Introduction
Estimates of galling insect richness calculates ~133,000 species distributed around the world (Espírito-Santo & Fernandes, 2007). The galling habit is widely spread among insects and is mostly comprised of Diptera, Hymenoptera, Hemiptera, Thysanoptera, Lepidoptera, and Coleoptera (Fernandes & Carneiro, 2009). These organisms induce abnormal structures on the plant, known as galls, cecidias or plant tumors (Fernandes & Carneiro, 2009). These structures are composed of plant tissue characterized by the increase in the number of cells (hyperplasy) and/or the increase in cell size (hypertrophy) (Mani, 1964).
Galls can be induced on any vegetative structure (leaves, stems, branches and roots) or reproductive organ (flowers, fruits and seeds) (Mani, 1964). The growth mechanisms of the plant organs are modified in response to stimuli from the galling insects (e.g., salivary secretion during feeding or maternal secretion in the oviposition), which alters the architecture and physiology of the plant in order to benefit it or its progeny (Oliveira et al., 2016; Raman, 2007; Stone & Schönrogge, 2003). The gall provides food, shelter, and protection against natural enemies for the galling insects (Fernandes & Santos, 2014; Price et al., 1987; Stone & Schönrogge, 2003).
Galling insects are found on specific host plants in natural communities across most biogeographical regions (Fernandes & Price, 1991; Price et al., 1998). However, their species richness has been reported to be higher in tropical regions (Fernandes & Price, 1988; Gonçalves-Alvim & Fernandes, 2001), with more species ocurring in xeric environments (Price et al., 1998) than in temperate and cold regions. At the global scale, most studies addressing the richness of galling insects have been carried out in the Cerrado (Brazilian savanna) (Araújo et al., 2014; Carneiro et al., 2009; Coelho et al., 2009; Fernandes et al., 1997; Gonçalves-Alvim & Fernandes, 2001), but also in tropical savanna (Blanche, 2000) and humid subtropical forests (Blanche & Westoby, 1995) in Australia, tropical humid and dry forests in Panama (Medianero et al., 2003) and in montane forest and shrublands in Texas, USA (Blanche & Ludwing, 2001). In Mexico, there is a scarcity of research studies on the ecology of galling insects. There are only a few studies reporting galling insect richness in 2 tropical rainforests (Los Tuxtlas Biosphere Reserve and in Lacandonia rainforest; Oyama et al., 2003) and in a tropical dry forest (Chamela-Cuixmala Biosphere Reserve; Cuevas-Reyes et al., 2004).
Buds, flowers, and fruits are poorly represented as host organs for galling insects, since these structures depend on the phenological stage of the plant (i.e., they may be unavailable to gallers throughout the year). The phenological dependence is more evident in tropical dry forests and xeric environments (Pezzini et al., 2014). Despite the low number of studies reporting galls on reproductive organs (Fernandes & Santos, 2014), the effects of galling on these organs could pose serious threats to the plants due to the potential impact they would have on plant performance and fitness (Fernandes, 1987). Galls on fruits exhibit noteworthy morphotypes, such as irregular (Santos et al., 2016), spherical (Quintero et al., 2014), amorphous, fusiform, globoid, ovoid, swollen or triangular (Isaias et al., 2014).
The impact of galling insects on their host plants is variable. Galling insects are known to stimulate the metabolism of nearby sources by increasing the sink demand relative to source supply (Fay et al., 1993). This leads to negative effects on the plant, such as a reduction in branch length (Fernandes et al., 1993; Kurzfeld-Zexer et al., 2010; Silva et al., 1996), lower quality seeds that affect germination (Santos et al., 2016; Silva et al., 1996), decrease in CO2 assimilation (Haiden et al., 2012; Larson, 1998), limitations on flower and fruit production (Fernandes et al., 1993; Silva et al., 1996), and a reduction of entire plant biomass (McCrea et al., 1985). Galls on leaves of Acer sacharum (Sapindaceae) diminish approximately 60% of the stomatal conductance (Patankar et al., 2011). In contrast, the stomatal conductance of leaf galls on Acacia longifolia (Mimosoideae) shows no change in response to light intensity, but the immature galls had higher rates of stomatal conductance (Haiden et al., 2012). To our knowledge, beyond these reports, there are no studies that quantify the stomatal conductance in fruit with galls. Since these structures are physiological sinks and could have a negative effect on the fruit set, studies related to fruit galls become particularly important in order to understand how the presence of galls affects the reproductive input and fitness of the plant. The approach of this study involves the anatomical and physiological features in galled and healthy fruits.
