Introduction
One of the fundamental challenges of ecology is to determine which factors influence the distribution of organisms on Earth (Sanders & Rahbek, 2012). Gradients are the most commonly utilized tool for analyzing the response of the biota to environmental change, and elevation gradients are well-studied systems, since there are diverse life zones along these gradients, with particular and diverse collections of organisms, where different types of vegetation can be observed. Due to strong climatic variation over short distances, biotic zones and vegetation types are both compressed into a small area (Liao et al., 2014).
Biodiversity along elevation gradients shows variation in patterns depending on the group under study and the geographic location of the gradient itself (Grytnes & Beaman, 2006). Among the diverse patterns of elevation variation, the unimodal pattern is the most common (Rahbek, 1995) although there are many diverse responses of the biota to environmental changes over elevation gradients. For example, in a study on Mt. Rinjani, Lombok, Indonesia, it was determined that the alpha diversity of understory, low-canopy and canopy plants decreases with increasing elevation, while that of the creeping plants shows a unimodal pattern (Dossa et al., 2013). Along a 2,700 m elevation transect in Costa Rica, the maximum diversity of woody species was found at 400-600 m elevation (Clark et al., 2015) whereas in Nepal, maximum diversity of tropical genera was found below the midpoint of the elevation gradient, and the diversity of temperate genera presented a unimodal pattern (Li & Feng, 2015).
The range of patterns of elevation variation challenges to propose a general model; the variation has been attributed to the use of different sampling methods (Nogués-Bravo et al., 2008), analysis of incomplete elevation gradients (Grytnes & Vetaas, 2002) and the effect of scale (Rahbek, 2005). To obtain a general explanation of the underlying causes of the patterns of elevation variation, it is advisable to adopt similar sampling methods, standardize both the area sampled and the monitoring of environmental data and include complete elevation gradients (Lomolino, 2001). Studies based on only part of a gradient face an important limitation and, their results can only apply to that part of the gradient (Grytnes & Vetaas, 2002). Frequently, the lowest and highest parts of mountains have been severely disturbed by human activities (Nogués-Bravo et al., 2008), such that the native vegetation has largely been altered and replaced by other land uses (Arévalo et al., 2010; Da et al., 2009; González-Abraham et al., 2015; Piperno, 2006).
Diverse studies have documented patterns of elevation variation of species richness, diversity and environmental factors (e.g., Salas-Morales & Meave, 2012; Sanders & Rahbek, 2012; Toledo-Garibaldi & Williams-Linera, 2014). Several hypotheses have been proposed to explain which factors underlie the elevation variation of organisms. The hypotheses include area, biogeographic interpretations, climate, environmental heterogeneity, geological and climatic history, geometric restrictions, productivity, and soil characteristics (Colwell & Lees, 2000; Hawkins et al., 2003; Kitayama & Aiba, 2002; Latham & Ricklefs, 1993; Li & Feng, 2015; Rowe, 2009; Sanders, 2002; Wang et al., 2009). More recently, elevation gradients are central to study plant and animal responses in the face of global climate change since some species could potentially migrate upslope, but others will go extinct under most projections of global temperature increases (Clark et al., 2015; Colwell et al., 2008; Feeley et al., 2013).
Few studies have compared patterns of change over several elevation transects. Sanders (2002) analyzed ant species richness along elevation transects in 3 states in the USA: Colorado, Nevada and Utah. Grytnes (2003) compared 7 transects in Norway in order to determine patterns of elevation richness variation in vascular plants. Rowe (2009) studied patterns of richness of non-flying mammals over 4 elevation gradients located close together in North America. In northeast China, Wang et al. (2009) analyzed regional patterns of forest plant species on 6 elevation gradients, and Kessler et al. (2011) determined patterns of elevation variation in ferns over 20 gradients located at diverse sites around the world. To the best of our knowledge, there is no study yet comparing extended gradients facing 2 different oceans.
Mexico is a land of mountains flanked by the Pacific and Atlantic Oceans on the western and eastern sides, respectively, and thus offers a great opportunity to compare gradients in the Neotropics. The objective of this study was to contrast the variation in vegetation structure and characteristics of the arboreal component of 2 extended and environmentally distinct elevation gradients. We hypothesized that if precipitation, air temperature and potential evapotranspiration (PET) vary over elevation gradients then differential patterns in vegetation structure and tree species composition would relate to different climatic variables. Alternative explanations would be related to mountain range location and disturbance history.
Materials and methods
We studied a Pacific coast elevation gradient in the state of Oaxaca, and a Gulf of Mexico coast gradient in the state of Veracruz, both in Mexico (Fig. 1). In both gradients, selected sites were distributed along the entire elevation gradients, had relatively little disturbance, and field work was conducted during the same years, 2010 and 2011. The Oaxaca elevation gradient is located on the southern slope of the Sierra Madre del Sur and vegetation is well conserved; 21 sites were located from 70 to 3,600 m elevation at the summit. The climate is sub-humid with a marked rainy season in the summer months. Mean annual temperature decreases from 27 °C at the lowest to 9 °C at the highest sites, total annual precipitation varies from 437 mm at the lowest site to 1,632 mm at mid-elevations (Salas-Morales & Meave, 2012). The Veracruz elevation gradient is located in the central part of the state; 21 sites were located from 97 m to 4,000 m elevation at the tree line on the Cofre de Perote Volcano. The vegetation along the gradient has historically been disturbed, but well-preserved sites are scattered throughout the landscape. Mean annual temperature decreases from 25 °C at the lowest to 8 °C at the highest sites, total annual precipitation ranges from 932 mm at lower elevations, to ca. 2,000 mm at mid-elevations (Toledo-Garibaldi & Williams-Linera, 2014). Hereafter, the Oaxaca and the Veracruz elevation gradients will be referred as Oaxaca and Veracruz, respectively.
