Introduction
The polyphasic approach has allowed a better assessment of cyanobacteria diversity and has been especially useful to discriminate between cryptotaxa, which are almost impossible to differentiate morphologically (Komárek, 2003, 2016). An example of the above is the genus Lyngbya which exhibits cytological (ultrastructural) and ecological variations, high morphological plasticity, and in some cases, lack of precision of the 16S rRNA gene at a specific level (Engene et al., 2010). Accordingly, Komárek et al. (2013) reviewed the Lyngbya species with planktic habitat and separated them into the genus Limnoraphis. This genus currently comprises 4 species: Limnoraphis hieronymusii (Lemmermann), L. birgei (G.M.Smith), L. cryptovaginata (Škorbatov), and L. robusta (Pakutty) (Komárek et al., 2013).
The genus Limnoraphis has 4 known species, of which only L. robusta produces blooms in freshwater bodies; these have been reported in Guatemala (Komárek et al., 2013), Peru (Komárková et al., 2016; Montoya et al., 2014), Cuba (Comas-González et al., 2017) and USA (Kurobe et al., 2013). It is worth noting that L. robusta blooms, both in Atitlán Lake, Guatemala, and Clear Lake, California, were accompanied by Microcystis aeruginosa (Kützing) Kützing 1846.
Cyanobacteria blooms are considered biological threats because their mass production often causes anoxia, but mainly because they can synthesize a wide variety of secondary metabolites known as cyanotoxins (Deblois & Juneau, 2010; Komárková et al., 2016). The presence of cyanotoxins is especially dangerous when the water body is used as a source of drinking water (Kuiper-Goodman et al., 1999).
Once a cyanobacteria bloom is detected, the main element for assessing the potential risk associated with the presence of cyanotoxins is the correct identification and quantification of cyanobacteria species (Lawton et al., 1999). However, cyanobacteria are organisms with very successful biological and ecological life strategies, with repeated rapid adaptation to various environmental conditions (Komárek, 2016), and so, many morphological characteristics used for identification have variations influenced by environmental conditions (Stoyanov et al., 2014; Wilmotte, 1994), leading to difficulties and confusion in the identification process. The way to address this problem is to combine morphological, ecological, and molecular traits (Komárek, 2003).
In Mexico, Cyanobacteria is the second most diverse group of freshwater phytoplankton, and blooms of these organisms, associated with eutrophic water bodies, are relatively common but poorly studied (Oliva-Martínez et al., 2014). According to Pérez-Morales et al. (2016), the main bloom-forming cyanobacteria are species of Microcystis, Cylindrospermopsis, Anabaena, Anabaenopsis, Nodularia, Phormidium, Planktothrix and Pseudoanabaena. In contrast, blooms of any Lyngbya or related species are uncommon, with the only report being in 2005 for Valle de Bravo, which is a reservoir used for recreational activities and water supply for Mexico City. This bloom was caused by an unidentified Lyngyba species along with Microcystis incerta and Anabaena (Dolichospermum) tenericaule (Nygaard) (Zapomelová et al., 2012), and was so intense that it forced Mexican authorities to close the reservoir to all water activities (Mercado-Borrayo, 2007).
Santa María del Oro crater lake is located in the State of Nayarit. It is an important place for tourism that attracts thousands of visitors who perform ecotourism and recreational activities. During 2015, the presence of a possible microcystin-producing cyanobacteria bloom represented a risk for lake users; thus, arose the need to identify the species that generated the bloom. Hence, this work aims to identify the bloom-forming cyanobacteria species with the utmost precision using a polyphasic approach.
Materials and methods
Santa María del Oro crater lake, is a subtropical water body located southeast from the City of Tepic, in Nayarit, Mexico, (21°23’ N, 104°35’ W; 750 m asl) (Fig. 1). The lake is classified as warm monomictic, stratified for 7 months with anoxic hypolimnion (May to December) and complete mixing between January and March (Vázquez-Castro et al., 2008). It has a surface area of about 3.7 km2 and a maximum depth of 65 m. The annual average precipitation in the region is 1,214 mm year-1 and a pH mean of 8.8 (Vázquez & Caballero, 2013).