We investigated the effects of galls on fruits of Parkinsonia praecox (Ruiz & Pav. ex Hook.) Hawkins based on morphological traits (length, diameter, thickness, water content and biomass content); anatomical features (trichomes, stomatal, and pavement cells), and physiological characteristics (stomatal conductance, gs).
Materials and methods
The study was conducted in the Zapotitlán Salinas Valley, located in the Tehuacán-Cuicatlán Biosphere Reserve, Puebla, Mexico. The data sampling was carried out in the nearby surroundings of the “Helia Bravo-Hollis” Botanical Garden (18°20’ N, 97°28’ W, at 1,500 m asl). The main plant association is the tetechera, dominated by the columnar cactus Neobuxbaumia tetetzo (Zavala, 1982); the vegetation corresponds to semi-arid scrubland (Rzedowski, 2006). The mean annual precipitation is 381.21 mm, and the annual mean temperature is 18.04 °C. The dry season is from September to April, and the rainy season is from May to August (Zavala, 1982). The climatic data were obtained from Conagua (2010) for the period 1964-2010.
Parkinsonia praecox (Fabaceae-Caesalpinoideae) is known in the study area as “manteco” or “palo verde”, it is a 7 m tall tree (Fig. 1A). In Mexico, it is distributed in the Northwest (Baja California Sur, Sonora, Chihuahua, Sinaloa, Durango, Zacatecas and Nayarit), East Center (Jalisco, Colima, Michoacán, Guanajuato, Morelos, Guerrero, Puebla, México), East (Tamaulipas and Veracruz), and South (Oaxaca and Chiapas) (Villaseñor, 2016). It is a common species in some vegetal associations in the Zapotitlan Salinas Valley (López-Galindo et al., 2003; Montaña & Valiente-Banuet, 1998). P. praecox fruits are flattened brown pods 10 cm length and 1 cm width, arranged in racemes, generally in pairs (Fig. 1B). Seeds are shiny-brown color and 7 mm mean length (Pennington & Sarukhán, 2005). The flowering season occurs between December-May, and the fruiting period is between January-September (Arias et al., 2001; Pennington and Sarukhán, 2005). Infructescences of P. praecox showed an important incidence of galls (Fig. 1C). Galled fruits had 1 larval chamber located at the center surrounded by parenchymatous tissue (Fig. 1D) (Contreras-Varela pers. obs.). Asphondylia sp. (Diptera: Cecidomyiidae) is the galling insect. From November to March, the branches are leafless when flowering and fruit set occurs (Pavón & Briones, 2001), indicating that it is a totally deciduous species during this period.
