requirements accepted by suppliers, users, producers, third-party laboratories, academics, and scientists. Corrosion control standards are developed by international organizations such as ASTM International, the International Organization for Standardization (ISO), and NACE International (AMPP)."
Standardization is a collaborative effort involving many individuals' contributions and voluntary participation in a shared field. Standards are typically revised every four to five years to reflect new information and experiences. They can be used to develop products or processes, draft specifications, implement government regulations, promote technology or knowledge, and educate the next generation of professionals. Adopting standards helps the community and industry meet a minimum value, metric, or practice. Additionally, many regulations are based on criteria or standards. Using standards in academic institutions is beneficial for educating students at the level of corrosion science. Introducing corrosion science standards can adequately prepare students for corrosion engineering and corrosion management in the real world.
These tools are developed based on many people's inputs and voluntary participation in a common field. They are updated regularly (typically every 4 to 5 years) based on new knowledge and experience. Standards are valuable for developing a product or process, writing specifications, implementing government regulations, promoting technology or expertise, and educating next-generation professionals. The adoption of standards helps the community and industry meet a minimum value, parameter, or practice. Many regulations are also based on criteria, or they refer to standards. Then, the standards are valuable to educate students in academic institutes at the corrosion science level. Introducing standards at the corrosion science level will prepare the students well before they enter the practical world of corrosion engineering and corrosion management.
Some pending corrosion tasks toward a sustainable society
Corrosion is a significant challenge that must be addressed to achieve a sustainable society. Corrosion is a process in which usually a metal degrades over time due to a chemical reaction with its environment. This process can cause structural damage to buildings and infrastructure, such as bridges and roads, and release hazardous substances into the environment. One of the main challenges of corrosion is that it is a complex process influenced by various factors, including the material being corroded, the environment it is in, and the presence of other substances. This makes it difficult to predict and control.
To address corrosion and move towards a more sustainable society, it is essential to invest in research and development of corrosion-resistant materials and technologies and implement effective corrosion prevention and management strategies. This may include using protective coatings, corrosion inhibitors, and regular maintenance and inspection programs. By taking these steps, we can help extend our infrastructure's lifespan and reduce the environmental impact of corrosion.
Corrosion is a significant problem that has been the subject of scientific study for more than 150 years. In the United States, it is estimated to cost approximately $276 billion annually, or 3.1 % of the country's GDP, according to a study by the U.S. Federal Highway Administration. Other studies in China, Japan, and the United Kingdom have also found significant costs associated with corrosion, with estimates of the worldwide direct cost exceeding $1.8 trillion. While eliminating corrosion is impossible, it is estimated that effective corrosion management practices could save 25%-30 % of annual corrosion costs.
Although I am not an expert at the level of being able to propose the challenges that society must face to achieve a more sustainable world, be enough to point out that the European Federation of Corrosion has just created a panel with 38 internationally recognized experts with the same objective [33]. For this reason, I will limit myself to pointing out two or three challenges that I believe are important for Mexico from the perspective of a university professor such as myself.
Effect of climate change on reinforced concrete infrastructures
The New Jersey Transit Board recently approved a $1.5 billion contract to replace the 110-year-old rail bridge Portal North Bridge [34]. Given the American Society of Civil Engineers' recent report that 7.5 % of United States bridges are structurally deficient and that the nation's backlog of bridge repairs requires an estimated $125 billion [35], the fundamental question for a world citizen could be:
Do you believe bridge replacement, rather than repair, is more time- and cost-efficient to solve the infrastructure problem?
In the specific instance of Mexico, it's crucial to highlight the National Plan for the Evaluation of Bridges Damaged by Corrosion in Mexico (PNEBDC) [36]. This comprehensive plan is designed to identify, assess, and mitigate the risks corrosion presents to bridges throughout the country. The Mexican Ministry of Communications and Transportation (SCT) spearheaded the project, working in conjunction with the Mexican Institute of Civil Engineers (IMCC) and the Mexican Transport Institute (IMT).
The PNEBDC is a four-phase plan that includes the following actions:
Phase 1: Identification of bridges at risk. This phase involves a comprehensive survey of all bridges in Mexico to identify those at risk of corrosion. The survey will consider various factors, including the bridge's age, materials, and location.
Phase 2: Assessment of the risk of corrosion. This phase involves a detailed assessment of the bridges identified in Phase 1 to determine the extent of the corrosion damage. The evaluation will consider various factors, including the type of corrosion, the damage's area, and the damage's location.
Phase 3: Mitigation of the risk of corrosion. This phase involves implementing various measures to mitigate the risk of corrosion to the bridges identified in Phase 1. The measures may include repairing the damage, strengthening the structure, or replacing the bridge, Fig. 4.
Phase 4: Monitoring of the bridges. This phase involves the ongoing monitoring of the bridges to ensure that the measures implemented in Phase 3 are adequate. The monitoring will include regularly inspecting the bridges to identify any new damage.
The PNEBDC is a significant step forward in addressing the corrosion problem of bridges in Mexico. The plan is comprehensive and includes a variety of measures to identify, assess, and mitigate the risks posed by corrosion. Implementing the PNEBDC will help ensure the safety of bridges in Mexico and protect the lives of those who use them.
