Introduction
Epibiosis is one of the most common ecological relationships among marine organisms. This term is defined as the spatial association between a substrate organism call as a basibiont and a sessile organism or epibiont attached to the outer surface of the basibiont without being trophically dependent on it (Wahl, 2009). Within the biotic interactions among marine populations, epibiosis has been well and intensively studied over the past decades (Wahl, 1989). Marine epibionts are an assemblage of organisms including different groups of algae, barnacles, bryozoans, hydroids, polychaetes, sponges, and mollusks; among other taxa (Connelly & Turner, 2009; Harder, 2009; Wahl, 2008).
Several organisms have been recorded as basibionts, being the main ones: corals, seagrasses, algaes, ascidians, sponges, bryozoans, and several species of mollusks (Connelly & Turner, 2009). According to Wahl (2008) this last group may harbor the biggest number of basibiont species. The variability, quantity, and quality of the epibiosis within a specific basibiont are determined by the seasonal availability of the colonizers, the differential colonization in the basibiont body, as well as the quality of the basibiont regarding body texture, consistency, shape, inclination, substance excretion, among others (Wahl, 2008).
Interactions such as epibiosis can generate benefits (decreased desiccation and reduced predation) and in some cases, harm (mechanical and chemical damage) for both the basibionts and the epibiont organisms, so this relationship is quite complex (Donovan et al., 2003; Harder, 2009; Laudien & Wahl, 1999; Marin & López-Belluga, 2005; Manning & Lindquist, 2003; McAllen & Scott, 2000; Penhale & Smith, 1977; Wahl, 2009). Therefore, all these effects are very costly and can reduce the adequacy of the basibionts and their ability to defend against predators or their possible response by interacting with other organisms through intra-specific relationships such as competition (Wahl, 2008).
In mollusks, the epibionts more commonly recorded have been oysters, barnacles, and algae (Creed, 2000). Epibiosis has been documented in various members of the class Polyplacophora, especially among the genera: Ceratozona, Leptochiton y Mopalia (Connelly & Turner, 2009). There are no studies that indicate the arrangement of the valves of chitons, except for the work of Álvarez-Cerrillo et al. (2017), where it is mentioned in which valve the epibionts were found, but it is not detailed whether these are algae or animals. Even though epibiosis is common in the genera mentioned above, the relation between chitons and their epibionts is still understudied.
The mollusk Chiton articulatus G. B. Sowerby I, has a geographic distribution restricted to the Pacific Ocean from Mazatlán, Sinaloa, to Huatulco Bays, Oaxaca, including Socorro Island and the Revillagigedo Archipelago, Mexico (Reyes-Gómez, 2016). This species is a herbivore and inhabits the intertidal rocks (Keen, 1971). These mollusks play an important role in the marine trophic net; it is part of the diet of fishes, crabs, octopuses, and other gastropods (Sampedro et al., 2012). Chitons represent a link between the producers (algae) and the consumers (carnivores). It is also important to mention that C. articulatus is considered a commercially important species in the tropical Mexican Pacific at a local level. Regarding the knowledge of C. articulatus in Mexico, only the studies regarding their populations in Socorro Island and in the Revillagigedo Archipelago (Holguín-Quiñones & Michel-Morfín, 2002), in the Venados and Pájaros Islands in Sinaloa (Flores-Campaña et al., 2007) and Oaxaca, can be mentioned (Ávila-Poveda, 2013; Ávila-Poveda & Abadia-Chanona, 2013). Also, the only study regarding C. articulatus epibionts is the contribution of Álvarez-Cerrillo et al. (2017) which includes the first approach to the epibionts and endobionts on this species on the shores of the state of Guerrero. Yet, this study focuses on animal epibionts leaving aside other communities such as algae, even though they are often found as epibionts in these chitons. The knowledge of macroalgae communities has usually been developed from a floristic approach, without considering their ecological interactions with other organisms, such as epibiosis relationships. Recently, this has changed a bit and more ecological studies have been published (Corado-Nava et al., 2014; López et al., 2017). Studies including the interactions among both communities are still scarce; one being the research of Aguilar-Estrada et al. (2017), who documented the mollusks associated with the macroalgae assemblages in the rocky intertidal from Ixtapa-Zihuatanejo, Guerrero. Given the importance of mollusks as a structuring component of the rocky intertidal communities and the scarce information available about their association with algae, this study aims to describe the composition and coverage of the epibiotic algae associated with Chiton articulatus. Also, we analyze the micro-spatial distribution of the algae on the shell of C. articulatus specimens.