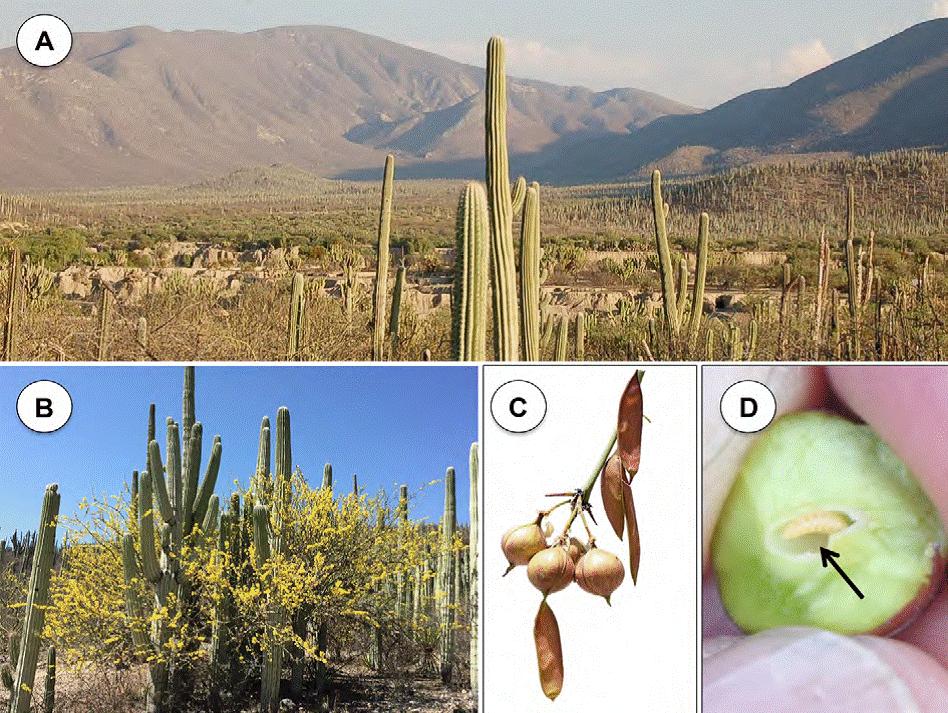
Figure 1 Parkinsonia praecox at the Zapotitlán Salinas Valley in Tehuacán-Cuicatlán Biosphere Reserve, México. A) Panoramic view of the study site; B) tree in flowering associated with Neobuxbaumia tetetzo; C) galled and healthy fruits; D) transversal cut of a gall. The black arrow indicates the position of the larval chamber.
The number of fruits affected by galls was determined in 30 randomly selected branches with galls and fruits of 50 cm long (2 per tree) in 15 different trees. In 10 individuals of P. praecox it was determined macroscopical characteristics of galled and healthy fruits, 50 pairs of a galled and a healthy fruit were randomly selected. A digital caliper (CD-s6, Mitutoyo Corp., Kawasaki, Japan) was used to measure length, diameter (distance considered starting in the abscission line in the middle of the fruit) and thickness of galled and healthy fruits. Aditionally, in transversal sections of galled fruits we measured parenchymatous tissue, and larval chamber size. In healthy and galled fruits, we quantified the water and biomass content by recording the fresh weight (FW), and then these were oven-dried for 36 hours at 70 °C. Later, the dry weight (DW) was quantified using a weighing scale (CP-225D, Sartorius, Germany, 0.01 g of accuracy). The water content was calculated as (FW-DW)/FW. The biomass content was defined as DW/FW. The water and biomass contents are expressed as a percentage.
In 3 individuals of P. praecox, 5 galled fruits (n = 15) and 5 healthy fruits (n = 15) were used to determine trichomes, stomatal, and pavement cell density, as well as stomata and pavement cell size. We applied a thin layer of clear nail varnish on the surface of the galled and healthy fruits in order to obtain permanent impressions of trichomes, stomata, and pavement cells. The area covered by the varnish layer was approximately 1 cm2. We determined the area of the 150 stomata and 150 pavement cells by measuring microphotographs of the samples with the software ImageJ (Rasband, 2017). The trichomes, stomatal, and pavement cell density were calculated in 2.7 mm2 in 3 randomly selected visual fields of an optical microscope per varnish layer (Carl Zeiss Inc., Thornwood, N.Y.).
Measurements of gs were determined in galled and healthy fruits in field using a porometer (SC-1, Decagon Devices Inc., Washington, USA). The sensor head of the porometer was situated in the middle section of the samples. Galled and healthy fruits were carefully placed on the sensor head without pressing, to avoid an overestimation of the gs. Seven measurement periods of gs were made between 07:00 h and 21:00 h on 3 different pairs of galled (n = 21) and healthy fruits (n = 21); 1 pair per individual of P. praecox.
Generalized linear mixed models (GLMMs) were used to evaluate the relationship between galled and healthy fruits in each of the morphological traits (length, diameter, thickness, water content, biomass content) and anatomical features (trichomes, stomata, pavement cells). As fixed effects, we entered the galled and healthy fruits into the model. As random effect, we used intercepts for individuals of P. praecox, as well as random slopes for the effect of galled and healthy fruits.