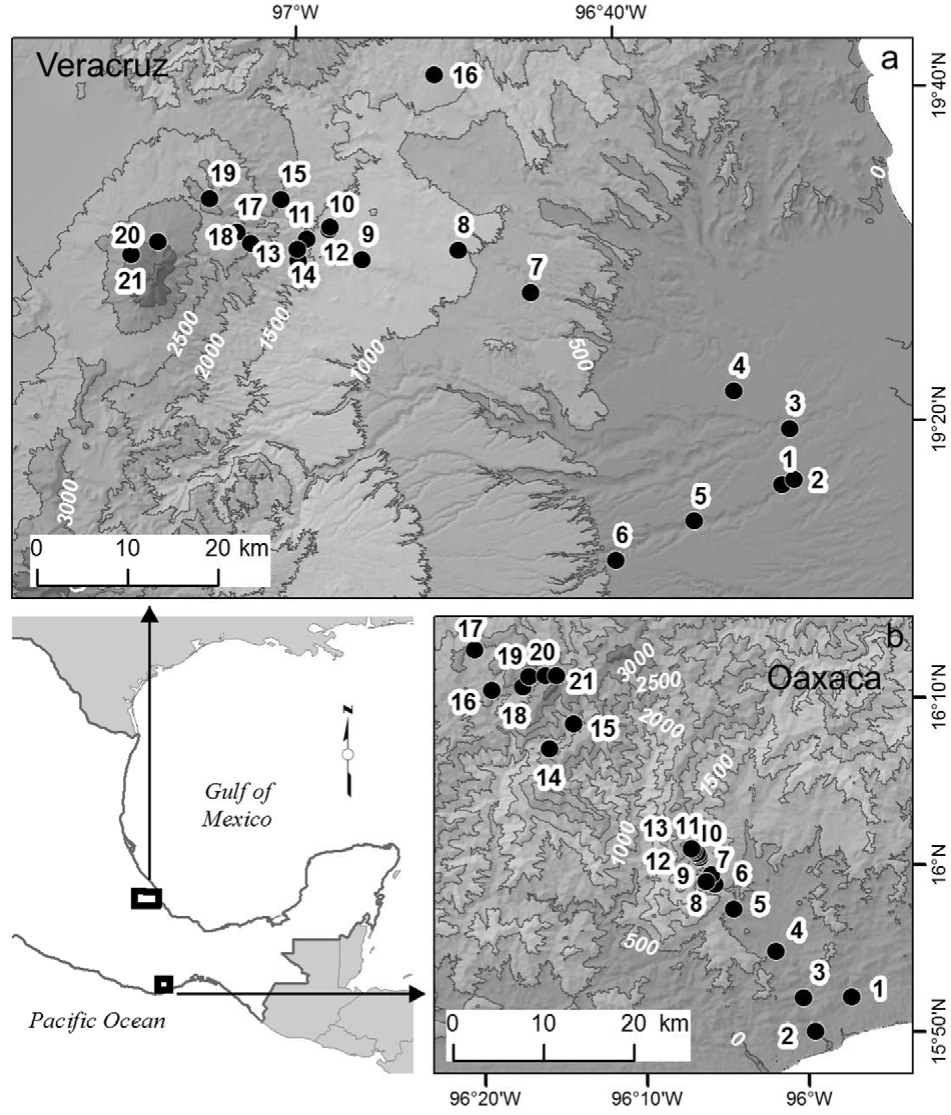
Figure 1 Map showing the geographic location of the 42 study sites along the 2 elevational gradients in Oaxaca (below right) and Veracruz (above), México.
Meteorological stations are scarce along the elevation gradients; however, we used the few that are available to corroborate data obtained from WorldClim (Hijmans et al., 2005) for each study site. We analyzed 8 variables extracted from WorldClim at a 1-km spatial resolution (mean temperature of the warmest and coldest quarter, annual mean temperature, precipitation of the warmest and coldest quarter, precipitation of the wettest and driest quarter and annual precipitation). In addition, we estimated PET as an indicator of dryness where the annual PET exceeds annual precipitation (Harris et al., 2013).
At each site, a 0.1 ha plot was established. In each plot, we counted the number of individuals and identified the species of all trees ≥ 5 cm diameter at 1.3 m in height (dbh). Vouchers were deposited at the SERO herbarium of the Sociedad para el Estudio de los Recursos Bióticos de Oaxaca, and the XAL herbarium of the Instituto de Ecología, A.C.