Materials and methods
Samples were collected under a collection permit processed by the Facultad de Ciencias, UNAM (Registro Nacional de Pesca y Acuacultura, Folio DF00000208) in 2 rocky shores: one in Ixtapa and a second one at the Zihuatanejo Bay (Fig. 1). Ixtapa is characterized by a sandy beach with rocky ends, with rocks of different sizes (0.5 to 2 m). The northern end has an artificial breakwater forming an inner channel used as a marina entrance. The southern end has a touristic complex, and there is a 10 m wide rocky shoreline around the hills. Zihuatanejo Bay is small, mainly with sandy beaches in the inner portion separated by rocky points. This locality is the Municipal pier of the city, being an assemblage of rocks of different sizes, located at the mouth of Las Salinas lagoon. Samples were collected in January (dry), May (dry), July (rainy), and November (storms) 2014 in El Palmar beach (17°39’0.4” N, 101°36’ 2.79” W) in Ixtapa and in the Municipal pier (17°38’13.88” N, 101°33’31.87” W) in Zihuatanejo. In each locality we collected randomly individuals of Chiton articulatus that were observed; the organisms were taken manually with a spatula in the intertidal rocky zone between the mid and high-water marks. Samples were preserved in ethylic alcohol (70%) until their revision at the laboratory. The collected chitons were identified at species level using the identification keys of Keen (1971) and Kaas et al. (2006). The length, width, and height of specimens were measured with a vernier (± 0.01 mm). Following the measures registered by Keen (1971), juveniles and adults were recognized. The algae epibionts observed were recorded from each valve present in the chitons (I to VIII) according to Ávila-Poveda et al. (2019). The epibiont algae identification was done following the protocol by Quiroz-González et al. (2020). The nomenclature and taxonomic update as well as the list of species were carried out using Algaebase (Guiry & Guiry, 2022). The epizoic algae were deposited in the “Invertebrados Asociados a Macroalgas, Laboratorio de Ficología (Biodiversidad Marina)” collection of the Facultad de Ciencias, UNAM. Since only a portion of the thallus was used for the identification of the species, semi-permanent preparations were used. Photographs were taken in dorsal view of the chitons that presented epizoic algae, and processed with Sigma Scan-Pro software to estimate the coverage in cm. Additionally, the algal functional group was determined for each algae species using the groups proposed by Steneck & Dethier (1994).
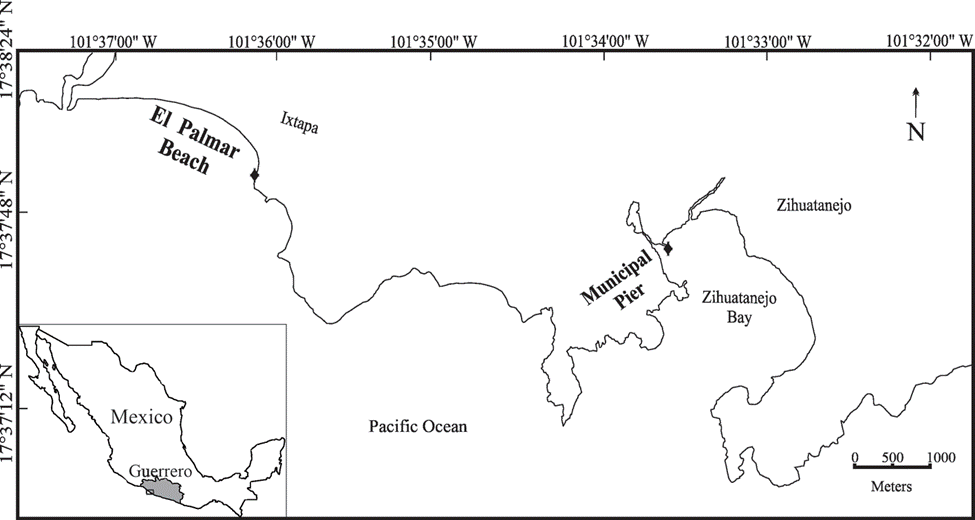
Figure 1 Location of the 2 sampling sites in Ixtapa-Zihuatanejo, Guerrero, Mexico. A) Palmar beach, B) Municipal pier.