A GLMM was used to assess the effects of the time of the day, galled and healthy fruits on the gs. The fixed effects were the galled fruits, healthy fruits and the time of the day (07:00 to 21:00 h), while the random effects were the individuals of P. praecox, as well as random slopes for the effect of galled and healthy fruits. We assumed a gamma distribution for the model.
Visual inspection of residual plots indicates there was no deviation of homoscedasticity or normality. P-values were obtained by a likelihood ratio test of the full model with the effect against the model without the effect (Zuur et al., 2009). All statistical analyses were performed in R version 3.2.2 (R Development Core Team, 2017) with the package lme4 (Bates et al., 2015) and multicomp (Hothorn et al., 2008). For all statistical analyses, we reported the mean ± SE and the differences in magnitude (i.e., mm2, %, and density) between galled and healthy fruits, referred as estimated values calculated in the GLMMs.
Results
Twenty percent of the P. praecox fruits had galls, although some affected fruits had a few seeds in the apical portion of the fruit. The branches with only healthy fruits had on average 28.1 ± 1.38 fruits per branch (n = 299), while branches with galled fruits had 21.5 ± 2.04 fruits (fruits ± SE) per branch (n = 138). Galled fruits had a spherical shape and were green-reddish. Each galled fruit was composed of parenchymatous tissue with 0.4 ± 0.06 cm (cm ± SE) of thickness. This tissue surrounds a larval chamber, which was 0.1 ± 0.01 cm of diameter that holds only 1 galling insect larva.