To identify groups of taxa that are specialists in each gradient, we used a classification model (CLAM; Chazdon et al., 2011). This is a multinomial statistical method that uses the relative abundance of taxa to classify specialists and generalists (Chazdon et al., 2011). We used a K-level of 0.5 for the simple-majority rule or liberal threshold, with a P-level of 0.005 as has been suggested when the objective is whole community analysis (Chao & Lin, 2011; Chazdon et al., 2011). We excluded morphospecies from the classification analysis; for analysis of the species and genera, we also excluded individuals identified to family level.
For each site, we calculated basal area (m2/ha), density (individuals/ha), species, genus, and family richness, and the Shannon diversity index (H'). Differences in climate variables between the gradients were analyzed using analyses of variance. To determine patterns of distribution, vegetation structure, taxa richness, diversity and climatic data, we fitted each variable to linear and polynomial models using generalized linear models. The best model was selected with the Akaike Information Criterion for small sample size, AICc (Burnham & Anderson, 2002). For the number of taxa (counts), we used a Poisson distribution and log link function. Data were analyzed using R project software version 3.4.2 (R Core Team, 2017).
Canonical correspondence analysis (CCA) was used to examine the relationship between plant taxa and climate variables along environmental gradients. The species, genus and family matrices consisted of the number of individuals of each taxa recorded in each of the 42 sites. The environmental data matrix included elevation and 8 climatic variables. Monte Carlo permutation tests were performed to determine whether the observed patterns differed from a random relationship. The forward selection procedure was used to determine the statistical significance of each environmental variable. Analyses were performed with CANOCO software version 4.5 (ter Braak & Šmilauer, 2002).
Results
Mean annual temperature decreased linearly with increasing elevation in both Oaxaca and Veracruz (Fig. 2a, b). However, in Oaxaca, the mean temperature values in both the warmest and coldest quarters were higher than those in Veracruz. The temperature difference between the coldest and the warmest quarters was smaller along the Oaxaca gradient (1.1 to 2.7 °C) than in Veracruz (3.2 to 5.7 °C; F7 34 = 207.10, p < 0.0001). Mean rainfall presented a unimodal relationship with elevation (Fig. 2c, d). A slight peak was observed between 500 and 1,200 m asl in Oaxaca and between 1,500 and 1,800 m asl in Veracruz. Climatic differences between the gradients were clear for PET below 2,000 m asl in elevation (Fig. 2e, f). These values indicated that Oaxaca is drier than Veracruz.
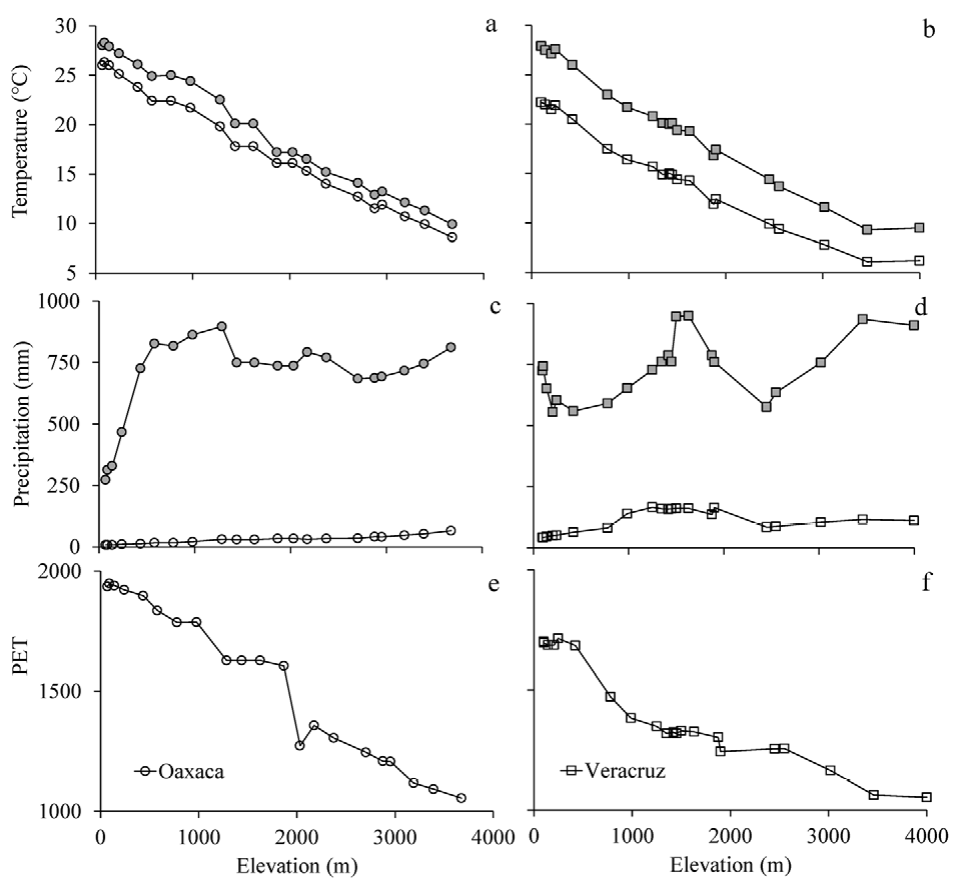
Figure 2 Climatic variables along elevation gradients in Oaxaca (left panels) and Veracruz (right panels), Mexico. a and b) mean temperature of the warmest (gray symbols) and coldest (open symbols) quarters; c and d) precipitation of the wettest (gray symbols) and driest (open symbols) quarters, and PET in e) Oaxaca (circle) and f) Veracruz (square). Labels on the far-left y-axes apply to both panels within a row.
