Introduction
The ants (Hymenoptera: Formicidae) are among the most diverse insect groups and are dominant arthropods in most terrestrial ecosystems, interacting direct and indirectly with various animal and plant species (Silva et al., 2017). Ants occupy different microhabitats including the subsoil, soil, litter, trunks and canopy of different plant species, where they can act as defoliators, predators of arthropods and small vertebrates, and participate in mutualistic interactions with some plants and other animal species (Davidson et al., 2003; Fagundes et al., 2005; Ribas et al., 2003). Therefore, ants play important ecological functions such as structuring natural communities, stabilizing food webs and maintenance of terrestrial ecosystem services (Chen et al., 2016; Dyer & Letourneau, 1999). In addition, ants are very sensitive to environmental changes, being suitable bioindicators of habitat quality to evaluate how natural or anthropogenic disturbances affect ecosystem services (Prado-Junior et al., 2020; Queiroz et al., 2017).
Some studies suggest that ant communities can be structured by environmental factors and by interspecific interactions (Fagundes et al., 2005; Shoereder et al., 2004; Silva et al., 2017; Vargas et al., 2007). Vegetation traits, such as plant size, canopy cover and abundance and richness of plant species have been used as environmental heterogeneity measures to explain ant diversity (Fonseca & Benson, 2003; Queiroz et al., 2017; Silva et al., 2017). In general, more heterogeneous habitats have greater diversity of ants because they have more microhabitats for nesting and greater availability of food resources (Davidson et al., 2003; Fonseca & Benson, 2003; Souza & Araújo, 2020). Positive relationship between ant species diversity and habitat heterogeneity seems to be a common phenomenon, but some studies also have shown negative or absence of a relationship between habitat heterogeneity and ant species diversity (Lassau & Hochuli, 2004; Ribbons, 2014). Therefore, it is possible to expect a negative relationship between habitat heterogeneity and ant diversity in habitats with less structural complexity, dominated by small and sparse plants, with low canopy connectivity among canopies (Queiroz & Ribas, 2016).
Interspecific interactions can also affect the structure of ant communities (Fagundes et al., 2005; Schoereder et al., 2004). Interspecific interactions among ant species can vary from negative, neutral to positive. In some cases, the diversity of interactions associated to high local ant richness can generate a structure in the mosaic of ant communities (Ribas & Schoereder, 2002). However, interactions involving ant species are malleable and may experience spatial and temporal changes even in small timescales in response to variations in abiotic and biotic factors (Thompson, 1999, 2012). In addition, other studies also suggest that habitat complexity and interspecific interactions may act synergistically to shape ant communities (Lassau & Hochuli, 2004). In fact, some studies suggest that the low availability of resources should intensify interspecific competition while greater resource availability allows the coexistence of several ant species (Fagundes, Santos et al., 2020; Ribas et al., 2003). However, some authors have questioned the role of deterministic events in the organization of ant communities (Floren et al., 2001).
The Cerrado (Brazilian savanna) is the second largest biome in Brazil covering 21% of the Brazilian territory, and it is considered one of the main hotspots of the biodiversity in the world (Colli et al., 2000). The Cerrado is better described as a mosaic of plant formations that include grasslands, shrublands, typical savannas, and woodland savannas, differing in grass cover, canopy cover, plant species composition as well as fire dynamics, and availability of nutrients and water in the soil (Fagundes, Cuevas-Reyes et al., 2020; Oliveira-Filho & Ratter, 2002). This biome presents a high diversity of ants, including endemic and rare species (Lopes & Vasconcelos, 2008). However, it is still necessary to characterize the role of biotic and abiotic factors in the organization of ant communities to understand the patterns of distribution and abundance of these important arthropods in Cerrado. The objective of this study was to evaluate the role of habitat heterogeneity and interspecific interactions in the organization of the ant community, associated with shrubs of a regenerating cerrado area by testing 2 hypotheses: 1) the vegetation structure negatively affects the diversity of ants in simpler habitats (Queiroz & Ribas, 2016), since open areas generally present a decrease in the number of ant species sensitive to more stressed environmental conditions, and 2) interspecific interactions shape the organization of ant communities in simpler environments (Ribas et al. 2003), since resources available such as food and sites for nesting are more limited in simpler environments (Fagundes et al., 2021).