The morphological traits of galled and healthy fruits (length, diameter, thickness, water content, and biomass content) exhibited significant differences. The galled fruits were 2.91 ± 0.17 cm shorter than the healthy fruits (χ2 = 51.8, p < 0.001; Fig. 2A). On the other hand, galled fruits had 0.28 ± 0.04 cm larger diameter (χ2 = 23.2, p < 0.001; Fig. 2B) and were 0.87 ± 0.03 cm thicker (χ2 = 53.1, p < 0.001; Fig. 2C) than the healthy fruits. The water content was 4.24 ± 1.2% higher in the galled fruits (χ2 = 7.1, p < 0.01; Fig. 3), while healthy fruits had 4.2 ± 1.3% higher biomass content (χ2 = 7.16, p < 0.01; Fig. 3).
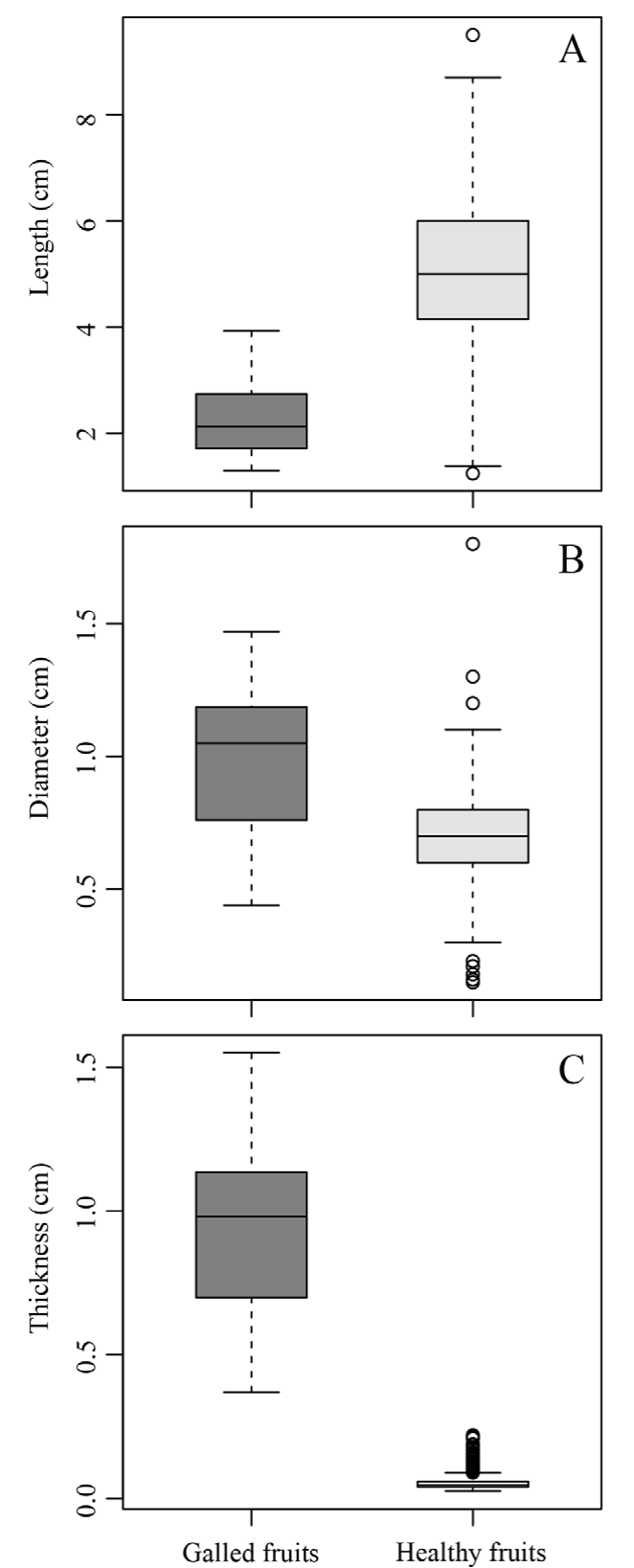
Figure 2 Morphological traits of galled and healthy fruits of Parkinsonia praecox. A) The length of a fruit from the base to the apex (p < 0.001); B) the diameter of fruits on the middle region (p < 0.001), and C) the thickness on the middle region (p < 0.001).
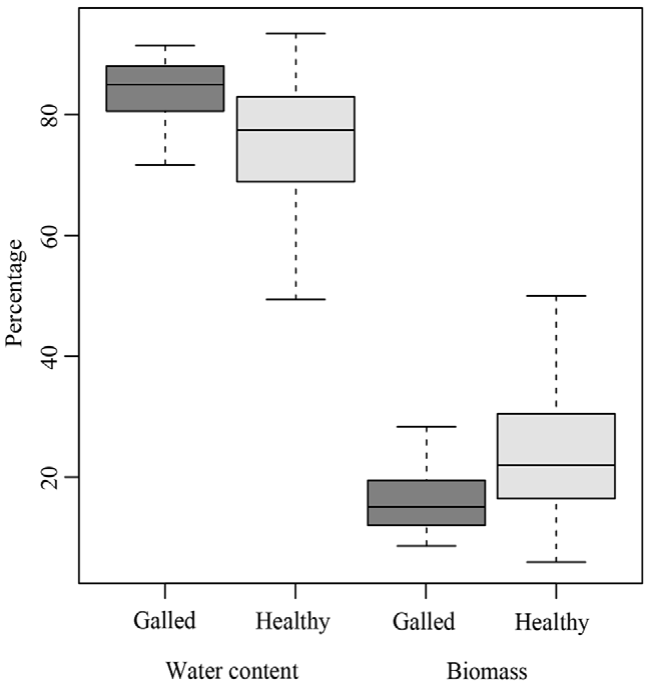
Figure 3 Comparison between galled and healthy fruits of Parkinsonia praecox in water content (p < 0.01) and biomass (p < 0.01).
The stomatal density (0.28 ± 0.81 stomata mm2, χ2 = 0.12, p = 0.72; Fig. 4A) did not differ between galled and healthy fruits. Contrarily, trichome density (4.76 ± 1.42 trichomes mm2, χ2 = 7.43, p = 0.006; Fig. 4C) and pavement cell density (19.4 ± 8.19 pavement cells mm2, χ2 = 4.34, p < 0.05; Fig. 4D) were statistically different between galled and healthy fruits. The area of pavement cells did not differ between galled and healthy fruits (0.005 ± 0.005 µm2, χ2 = 0.77, p = 0.37), while the stomatal size was 70.13 ± 21.51 µm2 larger in galled fruits than in healthy fruits (χ2 = 5.05, p < 0.05; Fig. 4B).