During 2015, we carried out monthly phytoplankton trawls with a 60 μm mesh net. One part of the sample was used for molecular analysis, and the other fixed with 4% formalin for morphological identification.
Observations were made with a phase-contrast microscope (Carl Zeiss Axioscope A1) and photographed with a digital camera (EOS Canon 6D); measurements were done with Adobe Photoshop CS6 (ver. 13.0.1.1). Observations were made immediately after collection, otherwise, during the next following days. Measurements and morphological traits were taken from the first 30 colonies. Diagnostic characteristics of the taxa were identified and enlisted using the taxonomic criteria of Komárek and Anagnostidis (1999, 2005), Komárek et al. (2013) and Comas-González et al. (2017).
Due to the extremely high density of the trawl samples, they were diluted with lake filtered water. After dilution, we used a stereomicroscope (Leica Zoom 2000) and natural bristles to isolate cyanobacteria filaments and coccoid colonies. Isolated colonies and filaments were washed in drops of filtered water to eliminate other organisms that may be present. The process was repeated until reaching ~50 mg of biomass. The biomass obtained in this way was stored in 1 ml tubes, and frozen at -80 °C until processed for molecular analyses.
DNA was extracted by the CTAB method (Allers & Lichten, 2000; Falcón & Valera, 2007). To improve cell lysis, due to a large amount of mucilage in samples, 1 mm diameter glass beads were used. For cell debris extraction a chloroform: octanol (24:1) solution was used.
The partial 16S rRNA gene region was amplified with cyanobacterial specific primers CYA106F and CYA781 (Nübel et al., 1997). The final volume of the PCR mixture was 50 μL and contained: 25 μL RedTaq Ready Mix PCR Reaction Mix [Sigma-Aldrich; 3 mM MgCl2, 20 mM Tris-HCl, pH 8.3, 100 mM KCl, 0.002% gelatin, 0.4 mM dNTP mix (dGTP, dCTP, dATP, dTTP), and 0.06 unit/μL of Taq DNA Polymerase], 1 μmol of each primer and 2 μL of template DNA. The amplification was carried out with the following setup: a cycle of 5 min at 94 °C; 35 cycles of 1 min at 94 °C, 1 min at 50 °C, 1 min at 72 °C and 5 min at 72 °C of final extension. Purification and sequencing were done by Macrogen (Seoul, Republic of Korea). The 16S rRNA partial sequences were manually edited to avoid reading errors, with the software BioEdit v.7.2.6 (Hall, 1999). Obtained sequences were compared with those in the GenBank NCBI (National Center for Biotechnology Information) using the Basic Local Alignment Search Tool (BLAST). Sequence alignment was performed with Clustal Omega 1.2.4 software (Sievers et al., 2011; Sievers & Higgins, 2018).
The analyses were performed based on maximum likelihood (ML) (Saitou & Nei, 1987) available in MEGA v7 software (Kumar et al., 2016). For ML, the evolutionary substitution model K2 + G was solved as the best one found using the MEGA v7 algorithms. The parameters (base frequencies, rate matrix of substitution types and shape of gamma distribution) were estimated from the data. One thousand bootstrap replicates were performed for ML analyses. Nucleotide sequences of the studied strains were deposited in GenBank under the accession numbers MH094657 (Limnoraphis robusta SAMAO-1), MH382818 (L. robusta SAMAO-2), and MH094658 (Microcystis aeruginosa SAMAO-3).
Results
The bloom lasted from January to April 2015, with a peak during March and April (Figs. 2, 3). Visual inspection of surface water revealed a brownish colour and uneven distribution of cyanobacteria, with macroscopic scums mainly on the shore (Fig. 4) and clusters in the center (Fig. 5).