Materials and methods
The study was carried out in a protected area (-17.215, -44.414 UTM) of approximately 98 hectares located in Jequitai Municipality, northern region of Minas Gerais state, Brazil. The study area was used for cattle raising until 2008. After this period, this area was purchased by Rio Jequitaí Consorce (Jequitaí Project) and transformed into a protected area. The region is included in the transition between Cerrado and Caatinga domains in the central region of Brazil, presenting semi-arid climate with well-defined dry and rainy seasons. The average annual temperature is 23 °C, and rainfall is 1,000 mm/year, with rainfall concentrated mainly in the months of November to January (Kuchenbecker & Fagundes, 2018). Specifically, the dominant vegetation in the study area is a regenerating “sensu stricto” Cerrado with sparse and low-sized plants.
A total of 16 plots of 100 m2 (10 × 10m), distant from each other at least 50 m, were randomly delimited in the study area during January to February 2016. In each plot, all plants with CBH (circumference at breast height) greater than 10 cm were marked with metallic platelets and their CBH and height was determined. Plants samples were collected for identification based on comparisons with botanical collections, herbarium, and by consulting specialists. Thus, the richness, abundance and height average of the plants of each plot were determined and used as measures of environmental heterogeneity (Table 2).
Table 1 Abundance (average individuals per plot), frequency (percentage of species occurrence per plot) and foraging habit of the ant species collected in a regenerating cerrado area at Jequitaí municipality, Minas Gerais state, Brazil.
| Subfamily | Specie | Abundance | Frequency | Foraging habit | |
|---|---|---|---|---|---|
| Arboreal | Soil* | ||||
| Dolichoderinae | Azteca sp. | 2.6 | 12.5 | X | |
| Formicinae | Brachymyrmex adenotus | 14 | 6.2 | X | |
| Brachymyrmex admotes | 39.4 | 50 | X | ||
| Camponotus arboreus | 0.2 | 6.2 | X | ||
| Camponotus blandus | 14.6 | 81.2 | X | ||
| Camponotus novogranadensis | 1.5 | 37.5 | X | ||
| Camponotus sp. | 0.2 | 6.2 | X | ||
| Camponotus vittatus | 1.3 | 6.2 | X | ||
| Pseudomyrmecinae | Pseudomyrmex gracilis | 0.7 | 18.7 | X | |
| Pseudomyrmex simplex | 1.3 | 25 | X | ||
| Pseudomyrmex termitarius | 0.4 | 12.5 | X | ||
| Myrmicinae | Solenopsis sp. | 7.9 | 6.2 | X | |
| Cephalotes liepini | 0.6 | 6.2 | X | ||
| Cephalotes pusillus | 5 | 56.2 | X | ||
| Crematogaster erecta | 1.7 | 12.5 | X | ||
| Crematogaster victima | 7 | 31.2 | X | ||
| Pheidole diligens | 1.3 | 12.5 | X | ||
* Forage habit in soil includes forage in more low vegetation and litter.
Table 2 Abundance of plant species collected in a regenerating cerrado area at Jequitaí municipality, Minas Gerais state, Brazil.