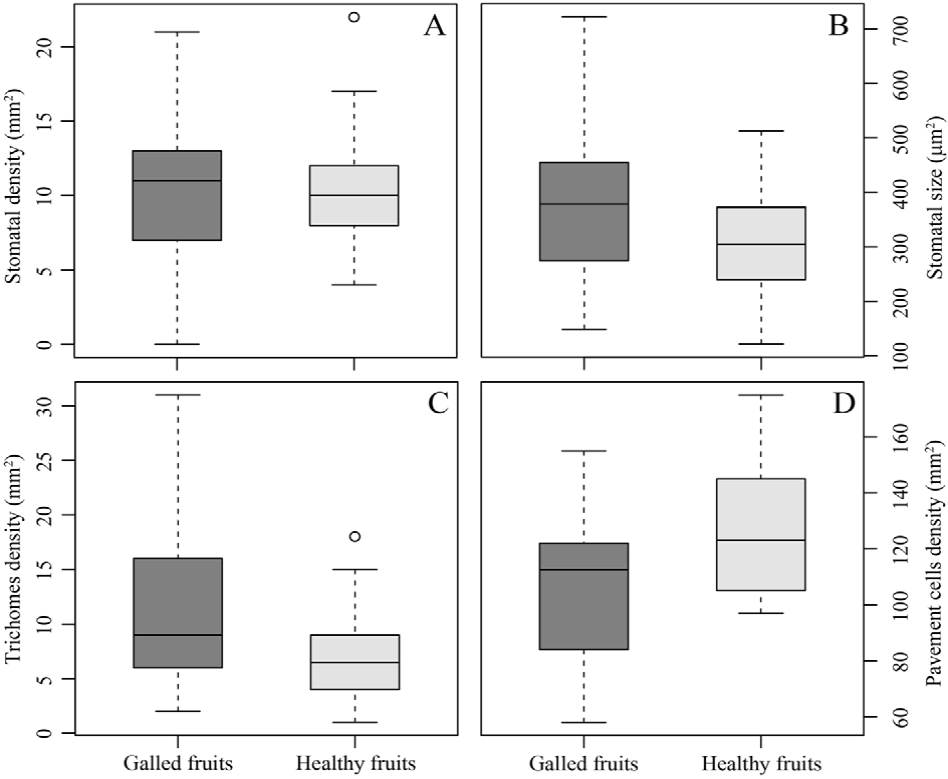
Figure 4 Anatomical features of galled and healthy fruits of Parkinsonia praecox. A) Stomatal density per area unit (p = 0.72); B) stomatal size (p < 0.05); C) trichomes density per area unit (p = 0.006), and D) pavement cells density per area unit (p = 0.37).
The gs was higher in galled fruits than in healthy fruits (χ2 = 12.6, p = 0.001). The interaction of daytime and the gall presence had a negative effect on the gs of galled fruits (χ2 = 36, p < 0.001). In the galled fruits, the highest values of gs were recorded at 07:00 h, while the lowest values were found in healthy fruits at 16:00 hrs. Likewise, the gs exhibited differences during the daytime (χ2 = 30, p < 0.001). Values of gs in galled and healthy fruits decreased throughout the day, with a slight increase in the last 2 records at night (18:00 to 20:00 h) (Fig. 5).
Discussion
In this study, we showed the incidence of galls in P. praceox fruits and its consequences on morphology and physiology. This is the first report of gall incidence on fruits in this semi-arid region of Central Mexico. In general, studies on galled fruits are scarce in the literature. We reviewed studies that report the presence of galls on reproductive structures, and we found 128 host plant species; Fabaceae was the most abundant host family (78 species), followed by Asteraceae (9 species), Boraginaceae (6) and Rubiaceae (6). The 2 most representative genera were Acacia (58 species) and Prosopis (14 species). Of the total number of host plant species found in our revision, 85.15% had galls on flowers (achene, buds, capitula, flowers, inflorescences) and the remaining 14.84% on fruits and seeds (Appendix).