Figure 2-5 Cyanobacterial bloom in Santa María del Oro crater lake. 2, Panoramic view of the crater lake showing the uneven distribution of the cyanobacterial bloom; 3, swimming competition during March 2015; 4, cyanobacteria scum at the shores of the crater lake; 5, clusters of cyanobacteria observed in the centre of the crater lake.
Microscopic observations of scums and clusters yielded 2 main cyanobacterial populations: one colonial coccoid form and one filamentous homocytous form. The colonial coccoid form was part of the planktonic ecosystem throughout the year. Colonies were macroscopic and mucilaginous with irregular or discoid form (Fig. 6). Cells were spherical, 3.4 to 7.9 µm (mean of 5.5 ± 0.8 µm) in diameter with many aereotopes, after division cells were hemispherical. Colour ranged between blue-green to pale green. These morphological traits correspond to those of Microcystis aeruginosa.
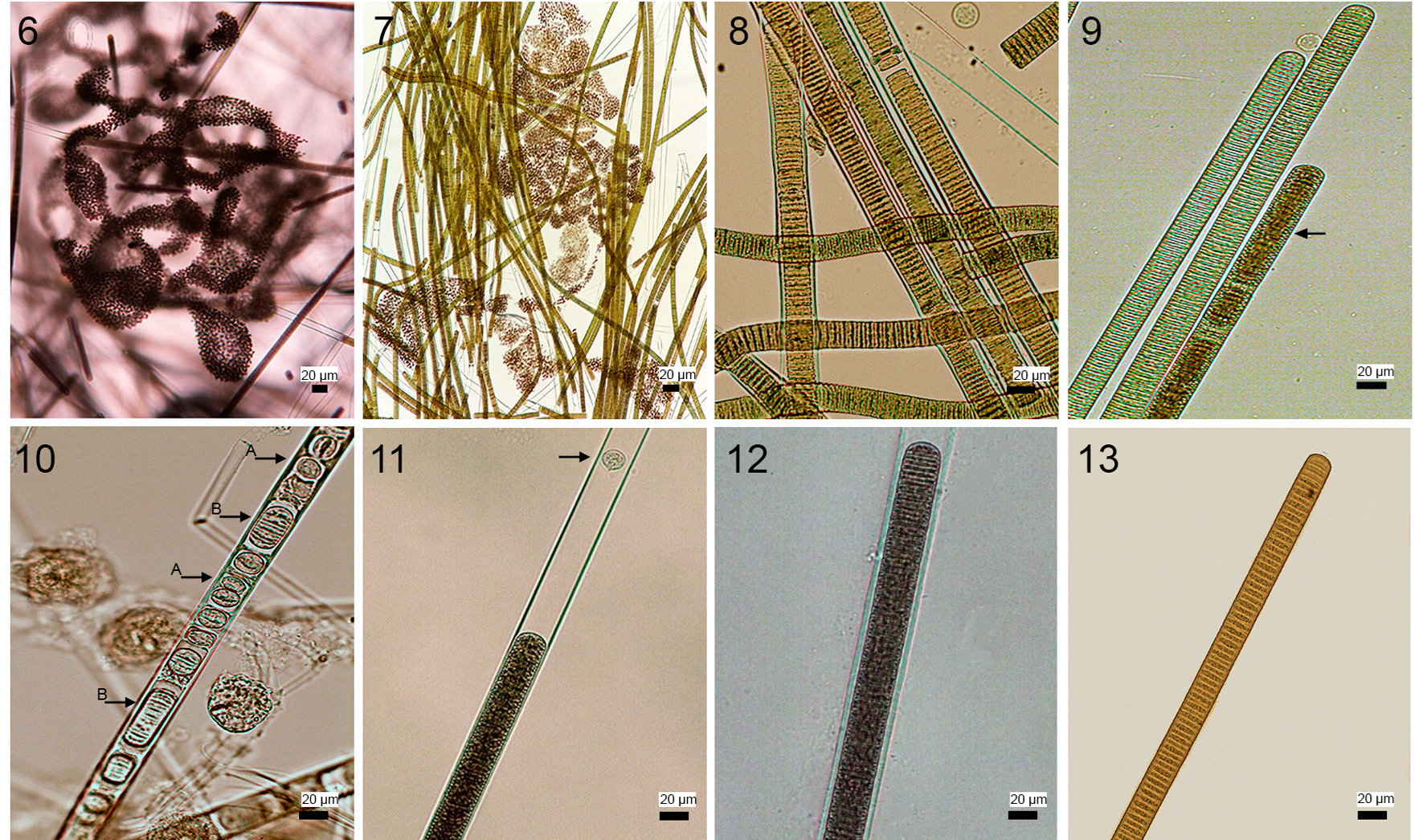
Figure 6-13 Microscope micrographs of the bloom-forming species in Santa María del Oro Crater lake. 6, Microcystis aeruginosa colony; 7, microscopic view of scums and clusters showing L. robusta and M. aeruginosa intermingled; 8, Limnoraphis robusta filaments; 9, aerotopes not distributed regularly along the trichome (arrow); 10, single cells (arrow a) and hormogonia with variable cell number (arrow b); 11, sheath surpassing trichome; 12-13, differences in filament and trichome width after the bloom (12, November) and during the most intense part of the bloom (13, April), note difference in the width of trichome and sheath. Scale bar 20 μm.