| Taxa | Plots (Quadrants) | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Species | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Q 10 | Q 11 | Q 12 | Q 13 | Q 14 | Q 15 | Q 16 | |
| Anacardiaceae | Astronium fraxinifolium | 1 | 1 | 1 | 1 | 11 | 15 | |||||||||||
| Annonaceae | Annona dióica | 1 | 11 | |||||||||||||||
| Apocynaceae | Aspidosperma tomentosa | 3 | 1 | 1 | 1 | 3 | 3 | 4 | 16 | |||||||||
| Apocynaceae | Himatanthus obovatus | 1 | 1 | |||||||||||||||
| Asteraceae | Eremanthus sp. | 1 | 1 | |||||||||||||||
| Bignoniaceae | Handroanthus ocharaceus | 1 | 1 | 2 | ||||||||||||||
| Caryocaraceae | Caryocar brasiliensis | 1 | 1 | 1 | 1 | 4 | ||||||||||||
| Clusiaceae | Kielmeyera coriaceae | 1 | 1 | |||||||||||||||
| Connaraceae | Connarus suberosus | 1 | 1 | 2 | ||||||||||||||
| Dilenaceae | Curatella americana | 1 | 1 | 1 | 13 | 1 | 17 | |||||||||||
| Dilenaceae | Davilla eliptica | 3 | 1 | 2 | 6 | |||||||||||||
| Erythroxylaceae | Erythroxylum suberosum | 2 | 2 | 8 | 3 | 4 | 2 | 6 | 2 | 4 | 6 | 1 | 3 | 43 | ||||
| Fabaceae | Acosmium dasycarpum | 3 | 12 | 1 | 2 | 1 | 2 | 3 | 7 | 1 | 1 | 6 | 39 | |||||
| Fabaceae | Acosmium subelegans | 2 | 2 | |||||||||||||||
| Fabaceae | Copaifera oblongfolia | 1 | 1 | |||||||||||||||
| Fabaceae | Dimorphandra mollis | 3 | 4 | 1 | 8 | |||||||||||||
| Fabaceae | Diptryx alata | 1 | 1 | |||||||||||||||
| Fabaceae | Enterolobium gunniferum | 1 | 1 | 2 | ||||||||||||||
| Fabaceae | Hymenaea courbaril | 1 | 4 | 3 | 1 | 1 | 10 | |||||||||||
| Fabaceae | Hymenaea stignocarpa | 3 | 3 | |||||||||||||||
| Fabaceae | Machaerium acutifolium | 1 | 4 | 5 | ||||||||||||||
| Fabaceae | Machaerium opacum | 1 | 1 | |||||||||||||||
| Fabaceae | Plathymenia reticulata | 19 | 1 | 1 | 5 | 5 | 4 | 3 | 3 | 2 | 3 | 46 | ||||||
| Fabaceae | Sclerobium aureum | 1 | 1 | 2 | ||||||||||||||
| Fabaceae | Smartzia sp. | 1 | 1 | |||||||||||||||
| Fabaceae | Stryphnodendron adstringens | 1 | 1 | 2 | ||||||||||||||
| Lythraceae | Lafoensia Pacari | 2 | 3 | 5 | ||||||||||||||
| Malpighiaceae | Byrsonima crasscifolia | 2 | 7 | 9 | ||||||||||||||
| Malpighiaceae | Heteropteris byrsominifolia | 1 | 1 | 4 | 6 | |||||||||||||
| Malvaceae | Eriotheca pubescens | 1 | 3 | 1 | 1 | 1 | 1 | 8 | ||||||||||
| Myrtaceae | Eugenia dysenterica | 10 | 2 | 3 | 6 | 1 | 1 | 5 | 9 | 3 | 40 | |||||||
| Myrtaceae | Myrciaria floribunda | 3 | 3 | |||||||||||||||
| Nictaginaceae | Guapira sp. | 2 | 1 | 1 | 1 | 1 | 1 | 7 | ||||||||||
| Opiliaceae | Agonandra brasiliensis | 1 | 1 | |||||||||||||||
| Polygonaceae | Cocoluba sp. | 4 | 1 | 1 | 6 | |||||||||||||
| Proteaceae | Roupala Montana | 1 | 3 | 4 | ||||||||||||||
| Sapindaceae | Magonia pubecens | 1 | 5 | 1 | 1 | 8 | ||||||||||||
| Sapotaceae | Pouteria ramiflora | 1 | 1 | |||||||||||||||
| Sapotaceae | Pouteria torta | 1 | 1 | 2 | ||||||||||||||
| Simaroubaceae | Simarouba versicolor | 2 | 2 | |||||||||||||||
| Vochysiaceae | Callisthene fasciculata | 1 | 5 | 3 | 9 | |||||||||||||
| Vochysiaceae | Callisthene major | 1 | 5 | 5 | 11 | |||||||||||||
| Vochysiaceae | Qualea grandiflora | 1 | 4 | 3 | 8 | |||||||||||||
| Vochysiaceae | Qualea parviflora | 9 | 6 | 4 | 21 | 7 | 7 | 6 | 19 | 2 | 7 | 5 | 8 | 1 | 1 | 1 | 2 | 106 |
| Vochysiaceae | Salvertea convallariodorea | 1 | 1 | 1 | 3 | |||||||||||||
| Vochysiaceae | Vochysia elíptica | 1 | 1 | |||||||||||||||
| Total | 40 | 34 | 39 | 28 | 24 | 25 | 30 | 31 | 28 | 23 | 25 | 27 | 14 | 18 | 31 | 55 | 472 | |