A total of 4,229 individuals were recorded belonging to 435 species, 212 genera, 85 families and 19 morphospecies on the 2 gradients. Along the Oaxaca gradient, 1,678 individuals were measured and 260 species, 146 genera and 66 families were identified (Appendix). Along the Veracruz gradient, 2,551 trees were measured and 210 species, 124 genera and 63 families were identified (Appendix). The families represented by the highest number of individuals and species were Leguminosae (53 species), Fagaceae (22 species), Euphorbiaceae (21 species), Rubiaceae (21 species), Malvaceae (19 species), Burseraceae (11 species) and Pinaceae (11 species). The genera with the highest number of species were Quercus (21 species), Bursera (10 species) and Pinus (10 species) (Appendix).
Classification of 388 species into groups of gradient specialization by CLAM indicated that, from 31 shared species, only 12 presented a relatively similar abundance in both gradients for classification as generalist (Appendix). The CLAM identified 58 species as Oaxaca specialists, while 41 were identified as Veracruz specialists (Appendix). Classification of 212 genera showed that, from 58 shared genera, 19 were generalist; 43 genera were Oaxaca specialists (e.g., Amphyterygium, Arbutus, Jacquinia, Phenax, Poeppigia) whereas 24 genera were Veracruz specialists (e.g., Fagus, Hedyosmum, Liquidambar, Savia, Turpinia). The classification of 85 families indicated that 16 families were generalists; 22 were Oaxaca specialists and 14 were Veracruz specialists (Table 1).
Table 1 Families in the elevational gradients of Oaxaca and Veracruz, Mexico, classified as generalists to both gradients, Oaxaca specialists and Veracruz specialists according to the CLAM analysis. Values are number of individuals recorded along each gradient. Superscripts indicate biogeographical distribution of each family: 1, tropical; 2, temperate; 3, cosmopolitan.
| Generalist | Oaxaca Specialist | Veracruz Specialist | ||||||
| Family | Oax | Ver | Family | Oax | Ver | Family | Oax | Ver |
| Actinidiaceae3 | 12 | 28 | Anacardiaceae3 | 62 | 21 | Betulaceae2 | 26 | 127 |
| Burseraceae1 | 61 | 111 | Annonaceae1 | 20 | 6 | Celastraceae1 | 1 | 11 |
| Caricaceae1 | 5 | 15 | Apocynaceae1 | 31 | 13 | Chloranthaceae1 | 0 | 40 |
| Lauraceae1 | 11 | 30 | Araliaceae1 | 24 | 15 | Clethraceae3 | 13 | 60 |
| Malvaceae1 | 101 | 84 | Bignoniaceae1 | 30 | 20 | Convolvulaceae3 | 0 | 15 |
| Moraceae1 | 9 | 16 | Boraginaceae3 | 14 | 3 | Fagaceae2 | 48 | 392 |
| Myrsinaceae1 | 5 | 10 | Clusiaceae1 | 14 | 0 | Hamamelidaceae2 | 0 | 84 |
| Myrtaceae3 | 37 | 51 | Combretaceae1 | 14 | 0 | Melastomataceae1 | 0 | 19 |
| Nyctaginaceae1 | 9 | 2 | Ericaceae3 | 48 | 8 | Pinaceae2 | 267 | 632 |
| Polygonaceae3 | 8 | 12 | Euphorbiaceae3 | 124 | 101 | Sapindaceae1 | 1 | 16 |
| Rhamnaceae3 | 2 | 11 | Hernandiaceae1 | 17 | 4 | Staphyleaceae2 | 0 | 75 |
| Rosaceae2 | 10 | 15 | Julianiaceae1 | 14 | 0 | Styracaceae2 | 0 | 27 |
| Rubiaceae1 | 66 | 70 | Leguminosae3 | 207 | 134 | Symplocaceae1 | 0 | 11 |
| Rutaceae1 | 4 | 13 | Meliaceae1 | 33 | 6 | Theaceae1 | 0 | 26 |
| Ulmaceae3 | 9 | 7 | Myricaceae3 | 16 | 0 | |||
| Verbenaceae1 | 8 | 15 | Oleaceae3 | 17 | 0 | |||
| Proteaceae1 | 9 | 0 | ||||||
| Salicaceae3 | 33 | 6 | ||||||
| Sapotaceae1 | 18 | 0 | ||||||
| Simaroubaceae1 | 12 | 1 | ||||||
| Theophrastaceae1 | 13 | 0 | ||||||
| Urticaceae1 | 37 | 0 |
Basal area showed a unimodal pattern in Oaxaca and a monotonic pattern in Veracruz (Fig. 3a; Table 2). Density of trees showed inverse linear patterns on the studied gradients; in Oaxaca, density decreased with increasing elevation, while in Veracruz density increased with elevation (Fig. 3b; Table 2).