Some species in South America have been reported as hosts of fruit-gall inducing insects, such as Conostegia xalapensis (Melastomataceae) (Chavarría et al., 2009); and Miconia calvescens (Melastomataceae) (Badenes-Perez & Johnson, 2007). Curiously, most studies are developed in the Costa Rican tropical dry forest (Janzen, 1982); Australian seasonally tropical forest (Kolesik et al., 2010), and Brazilian savanna (Santos et al., 2016). There are few studies on xeric environments that report the presence of galls on fruits, i.e. in Brazilian Restinga, Pithecellobium tortum have galls in seeds (de Macêdo & Monteiro, 1989). In our work we reported galls on P. praecox fruits and this is a pioneer study related to the study of galls in this area (Tehuacán-Cuicatlán, Mexico).
The galling insect Asphondylia sp. modifies the natural development of P. praecox fruits and negatively affects the fruit set of the host plant. In our bibliographical revision we found that the most representative taxonomic level of gall-forming insects on reproductive structures corresponded to the order Diptera (92.96%; 119 species) followed by Hymenoptera (6.25%; 8 species). The most important families of dipteran galling insects were Cecydomiidae (89.06%; 114 spp.) and Teprhitidae (3.9%, 5 spp.); of Coleoptera was Curculionidae (0.78%; 1 spp.); of Hymenoptera were Braconiidae (3.12%; 4 spp.), Agaonidae (0.78%; 1 spp.), Pteromalidae (0.78%; 1 spp.), Chalcidoidea (0.78%; 1 spp.) and Euritomidae (0.78%; 1 spp.). The most representative genus of galling insects was Asphondylia (44.87%; 60 spp.); followed by Dasineura (23.43%; 30 spp.), Allorhgas (3.12%; 4 spp.), Clinodiplosis (3.12%; 4 spp.), Urophora (3.12%; 4 spp.), Bruggmanniella (2.34%; 3 spp.), Eschizomia (1.56%; 2 spp.), and 13 genera correspond to 10.93%, each one represented by 1 species (Appendix).
Branches of P. praecox had 20% of gall incidence, indicating a high impact on fruit production. In association with pre-dispersal seed predation by coleopterans (Contreras-Varela, pers. obs.) and seed viability (Flores & Briones, 2001) the performance of P. praecox should be largely reduced.
Gall induction on P. praecox fruits should start inside the ovary where the cells are not yet differenciated, consequently the normal structure of the fruit is modified thus producing galls of spherical shape mainly without seeds (Fig. 1C). In general, galling insects require totipotent and undifferentiated tissues to induce galls development (Fernandes & Carneiro, 2009; Fernandes & Santos, 2014).
Galled fruits on P. praecox are mainly composed of parenchymatous tissue surrounding the larval chamber. We found that the biomass was greater in healthy fruits than in galled fruits. The water content in galled fruits (ca. 84.16%) suggests that galls are indeed important adaptations to live under harsh environments (Fernandes & Price, 1988; Price et al., 1987). The volumetric growth of fleshy fruits is the result of water and solutes accumulation that involves differences in water potential between the pedicel of the fruit and the rest of the plant (Matthews & Shackel, 2005). The gall formation in P. praecox involves the differentiation of a wide range of tissues that make the fleshy galls to superimpose an overall size. The water storage of galled fruits should be associated to solutes accumulation and to the increase of parenchyma cells turgor, these 2 conditions induce the cell hypertrophy and hyperplasia (Cosgrove, 2000). The parenchyma plays an important role for water and nutrient storage (Evert, 2006). To our knowledge, there is no information about the role of solutes content on the water movement to galls or if the chemical signals from the galling insects regulate the accumulation of solutes in galls. It has been reported that gall formation in vegetative structures comprise mainly the cellular elongation, however, cellular division and elongation has been associated to galls formation in reproductive structures (dos Santos et al., 2014). The sclerified tissue in galls and the high trichome density provide mechanical protection, the parenchymatous layer serves as a water and nutrient reservoir (Oliveira et al., 2014, 2016; Stone & Schönrogge, 2003).