| Average plant height (m) | 3.54 | 3.05 | 3.01 | 2.73 | 2.65 | 1.86 | 2.66 | 2.3 | 3.07 | 3 | 3.4 | 3.2 | 2.66 | 4 | 3.23 | 2.94 | ||
During the end of the wet season, period in which all plant species still have leaves, 5 shrubs with more than 10 cm of CBH were randomly selected in each plot for ants sampling. The collection of ants from the plants was performed using the beating technique with capture in entomological umbrella. Thus, 3 branches of similar size and shape of each plant were beaten 10 times and the dislodged insects were collected in the entomological umbrella (Kuchenbecker & Fagundes, 2018; Souza & Araújo, 2020). This was done in the morning from 8 to 11 h. All insects sampled from each plant were properly conditioned in individual plastic containers and transported to the Laboratory of Conservation Biology of the State University of Montes Claros, where they were screened. All collected ants were identified by using taxonomic key and by consulting an ant taxonomist. Exemplars of all species of plants and arthropods sampled during the study were deposited in the basic reference collection of the Rio Jequitaí Consorce - Jequitaí Project.
In order to test the first hypothesis (i.e., the vegetation structure negatively affects the diversity of ants in more simplified habitat), 2 generalized linear models were created and tested with ANOVA. In the formulation of these models, the richness or abundance of ants per plot were considered as response variables and the abundance and average height of plants per plot were used as explanatory variables, based on Poisson (corrected for quasi-Poisson) or Gaussian distribution, respectively. Since richness and abundance of plants are 2 highly correlated variables (F = 23.072, p < 0.001), we chose to remove the plants richness variable from the general models. The sequence of the explanatory variables in the models was determined according Akaike Information Criterion for small samples (AICc), using the MuMIn Packages (Bartón, 2015) on R Software (R Core Team, 2015).
The second hypothesis (i.e., interspecific interactions shape the organization of ant communities in stressed habitats) was tested by using null model analyzes. The null hypothesis predicts that the occurrence of one ant species in one plot does not interfere with the occurrence of another ant species in the same plot (Fagundes, Santos et al., 2020; Ramos et al., 2019). In this case, the ant community is randomly structured and interspecific interactions cannot be used to explain ant community organization (Ribas & Schoereder, 2002). We use the C-score index (Stone & Roberts, 1990) as a measure of co-occurrence of ant species in the 16 plots. The index was calculated on presence/absence matrix of ant species where the columns of the matrix represented the collection plots and the lines the species of ants. To investigate whether the C-score of observed data was different from a C-score found at random, we randomized the data matrix 5,000 times. During the randomization process, the number of species per line and the number of occurrence plots per columns was kept constant. Thus, a C-score different from found in randomized matrices suggests that the community may be structured by deterministic processes, such as interspecific interactions. These statistical procedures were performed using the EcoSim software (Gotelli & Entsminger, 2001).