Overall, species, genus and family richness and Shannon diversity index tended to decrease with increasing elevation along Oaxaca and Veracruz (Fig. 3c-f; Table 2). Richness and diversity were higher in Oaxaca than in Veracruz; however, above 1,800-2,000 m elevation, these parameters were similar on both gradients (Fig. 3c-f).
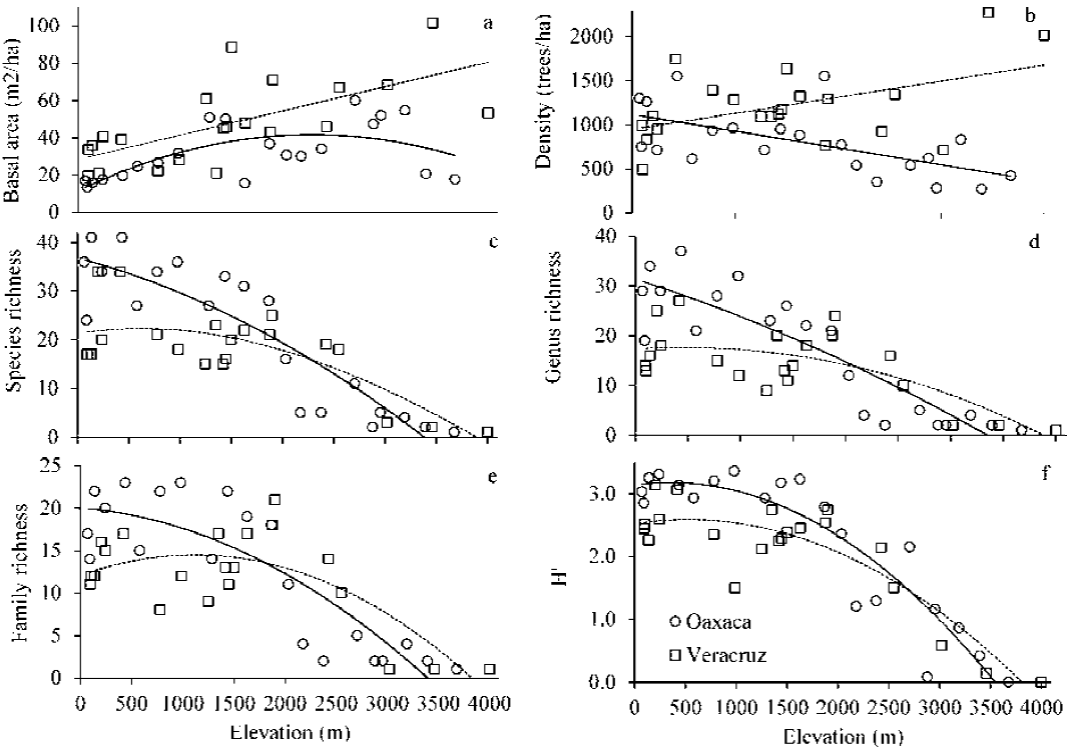
Figure 3 Fitted models of vegetation structure, richness and diversity changes along the elevation gradients of Oaxaca and Veracruz, Mexico. a) basal area, b) density, c) species richness, d) genus richness, e) family richness, and f) Shannon diversity. In each panel, circles are sites on Oaxaca, and squares are sites on Veracruz.
Table 2 Model fitting of species, genus and family richness, Shannon's diversity index, basal area and density in relation to elevation in Oaxaca and Veracruz, Mexico. Results shown are residual deviance, deviance explained (%), Χ2 and P. AICc is corrected Akaike information criterion and Ai is the AICc difference between the AICc of the best model and that of the model i. Note that models having AAICc within 2 of the best model have substantial support and receive consideration (Burnham & Anderson, 2002). Boldface indicates the best model. Model 1 is linear; Model 2 is quadratic, Model 3 is cubic. *p is < 0.05, ** is < 0.01, *** is < 0.001.