The filamentous individuals formed scums and irregular clusters intermingled with Microcystis aeruginosa (Fig. 7). Filaments were straight or slightly curved (Fig. 8), length ranged between 88.5 and 5,032.1 μm (mean of 912.7 ± 727.4 μm); width varied from 7.5 to 23.4 μm (mean of 16.8 ± 2.9 μm). Trichomes were straight, cylindrical and not constricted at cross-walls; width varied between 6.3 and 19.8 μm with a mean of 14.3 ± 3.2 μm; apical cells were widely rounded, without calyptra. Aerotopes were not regularly distributed along the whole trichome (Fig. 9). Hormogonia varied in length and cell numbers (2-12), also we observed monocytes (Fig. 10). Sheaths were firm, colorless, sometimes widened, surpassing the trichomes in form of a firm tube (Figs. 11, 12). The above morphological traits correspond to Limnoraphis robusta, however, morphometric characteristics show important variations over the year (Figs. 12, 13). Table 1 shows that during the bloom (February, March, and April), filament length and width and trichome width decreased considerably; then after the bloom, all morphometric characters stabilized. In April, we observed year minimum values for the filament mean length (377.1 ± 240.2 μm), width (9.9 ± 1.1 μm), and trichome width (8.2 ± 0.9 μm).
Table 1 Monthly range, mean and standard deviation values for Limnoraphis robusta filament length, width and trichome width measured in Santa María del Oro crater lake during 2015.
| Month | N | Filament length min-max (μm) | Filament mean length (μm) | Filament width min-max (μm) | Filament mean length (μm) | Trichome width min-max (μm) | Trichome mean length (μm) |
| January | 30 | 167 - 995 | 626 ± 255 | 16-20 | 17.8 ± 1.3 | 14- 18 | 16.2 ± 1.3 |
| February | 30 | 93 - 880 | 323 ± 243.6 | 9-23 | 16.6 ± 4.1 | 9-18 | 15.6 ± 1.3 |
| March | 30 | 89 - 1,608 | 472.2 ± 354 | 8-12 | 10.7 ± 4 | 7 - 9 | 8.3 ± 0.5 |
| April | 30 | 86 - 811 | 377.1 ± 240.2 | 7-20 | 9.9 ± 1.1 | 6 - 9 | 8.2 ± 0.9 |
| May | 30 | 145 - 3,069 | 690.2 ± 559.9 | 16-20 | 18.2 ± 1.4 | 12 - 20 | 16.4 ± 2.5 |
| June | 30 | 131 - 1,483 | 566.1 ± 326.3 | 16-21 | 17.9 ± 1.4 | 15 - 20 | 16.9 ± 1.6 |