Results
A total of 457 individuals belonging to 17 species and 8 genera of ants (Hymenoptera: Formicidae) were sampled in this study. The most common genus was Camponotus, being represented by 5 species. The most abundant species were Brachymyrmex admotes (39.4%), Camponotus blandus (14.7%) and Brachymyrmex adenotus (14%) while the most frequent species were Camponotus blandus and Cephalotes pusillus, occurring respectively in 81.25% and 56, 25% of the 16 plots (Table 1). We also sampled a total of 472 trees/ shrubs belonging to 46 plant species (Table 2). The more common plant species were Plathymenia reticulata (46 individuals) Erythroxylum suberosum (43 individuals), Eugenia dysenterica (40 individuals) Acosmium dasycarpum (39 individuals). The vegetation had a density of 0.29 plants/ m2 and an average height of 2.94 m.
Of all the explanatory variables tested, only the interaction of the variables like plant abundance and plant height average per plot affected the ant species richness (Deviance = 2.199, F = 5.013, p = 0.041). In fact, higher ant species richness was observed in plots with greater plant abundance and lower average height of plants (Fig. 1A). We also observed a negative relationship between ant abundance and the height average of plants per plot (Deviance = 7.289, F = 17.860, p = 0.006, Fig. 1B).
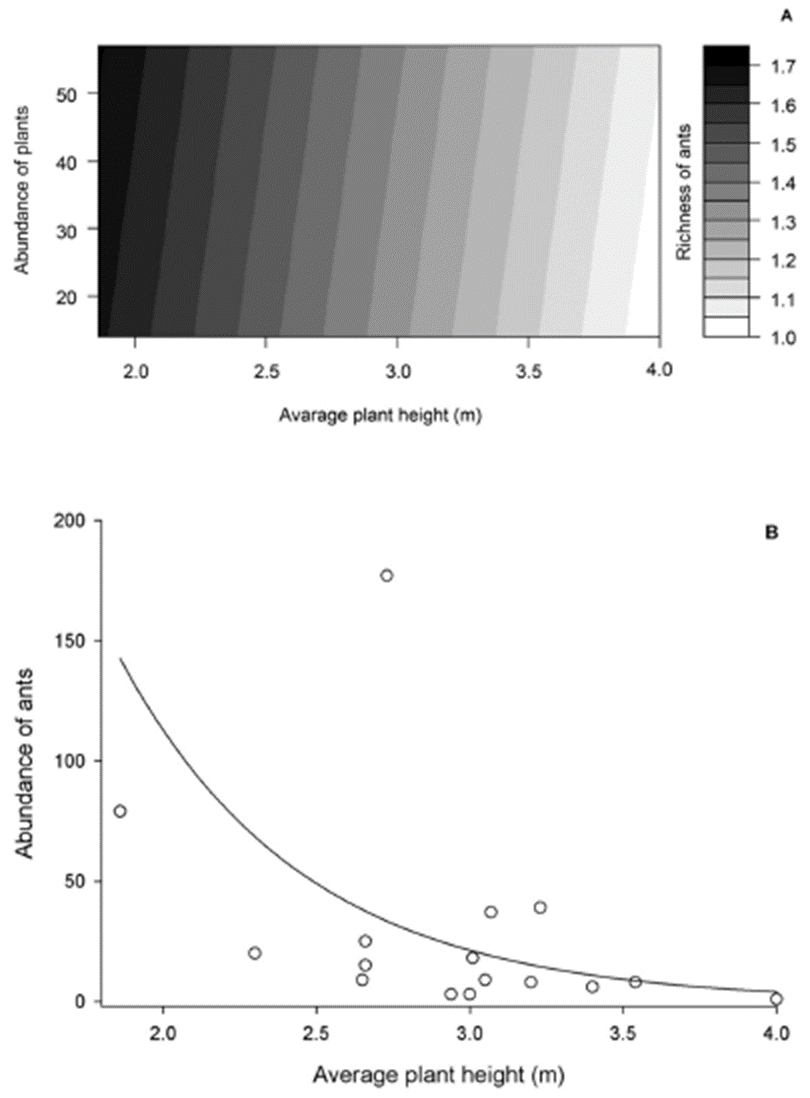
Figure 1 Effect of plant abundance and average plant height on ant richness (A) and average plant height on the abundance of ants (B) collected in a regenerating cerrado area, Jequitaí city, Minas Gerais State, Brazil.