| Oaxaca | Veracruz | |||||||||
| Model | Residual deviance | Percentage deviance explained | X 2 | AICc | ∆i | Residual deviance | Percentage deviance explained | Χ 2 | AICc | ∆i |
| Species richness | ||||||||||
| 1 | 74.9 | 69.84 | 173.45*** | 173.2 | 41.1 | 61.61 | 38.76 | 38.99*** | 161.1 | 25.4 |
| 2 | 31.12 | 87.47 | 217.24*** | 132.1 | 0 | 36.57 | 63.65 | 64.03*** | 138.8 | 3.1 |
| 3 | 31.07 | 87.49 | 217.28*** | 135.2 | 3.1 | 30.32 | 69.86 | 70.27*** | 135.7 | 0 |
| Genus richness | ||||||||||
| 1 | 63.91 | 71.56 | 160.81*** | 156.7 | 33.2 | 55.72 | 36 | 31.34*** | 150.4 | 19.2 |
| 2 | 27.99 | 87.54 | 196.73*** | 123.5 | 0 | 37 | 57.5 | 50.06*** | 134.4 | 3.2 |
| 3 | 26.43 | 88.24 | 198.28*** | 125.1 | 1.6 | 30.67 | 64.77 | 56.40*** | 131.2 | 0 |
| Family richness | ||||||||||
| 1 | 50.89 | 62.92 | 86.36*** | 139.6 | 26.4 | 53.02 | 24.17 | 16.90*** | 143.5 | 27.8 |
| 2 | 21.75 | 84.15 | 115.50*** | 113.2 | 0 | 28.08 | 59.84 | 41.84*** | 121.3 | 5.6 |
| 3 | 20.62 | 84.98 | 116.63*** | 115.2 | 2 | 19.4 | 72.25 | 50.52*** | 115.7 | 0 |
| H' | ||||||||||
| 1 | 6.44 | 76.38 | 30.31*** | 42.2 | 7.3 | 5.45 | 64 | 21.45*** | 38.7 | 7.78 |
| 2 | 3.93 | 85.59 | 40.69*** | 34.9 | 0 | 3.25 | 78.53 | 32.32*** | 30.9 | 0 |
| 3 | 3.41 | 87.5 | 43.69*** | 35.4 | 0.5 | 3.25 | 78.53 | 32.32*** | 34.4 | 3.5 |
| Basal area | ||||||||||
| 1 | 3413.5 | 24.66 | 5.95* | 173.9 | 3 | 5508.1 | 44.19 | 12.25** | 183 | 0 |
| 2 | 2549.2 | 43.74 | 12.08** | 170.9 | 0 | 5348.6 | 45.81 | 12.86** | 186.4 | 3.4 |
| 3 | 2375.1 | 47.58 | 13.56** | 172.9 | 2 | 4965.2 | 49.69 | 14.43** | 188.4 | 5.4 |
| Density | ||||||||||
| 1 | 1762528 | 36.61 | 9.57** | 305.1 | 0 | 2805447 | 23.33 | 5.58* | 314.9 | 0.6 |
| 2 | 1735128 | 37.59 | 9.90** | 307.9 | 2.8 | 2655046 | 27.44 | 6.74* | 316.8 | 2.5 |
| 3 | 1728317 | 37.84 | 9.98* | 311.3 | 6.2 | 1997907 | 45.4 | 12.71** | 314.3 | 0 |
Ordination by CCA of the 42 sites for species, genus and family abundance was significant for the first axis (Monte Carlo test, F = 1.74, 2.52, 5.29, respectively, p = 0.002) and all canonical axes (Monte Carlo test, F = 1.56, 1.95, 2.66, respectively, p = 0.002), showing that the observed patterns differed from a random relationship. For species, the first 2 axes accounted for 12.9 and 12.4% of the cumulative variance, respectively (Fig. 4a). For genus, axis 1 and axis 2 accounted for 18.6% and 16.3%, respectively (Fig. 4b). For family, axis 1 and axis 2 described 30.3% and 24.6% of the cumulative variance, respectively (Fig. 4c). For species, genus and family, the first axis may be interpreted by temperature gradients whereas the second axis was related to precipitation and PET (Fig. 4). The retained significant variables in each CCA are shown in Table 3.
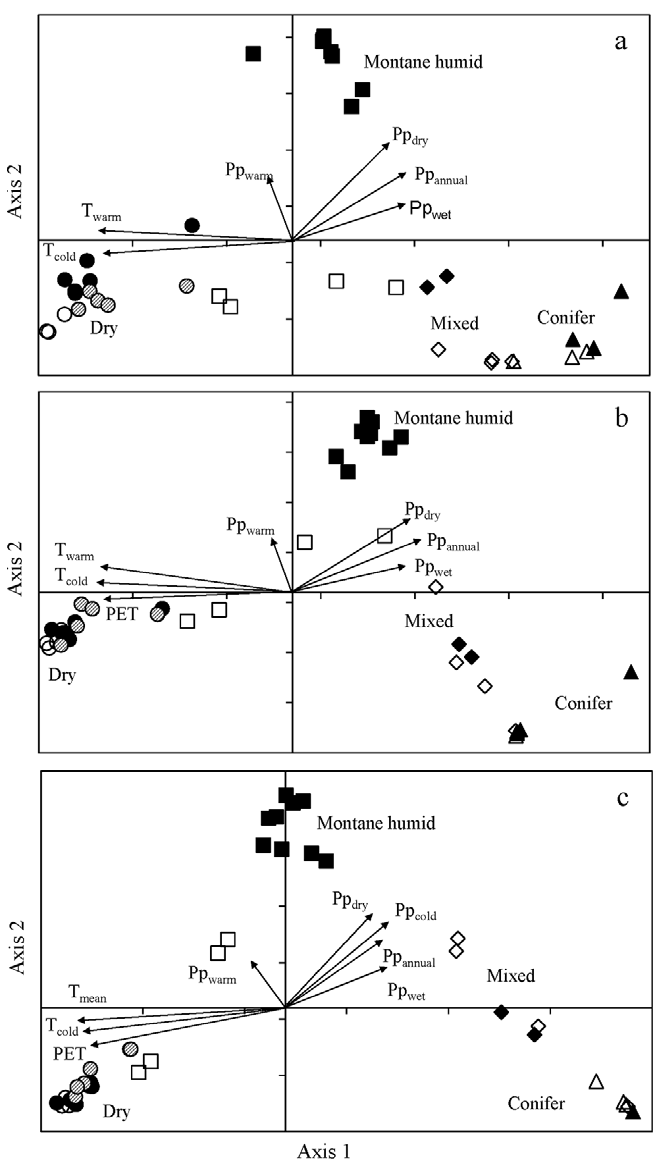
Figure 4 Canonical correspondence analysis biplots for 42 sites located along elevation gradients in Oaxaca (open and dashed symbols) and Veracruz (black symbols), Mexico. a) species, b) genus, and c) family abundance in sites along the 2 elevation gradients. In each panel, squares represent montane humid forests, circles are dry forest, diamonds are mixed forests, and triangles are conifer forests. Vectors are significant explanatory variables (Table 3), annual mean temperature (Tmean), temperature of warmest quarter (Twarm), temperature of coldest quarter (Tcold), annual precipitation (Ppannual), precipitation of wettest quarter (Ppwet), precipitation of driest quarter (Ppdry), precipitation of warmest quarter (Ppwarm), precipitation of coldest quarter (Ppcold), potential evapotranspiration (PET).