In addition to the actions outlined in the PNEBDC, several other things can be done to reduce bridges' corrosion risk. These include:
Using corrosion-resistant materials.
Providing adequate drainage to prevent water from pooling on the bridge deck.
Applying a protective coating to the bridge.
Regularly inspecting the bridge for signs of corrosion.
Taking these steps makes it possible to reduce bridges' corrosion risk and keep them safe significantly. The American Society of Civil Engineers' report highlights the need for increased investment in the maintenance and repair of bridges in the United States. The fact that 7.5 % of bridges are structurally deficient is a significant issue, as these bridges may not support the weight of vehicles safely and may be at risk of collapsing. Furthermore, the estimated $125 billion backlog of bridge repairs illustrates the significant amount of work that needs to be done to improve the condition of bridges in the country. This is a significant problem that needs to be addressed to ensure the traveling public's safety and the integrity of the country's infrastructure. As infrastructure ages, maintenance costs increase, and it is estimated that approximately 50 % of maintenance costs for traffic infrastructure in industrial countries are related to corrosion. The cost of corrosion in reinforced concrete structures in the United States is estimated to be between $20 billion and $40 billion [37].
Steel corrosion in concrete is a complex process influenced by various factors, including the materials used in the concrete and reinforcement and environmental factors. As a result, it is difficult to quantify and predict the corrosion of the reinforcement in concrete structures. In practice, making informed decisions about the best maintenance and repair methods is challenging due to a lack of understanding of corrosion and protection mechanisms. Then, it is essential to study the durability of various repair and protection methods to implement effective, life cycle-oriented management. Sensor-based monitoring systems integrated into digital building models can support building inspection and supervision of buildings to prevent unexpected corrosion problems and improve maintenance. Another critical area of research is using low carbon footprint binders in concrete and how they may affect concrete structures' corrosion behaviour and durability. Further research in these areas can help reduce the high corrosion costs in our infrastructure.
The most promising approach. Corrosion prediction using Finite Element Modeling (FEM) and Artificial Intelligence [38-42]
Finite element modeling (FEM) and artificial intelligence (A.I.) are promising tools that can be used to predict corrosion in the automotive industry. FEM is a numerical analysis method that can simulate the behavior of materials and structures under different loads and conditions. It can be used to model the corrosion process and predict how a material will behave over time. On the other hand, A.I. is a computer science field that focuses on developing intelligent systems that can perform tasks without being explicitly programmed to do so. A.I. can evaluate large amounts of data and identify patterns that may not be visible to humans. The above can be particularly useful in predicting corrosion, as it can help identify factors that may contribute to corrosion and allow for more accurate predictions.
The use of FEM and A.I. for corrosion prediction in the automotive industry has the potential to improve the efficiency and effectiveness of corrosion prevention and management efforts. It can help reduce the need for expensive and time-consuming physical testing and allow companies to better understand the factors contributing to corrosion and take steps to mitigate its impact. Using these technologies is a promising step towards a more sustainable and efficient automotive industry. Thus this collaborative approach is being explored as potential tools for improving the prediction of metal corrosion and lifespan. Traditional corrosion experiments often assume that the electrolyte layer surrounding the metal is thick enough to be treated as "infinite," ignoring the local corrosion effects that can be introduced by dynamic electrolyte dimensions such as droplets and puddles. To generate more accurate numerical models is necessary to abandon this assumption and consider the influence of dynamic and finite electrolyte dimensions on metal surfaces [42].
By using FEM modeling in combination with experimental approaches, it is possible to better understand and predict corrosion under dynamic electrolyte geometries ranging from micrometers (droplets) to millimeters (continuous film). The interaction between experimental and numerical tools can provide insights into the atmospheric corrosion process and help to improve prediction tools. Accurate and real-time evaluation of atmospheric corrosion can also aid in materials selection and engineering design for corrosion mitigation.
One alternative to using finite element modeling (FEM) and artificial intelligence (A.I.) for corrosion prediction could be advanced experimental techniques and data analysis methods. This approach could involve the development of new methods for corrosion testing and measurement methods, as well as using advanced statistical and machine-learning techniques to analyze and interpret the resulting data. The above could allow for identifying key factors that influence corrosion and the development of more accurate prediction models. Additionally, using sensors and monitoring systems could enable real-time corrosion monitoring and allow for the development of more effective prevention and management strategies.
The carbon footprint of steel corrosion [43]
The direct corrosion cost is estimated to be between 3 and 4 percent of the world GDP when just direct expenses are considered. No study has measured the environmental impact of steel corrosion, however. Thinking European Union and current U.S. greenhouse gas reduction targets, Iannuzzi and Frankel [43] projected that the CO2 emissions associated with the steelmaking necessary to replace corroded steel will account for 4.1 % to 9.1 % of the total by 2030. According to the authors, better corrosion management procedures might significantly reduce greenhouse gas emissions associated with replacing rusted steel, which also underlines the need for coordinated international initiatives.











 nueva página del texto (beta)
nueva página del texto (beta)