| July | 30 | 236 - 3,013 | 830.8± 538.6 | 15-22 | 17.1 ± 1.5 | 13 - 18 | 14.7 ± 1.8 |
| August | 30 | 182 - 3,069 | 1,026.9 ± 854.9 | 15-19 | 16.2 ± 0.9 | 14 - 18 | 14.8 ± 1.5 |
| September | 30 | 150 - 2,692 | 931.9 ± 664.7 | 16-23 | 19.8 ± 1.5 | 15 - 18 | 16.5 ± 1.2 |
| October | 30 | 131 - 1,855 | 800.7 ± 398.1 | 15-19 | 16.6 ± 0.8 | 13 - 17 | 15.1 ± 1.1 |
| November | 30 | 475 - 3,679 | 1,409.8 ± 825.2 | 14-23 | 17.7 ± 2.3 | 13 - 18 | 15.5 ± 1.5 |
| December | 30 | 236 - 5,032 | 1,427.2 ± 1,044.9 | 15-22 | 17.1 ± 1.5 | 15 - 18 | 15.7 ± 0.9 |
Sequences of ~ 640 bp were obtained from the morphological isolates of Limnoraphis robusta and Microcystis aeruginosa. For L. robusta SAMAO-1 and SAMAO-2, a 100% BLAST sequence identity was obtained with L. robusta (JX006093), while for M. aeruginosa SAMAO-3, BLAST identity was 99% with M. aeruginosa (KF287004.1). The phylogenetic analysis indicated that Limnoraphis from Santa María del Oro formed a monophyletic clade, with 99% of support, along with other species of the genus (Fig. 14). In contrast, Microcystis formed a clade with 98% bootstrap support; while M. aeruginosa formed a defined group with 71% bootstrap support (Fig. 15).
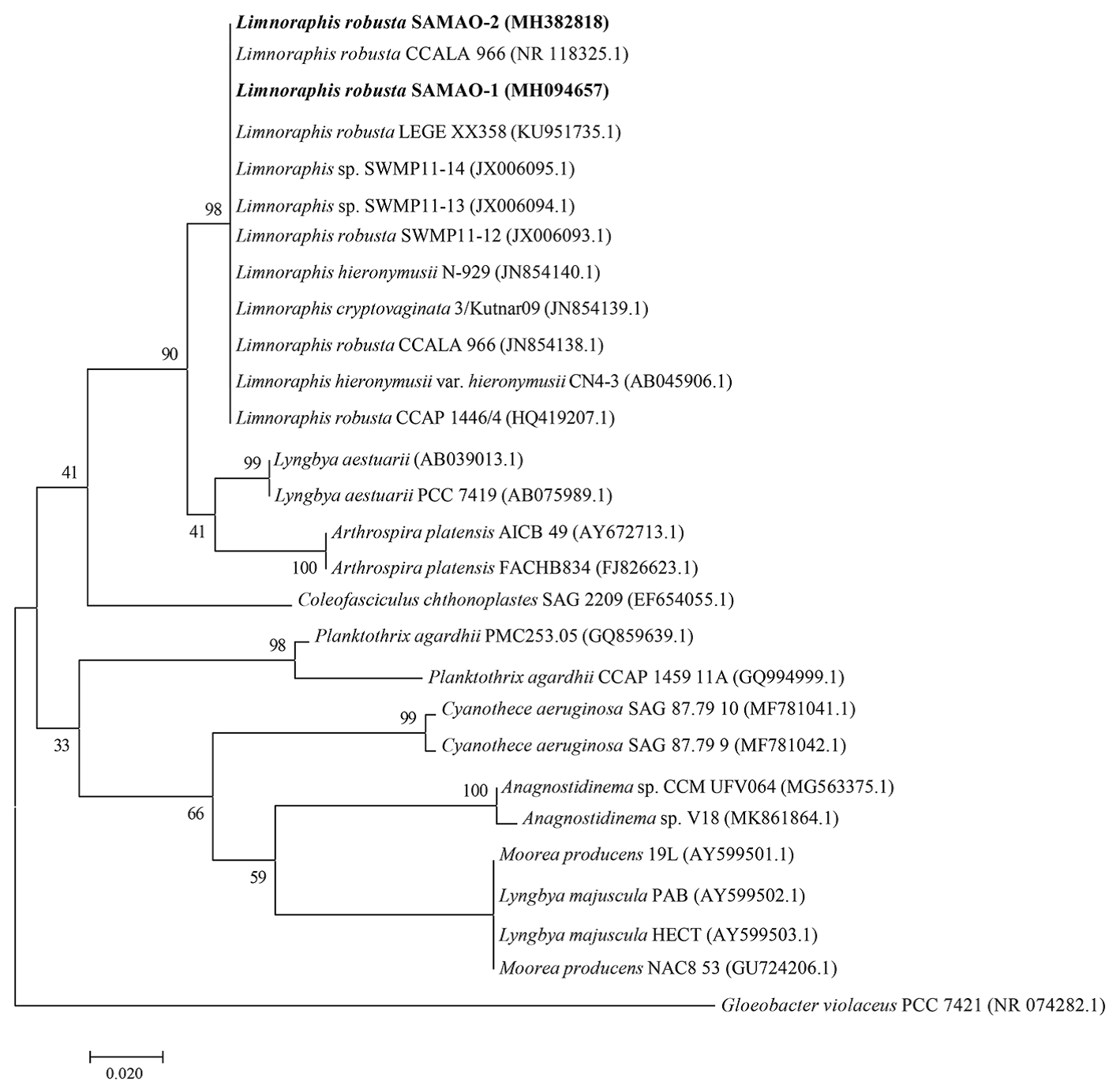
Figure 14 Maximum likelihood tree based on 16S rRNA sequences (640 and 643 pb; SAMAO-1 and -2 respectively) from Santa María del Oro morphological isolates of Limnoraphis (indicated in bold). Numbers near nodes indicate bootstrap value (percentages of 1,000 replications).
Discussion
Besides being the first record of Limnoraphis robusta for Mexico, it is also the first record of a bloom of this species in a Mexican freshwater body. Hence, this study widens the biogeographic distribution of L. robusta and the bloom records for this species. It is also the first record for Nayarit of Microcystis aeruginosa, which is the main bloom-forming species in Mexican freshwater bodies (Pérez-Morales et al., 2016).