Table 3 Results of the forward selection procedure to choose climate explanatory variables for CCA analyses of species, genera and family along the elevation gradients of Oaxaca and Veracruz, Mexico. Boldface indicates significant p values.
| Species | Genus | Family | |||||||
| Variable | λΑ | F | p | λΑ | F | p | λΑ | F | p |
| Elevation | 0.65 | 1.41 | 0.058 | 0.33 | 1.37 | 0.128 | 0.06 | 0.63 | 0.900 |
| Annual mean temperature | 0.46 | 1.02 | 0.434 | 0.26 | 1.1 | 0.326 | 0.85 | 6.75 | 0.002 |
| Temperature of warmest quarter | 0.97 | 1.95 | 0.002 | 0.92 | 3.27 | 0.002 | 0.12 | 1.12 | 0.292 |
| Temperature of coldest quarter | 0.79 | 1.66 | 0.002 | 0.34 | 1.38 | 0.044 | 0.15 | 1.55 | 0.040 |
| Annual precipitation | 0.75 | 1.58 | 0.002 | 0.52 | 1.98 | 0.002 | 0.27 | 2.42 | 0.002 |
| Precipitation of wettest quarter | 0.63 | 1.39 | 0.016 | 0.46 | 1.8 | 0.006 | 0.24 | 2.21 | 0.002 |
| Precipitation of driest quarter | 0.93 | 1.89 | 0.002 | 0.72 | 2.65 | 0.002 | 0.55 | 4.74 | 0.002 |
| Precipitation of warmest quarter | 0.69 | 1.47 | 0.010 | 0.44 | 1.72 | 0.008 | 0.19 | 1.83 | 0.008 |
| Precipitation of coldest quarter | 0.51 | 1.14 | 0.288 | 0.35 | 1.43 | 0.102 | 0.17 | 1.68 | 0.048 |
| Potential evapotranspiration | 0.62 | 1.36 | 0.064 | 0.39 | 1.59 | 0.048 | 0.19 | 1.87 | 0.028 |
In general, the CCA for species, genus and family, consistently separated 3 groups of sites (Fig. 4). The biplots indicated that, on axis 1, pine-oak and coniferous forests in Oaxaca and Veracruz had positive scores while dry forest sites had negative scores. On axis 2, all montane cloud forests of Veracruz had positive scores; however, montane forest sites on Oaxaca gradient had negative scores for species and were separated from the Veracruz group.
Discussion
Differences in climate along the gradients of both, Oaxaca and Veracruz, are related to their geographic location and the meteorological phenomena affecting them (Espinosa et al., 2008). On Veracruz gradient, the frequent presence of mist is attributed to the dominant warm marine current of the Gulf of Mexico, whereas the Oaxaca gradient on the Pacific side is influenced by dry wind and cold marine currents (Espinosa et al., 2008). Moreover, from November to March, cold northerly winds across the Gulf of Mexico, contribute to the lower temperatures reported for the Veracruz gradient, and bring rains and fog during the relative dry season (Holwerda et al., 2010). PET plays an important role in determining community types since same amount of rainfall manifests different in warm than in cold environments. PET values clearly indicated that Oaxaca is drier than Veracruz, but only below 2,000 m elevation.
The CCA results evidenced the relationship between vegetation and climate on these 2 gradients. Several authors have emphasized the importance of climate, not only in large-scale patterns, but also at the local level (Francis & Currie, 2003; Hawkins et al., 2003). The groups of forest types were differentially related to precipitation or temperature variables (Toledo-Garibaldi & Williams-Linera, 2014). On the Oaxaca gradient, temperature was the most important environmental factor (Salas-Morales et al., 2015). In Oaxaca, groups of sites were related to low and high temperatures, separating tropical from temperate vegetation (Salas-Morales & Meave, 2012). Temperature is a variable that is highly associated with altitude and with elevation patterns in floristic and vegetation variation (Grubb, 1977; Sang, 2009). On the Veracruz gradient, 3 groups of sites were distinguished: lowland dry forests and highland temperate forests related to high and low temperatures, respectively, and montane cloud forests related to humidity. These forests are found in particular sites on the mountains of Mexico, in an elevation belt where there is a frequent influence of fog (Holwerda et al., 2010).