The density of trichomes found on galled fruits of P. praecox was 1.5 times higher than on healthy fruits. In xeric environments, an increase of leaf pubescence leads to reduced water vapor transpiration and increases the thickness of the boundary layer (Evert, 2006; Pallardy, 2008). It has been reported that the pubescence of fruits significantly reduces water loss (Fernández et al., 2011). In P. praecox galled fruits, the high density of trichomes could prevent excessive moisture loss in the larval chamber and may maintain the internal temperature (Oliveira et al., 2014; Price et al., 1987). In addition, an elevated density of trichomes could reduce the vulnerability of the galling insects to potential natural enemies (Fernandes et al., 1987; Stone & Schönrogge, 2003; Woodman & Fernandes, 1991).
Galling insects do not only affect plant architecture and host organ morphogenesis, but also can modify physiological conditions such as stomatal conductance (Fay et al., 1996; Florentine et al., 2005; Larson, 1998), transpiration and CO2 assimilation (Dorchin et al., 2006). Those modifications influence positively to the gall (Fay et al., 1996) or negatively to the host (Florentine et al., 2005; Larson, 1998). Galled fruits of P. praecox increase the gs and stomatal size but had lower density of pavement cells in relation to healthy fruits; thus, it is possible that the increase in trichome density is the result of a compensatory mechanism to the high gas exchange of the galls with the environment; otherwise, the gs could be even higher (Fernández et al., 2011). Stomatal conductance estimates the rate of gases exchange through the stomata; it involves the density and aperture of stomata (Pietragalla & Pask, 2012) to infer transpiration and photosynthesis (Hiyama et al., 2005). According to Lemos-Filho and Isaias (2004), the fruits of Dalbergia miscolombium (Fabaceae) have a photosynthetic activity that contributes to the carbohydrates required for the fruit development. The gs in both galled and healthy fruits of P. praecox exhibited a similar pattern throughout the day, the higher values were recorded after the sunrise, indicating that the variation in gs of galls is due to water conditions in stem. The stomatal opening regulates the transpiration and prevents excessive water loss in the environment (Farquhar & Sharkey, 1982).
The gs in galled fruits was 2.5 times higher than in healthy fruits. Stomatal conductance can vary due to environmental (e.g., light intensity, CO2 concentration, relative humidity, temperature, wind, atmospheric pressure), anatomical (e.g., foliar area, pubescence, size and stomatal density), and/or endogenous factors (e.g., phytohormones) (Farquhar & Sharkey, 1982; Pallardy, 2008). In our study, the environmental conditions were similar in galled and healthy fruits. We found that galled fruits increased significantly the gs rates, although the stomatal density was not statistically different. This could be influenced by the production of signaling molecules, like phytohormones that promote the stomata opening and water movement to galls, in order to favor the water and nutrient continuum from host plant to gall. A revision of the abscisic acid (ABA) role in gall formation made by Tooker and Helms (2014) indicates that this phytohormone promotes gall growth. Even though the exact function of this hormone has not been yet fully recognized in gall tissues, ABA has been acknowledged as an endogenous regulator of the transpiration rate that controls the stomatal closing (Xiong & Zhu, 2003). We also suggest that similar endogenous regulation may occur on P. praecox galled fruits, but further phytochemical analysis will be necessary to determine the presence of ABA.
In conclusion, we found an important incidence of galled fruits on P. praecox that negatively affect morphological features of the fruits, with consequences on performance of the fruits. In addition, galled fruits are water sinks for this host plant that inhabits xeric environments. Future research is required to evaluate if the incidence of galled fruits negatively affects the plant fitness at the population level, in the different environmental conditions that occur in the Tehuacán-Cuicatlán Biosphere Reserve.











 nueva página del texto (beta)
nueva página del texto (beta)