Despite the lack of specificity of phylogenetic analysis at the species level, morphological traits of the filamentous forms were those reported for L. robusta by Komárek et al. (2013). Regarding ecological data, in Santa María del Oro crater lake, Ochoa-Zamora (2018) observed that L. robusta was the dominant species whose density peak was on February, March, and April. Meanwhile, Salas-Betancourt (2018) estimated a mean temperature of 24.5 °C during the bloom, after it, rose to 26 °C in May and June, reaching its peak of 30 °C in August and September. On its side, Caballero et al. (2013) and Salas-Betancourt (2018) found that the trophic state of Santa María del Oro crater was mesotrophic, with elevated concentrations of phosphorus. Thus, based on our morphological measurements and ecological data of the study site, the identity of L. robusta is confirmed. Our result also supports Komárek et al. (2013) hypothesis that L. robusta blooms develop in oligo-to mesotrophic water bodies with increased phosphorus content.
Species of the genus Limnoraphis are not common in Mexico and most of the records are for Limnoraphis hieronymusii. Montejano et al. (2005) reported it for central Mexico, which comprises Balsas, Pánuco and Papaloapan rivers, although the authors did not specify a site. Novelo (2011) reported it for San Lorenzo spring (Tehuacán) and San Bernardino Lagunas and Laguna Mayor (Vicente Guerrero), Puebla. Finally, Nava-Ruiz and Valadez-Cruz (2012) and Valadez et al. (2013) reported it for Lagartos Lagoon, Quintana Roo. For Limnoraphis birgei, the only record was by Gaytán-Herrera et al. (2011) for the Valle de Bravo reservoir, Estado de Mexico; where L. birgei was one of the dominant species.