The observed value of co-occurrence index (C-scores) was lower than expected values of simulated matrices, suggesting that the organization of the ant species community does not occur randomly (Fig. 2). Thus, the null hypothesis that predicts that the presence of one ant specie does not interfere in the co-occurrence of another ant species was not corroborated and deterministic processes, such as interspecific interactions, can be responsible for organization of the studied ant community.
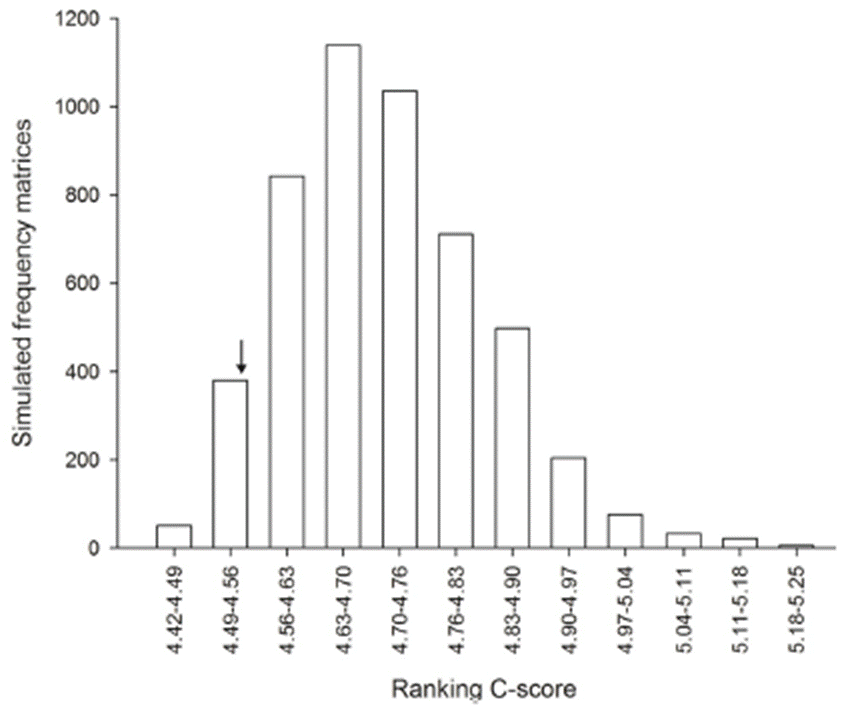
Figure 2 Co-occurrence patterns of the ants species sampled in a regenerating cerrado vegetation area of Brazil. The histogram shows the class frequency of simulated C-Score using the fixed-fixed model. The arrow indicates where the observed C-scores coincided with the frequency class of the C-scores of the 5,000 simulated matrices. The minimum and maximum values of the indices calculated for 5,000 randomized matrices were 4.42 and 5.25, while the observed C-score index was 4.54. The p- value was 0.0472 showing that observed index is outside of the 95% limit of the frequency distribution of randomized matrices, indicating ant species are not randomly distributed in the landscape.
Discussion
Previous studies indicate that Cerrado areas have high diversity of ant species (Neves et al., 2013; Silva et al., 2017). In contrast, some Cerrado physiognomies with low canopy cover such as rupestrian field and candeal forests (Lopes et al., 2012; Queiroz & Ribas, 2016), as well as cerrado areas affected by anthropogenic activities (Fagundes, Santos et al., 2020; Neves et al., 2012), generally harbor a lower ant diversity. Moreover, ant fauna of these open habitats usually is composed of opportunistic and generalist species adapted to foraging on sites subjected to high temperatures (Lassau & Hochuli, 2004). In general, most ant species sampled in our study are generalists and frequently found in open vegetation habitats with less litter accumulation and high levels of sunlight incidence (Costa et al., 2015; Lopes et al., 2012). Moreover, the majorly of 17 ant species belong to genera that are frequently associated with plants, while foraging on extrafloral nectaries or honeydew secreting homopterans (Fagundes et al., 2005; Rosumek et al., 2009).