Forests on the Oaxaca gradient display lower basal area and density of trees than on Veracruz. Differences become complicated because along the whole gradients, BA and density tend to increase in Veracruz whereas in Oaxaca tend to decrease with elevation. Differences are greater in vegetation structure in the temperate forests of higher elevations, and may be related to humidity, since during the warmest, coldest, and driest quarters, the Veracruz gradient receives twice the precipitation in the highest-altitude sites than Oaxaca. Different patterns of vegetation structure along gradients have been observed in a number of studies, and generality is not expected when comparing tropical elevation transects (Clark et al., 2015).
For example, on Mount Kinabalu in Borneo, stem density increased with elevation, and basal area in non-ultrabasic soils increased monotonically, while in the ultrabasic soils presented a unimodal pattern (Aiba & Kitayama, 1999). However, on an elevation gradient on the Barba Volcano in Costa Rica, there were 2 peaks in tree density, at 400 m and 2,800 m elevation, while the basal area varied little along the gradient and was the highest in the 2,800 m plot (Clark et al., 2015).
Variation in elevation patterns is not limited to vegetation structure. Elevation gradients display variation in richness, diversity and taxa composition. For both gradients, richness and diversity decrease with increasing elevation; however, the patterns differ from each other. On the Oaxaca gradient above 1,800 - 2,000 m occurs a rapid decreasing trend in species, genera and family richness and diversity related to the low tolerance of some tropical taxa to relatively cold temperatures (Salas-Morales et al., 2015). While in Oaxaca the elevation pattern seems to be related to a critical elevation, the richness pattern in Veracruz decreased smoothly related to the mixture of temperate and tropical taxa and more humid conditions (Challenger & Soberón, 2008). Likewise, in Eastern Asia forests with tropical and temperate genera are found in similar elevation gradients (Li & Feng, 2015; Liao et al., 2014). Li and Feng (2015) reported that tropical genera are found below and temperate genera above mid-point in Nepal.
Oaxaca was more diverse (260 species) than Veracruz (210 species), which was contrary to expected given that the Veracruz gradient is more humid. In the Neotropics, plant species richness is strongly correlated with total annual precipitation (Gentry, 1988), but the most diverse dry forest are not the wettest ones, but rather the western Mexico dry forests (Gentry, 1995). Thus, the dry forest is more diverse in the Pacific coast than in the Gulf of Mexico, contributing greatly to the high diversity of Oaxaca.
The family with the highest number of individuals for both, Oaxaca and Veracruz, was Pinaceae. In elevations above 2,200 and 2,450 m on the Oaxaca and Veracruz gradients, respectively, the forests are dominated by the genus Pinus, which shares dominance with Quercus in these elevation belts. This result is consistent throughout the mountains of Mexico where forests are dominated by pine-oak and pine forests, and although Mexico has more than 150 species of oaks and more than 40 species of pines (Gernandt & Pérez-de la Rosa, 2014; Valencia, 2004), in each particular site they were represented by a few species (Challenger & Soberón, 2008). Gentry (1988) indicated that Neotropical plant communities are together in nonrandom ways. In Oaxaca and Veracruz, families classified by CLAM as generalists (e.g., Lauraceae, Rubiaceae) were the families that contributed most to species richness in the Neotropics according to Gentry (1988). Oaxaca specialist families identified by CLAM were mainly of tropical affinity (e.g., Euphorbiaceae, Leguminosae), whereas in Veracruz, several of the indicator families were of temperate affinity (e.g., Betulaceae, Fagaceae, Pinaceae; Table 1).
It is likely that difference in temperature and precipitation was not the only factor to affect forest development and composition. Other variables, such as soil characteristics and land use history or legacy play a role, and they are alternative explanations as has been shown in previous studies (Aiba & Kitayama, 1999; Arévalo et al., 2010; Da et al., 2009; Kitayama & Aiba, 2002; Piperno, 2006). Fragmentation and worldwide forest disappearance are mostly due to a long history of human activities (Da et al., 2009; González-Abraham et al., 2015; Piperno, 2006). Veracruz has a long history of land use before and after the arrival of the Spaniards, and the center of Veracruz was intensively used and deforested, since this gradient is located along a major route to Mexico City (González-Abraham et al., 2015). In contrast, up until 50 years ago, the Oaxaca gradient lacked paved roads and the human impact on the vegetation is therefore more recent and confined to lower altitudes (Salas-Morales & Meave, 2012).
Our results support the hypothesis that climate is one of the main underlying factors related to differential patterns in vegetation structure and taxa distribution along elevation gradients. However, climate influence depends on other local factors such as mountain range location, physiography, slope, and disturbance. The results strongly indicate differential influence of climate, since humidity is apparently an important environmental factor for the vegetation of the Gulf of Mexico, while temperature is the determining factor on the Pacific coast. The Oaxaca gradient displayed higher taxa richness than Veracruz gradient, particularly in the lower elevations. In both gradients richness decreases with increased elevation, but in Veracruz there is a smooth transition from tropical to temperate vegetation whereas in Oaxaca richness at mid-elevation shows an abrupt decreased related to temperature.











 nueva página del texto (beta)
nueva página del texto (beta)