Cyanobacteria morphological variations due to environmental conditions are long known (Wilmotte, 1994); thus, trichome and filament width variations observed on Limnoraphis robusta fall within the expectations. According to Anagnostidis and Komárek (1988), cell dimensions are relatively stable within certain limits and, therefore, are useful for species-level distinctions; however, as our data clearly show, even cell size had wide variations in the same site under different natural conditions. In particular, cell width variations are influenced by environmental conditions. For example, Krüger et al. (1981) reported that Microcystis aeruginosa had larger cells under stress conditions, mainly caused by light intensity and changes in the state of the growth media. Tavera et al. (1994) found that temperature is the most important factor concerning morphological changes in some Phormidium strains (Oscillatoriales); they observed that above 30 °C cells in trichomes started to appear wider than average. Thus, considering the temperature variations (24-30 °C) observed in Santa María del Oro crater lake by Salas-Betancourt (2018), we can hypothesize that temperature could be the driving forces that produce cell width modifications in L. robusta. Therefore, the following questions remain: Is temperature the main driver of morphological changes? Which temperature range represents stress conditions for L. robusta, during the bloom or after it?
Recognition of some cyanobacteria species is not always possible with the 16s rRNA gene sequencing (Komárek, 2010); also, wrong strain identification is a common issue found in cyanobacteria phylogenetic analysis (Komárek, 2018). According to Engene et al. (2010), the above is related with at least 2 reasons: a) the short length of the gene sequences available in GenBank (minimally 600 bp), thus affecting the reliability of the analysis, and b) that cyanobacteria could contain multiple and variable copies of their 16S rRNA genes. Based on the above, our Limnoraphis robusta phylogeny results were not surprising; moreover, Komárek et al. (2013) and Kurobe et al. (2013) found similar results for their Limnoraphis strains. Meanwhile, Kurobe et al. (2013) found that their strains SWMP11-13 (JX006094.1) and -14 (JX006095.1) were distinct from any other sequence of L. robusta or L. cryptovaginata and tentatively named it as Limnoraphis sp. The above was unsupported by the present study since our results show that those strains form a single cluster along with all other species of the genus; nevertheless, a greater number of longer sequences are needed to perform more robust phylogenetic analysis.
For Microcystis, the outlook is almost the same. There are several efforts to disentangle the subgeneric relationships among the members of the genus, for example Kim et al. (2002), Komárek (2018), Neilan et al. (1997) and Otsuka et al. (2001); however, all attempts made with partial sequences of the 16S rRNA have not been capable of elucidating it. For example, Engene et al. (2012) found that the genus Moorea has relatively high levels of intra-genomic gene heterogeneity; that in combination with low subgeneric sequence divergence, makes the 16S rRNA gene inadequate for distinguishing species in some cyanobacteria genera.
Our findings showed that it is relevant to know cell size variation limits on natural cyanobacteria populations under different environmental conditions since, in most studies, the identification relies on morphology. So, even though cyanobacteria cultures are a common resource to make species description, it should be considered that descriptions based on enriched cultures alone may not indicate the natural limits of variation for in situ populations (Baker, 2007). Komárek et al. (2013) found that Limnoraphis robusta aerotopes are mostly reduced and mucilaginous sheaths are distinctly thinner and less developed in comparison with natural material. As stated by Krüger et al. (1981), the validity of cell size as a taxonomic character should be questioned unless environmental conditions are considered carefully; especially because it is known that modifications of the morphology in culture are directed by the selected culture conditions (Komárek & Anagnostidis, 1989; Tavera et al., 1994).
Based on morphological, ecological and molecular data, Limnoraphis robusta and Microcystis aeruginosa were identified as the species that formed the cyanobacterial bloom in the Santa María del Oro crater lake, Nayarit. Also, is the first bloom in Mexico with these 2 species associated.











 nueva página del texto (beta)
nueva página del texto (beta)