The results of our study showed that richness of ants was higher in plots with lower plant height average and high plant abundance. Moreover, the ant abundance was higher in plots with smaller plants. Therefore, these results corroborate our first hypothesis that predicts that vegetation structure negatively affects ant diversity in more simplified habitats (Queiroz & Ribas, 2016). The vertical stratification and interconnection of tree canopies in more complex habitats, such as tropical forests, generate different microhabitats where different species of ants can coexist (Fagundes, Santos et al., 2020; Fonseca & Benson, 2003; Silva et al., 2017). In simplified habitats such as the studied regenerating cerrado, vertical canopy stratification is not observed, and plants canopies are not interconnected. The lack of connection between the canopies hinders the movement of the ants between plants through canopy, and the displacement via soil should be more common in order to access different plants.
In open environments, where arboreal ant migration among plants should occur throughout the soil, associated to high level of sunlight incidence and temperatures due low canopy cover, determine that energy saving during resource searchs by ants should be prioritized. In fact, according to optimal foraging theory, animals will either attempt to maximize energy gained or minimize time spent to obtain a fixed amount of energy (Stephens & Krebs, 1986). In this scenario it would be reasonable to think that ants should seek resources on plants with lower heights in order to minimize time spent to find food resources, justifying the negative relationships between plant height and ant species richness and abundance observed in our study. Besides, the greater ant species richness observed in plots that contained greater plant abundance could be explained by higher shading promoted by increase in plant abundance. In fact, open habitats usually harbor opportunistic and generalist ant species (Lassau & Hochuli, 2004). The increase in canopy cover causes a decrease in sunlight incidence, promoting an increase in humidity and decrease in temperature, that would be allowing other ant species to colonize and coexist with more generalist ants, typical of open areas (Pacheco & Vasconcelos, 2012).
The results of the co-occurrence analysis found in this study demonstrated that the presence of one ant species interferes with the occurrence of another ant species. When experimental manipulations are not possible, comparing observed patterns of co-occurrence with patterns that may occur at random is a conventional technique for interspecific competition studies (Fagundes et al., 2019; Morin et al., 2011). Despite the wide-ranging discussion on the role of competition in the organization of natural communities, more recent studies using co- occurrence suggest that interspecific competition may be an important factor capable to shape the organization of natural communities especially in stressful habitats, where resources are scarcer (Ramos et al., 2019 and references therein).
The aggressive/territorial behaviors of many ant species also suggests that interspecific antagonist interactions may be shaping ant communities when resources are limited (Mezger & Pfeiffer, 2011; Parr & Gibb, 2010). However, while well-defined vertical stratification allows ant species to explore different resources on the same plant, increasing the number of coexisting ant species in a habitat (Silva et al., 2017; Tobin, 1997), low heterogeneous environments have a lower supply of food resources and nesting sites, increasing the likelihood of overlapping the resource used by ants (Fagundes, Santos et al., 2020; Parr & Gibb, 2010; Sant’Ana et al., 2008). Therefore, it would be reasonable to think that competition between ant species could be a force capable of shaping ant community in more simplified habitats as observed in our study.
In summary, our study showed that the relationship between environmental heterogeneity and ant diversity may not be a universal phenomenon since smaller plants can support greater ant diversity. In addition, the results of the null model tests showed that interspecific competition is an important biotic factor capable to shape the organization of ant communities in more simplified habitats. Finally, our findings illustrate the possibility that the mechanisms that regulate species diversity can change between different habitats. Understanding these mechanisms may help supporting future management strategies for the conservation of species in less heterogeneous (and often more neglected) habitats. We highlight the importance of conserving small trees especially in open environments because they represent key elements that can harbor high ant species diversity and contribute to the maintenance of the biological interactions of this important group of insects with plants and other animal species.











 nueva página del texto (beta)
nueva página del texto (beta)