All graphs were done using GraphPad Prism version 8.0.0. The multivariate analyses were performed using PRIMER6 with add on package PERMANOVA + software (PRIMER-E Ltd., Plymouth, UK) (Anderson et al., 2008). For all statistical analyses, algal species coverage table was log10 (x+1) transformed and a resemblance matrix was obtained using the Bray-Curtis similarities among all the chitons with epibionts on their shell. A distance- based linear modeling analysis (DistLM) and a distance- based redundancy analysis plot (rdRDA) were used to illustrate the relationship between shell length (basibiont) and the richness of the epibionts algae. The Permutational multivariate analysis of variance (PERMANOVA) was used to test the null hypotheses that algae coverage and species composition did not differ among chitons regarding locality, month, and functional algal group. Also, this analysis was used to evaluate differences among the algae species distribution in the chitons shell. One factor main and pairwise PERMANOVA, “Unrestricted permutation of raw data” and “Permutation of residuals under a reduced model” were used as the permutation method. To assess the contribution of the different algae species to the differences between chitons, we applied a similarity percentages analysis (SIMPER). A Principal Components Ordination analysis (PCO) was plotted to show the chitons’ patterns in relation to their algal epibionts and community similarities with superimposed epibionts species as vectors calculated as Pearson correlations with the PCO ordination coordinates.
Results
A total of 62 specimens of Chiton articulatus were collected in the Ixtapa-Zihuatanejo region. Sixteen organisms in El Palmar beach and 10 in the Municipal pier had algae in their valves. The length of the chiton shell ranged from 15.8 to 60 mm with a mean of 37.6 mm (SD ±8.3 mm). Of the total collected specimens, only 5 (8%) were adults and 57 (92%) were juveniles. Further, 36 (58%) did not register algae epibionts and only 26 (42%) had epibionts on their shells. Even though less than half of the chitons presented algal epibionts, 50 species of algae were identified; it would be convenient to mention that 37 of them were determined to species and 13 to genus level. Of the algal species identified, 22 were Rhodophyta, 17 Chlorophyta, 5 Ochrophyta-Phaeophyceae, and 6 were Cyanobacteria (Table 1). The algae inventory consisted of 50 species grouped in 33 genera, 23 families, 19 orders, 7 classes, and 4 phyla. The best represented family was Cladophoraceae with 10 species; followed by Erithrotrichiaceae with 5 species, and Rhodomelaceae with 4 species. Twelve species of algae are new records for the flora in Guerrero; 3 were new records for the Mexican tropical Pacific and 3 others for the Mexican Pacific (Table 1). The most frequent algal species was Grania pectinata (Kylin) Athanasiadis being present in 24% of all chitons with epibionts, followed by Lithophyllum sp. (8%), and Lithothamnion sp. (8%).
Table 1 Coverage of epibiont algal species in cm2 on the shell of Chiton articulatus individuals for each location and sampling month. This work. *New records only for state of Guerrero; **New records for the Mexican tropical Pacific; *** New Records for the Pacific of Mexico.
| El Palmar beach | Municipal pier | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Jan | May | Jul | Nov | Ma | Jul | Nov | |||||||||||||||||||
| Rhodophyta | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| Bangia fuscopurpurea (Dillwyn) Lyngbye | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - |
| Erythrotrichia carnea (Dillwyn) J. Agardh | - | - | - | 3.5 | 16.4 | - | - | 0.01 | 10.9 | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| E. porphyroides N.L. Gardner | - | - | - | - | 16.4 | - | 0.03 | - | - | - | - | - | - | - | - | 1.9 | - | - | - | - | - | - | - | - | - | - |
| E. tetraseriata N.L. Gardner* | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - |
| Porphyrostromium pulvinatum West & Zuccarello** | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | 0.06 | - | - | - | - | - | - | - | - | |
| Sahlingia subintegra (Rosenvinge) Kornmann | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | - | 1.9 | - | - | - | - | - | - | 6.0 | - | - | - |
| Chroodactylon ornatum (C. Agardh) Basson | - | - | - | - | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Acrochaetium pacificum Kylin | - | - | - | 3.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Grania pectinata (Kylin) Athanasiadis | - | - | - | - | - | 0.03 | - | 5.2 | 10.9 | - | 4.9 | 0.1 | 3.7 | 3.4 | 8.3 | 1.9 | - | 0.06 | - | 4.5 | - | 0.1 | 6.0 | - | - | 1.9 |
| Colaconema coccineum (K.M. Drew) P.W. Gabrielson*** | - | - | - | 3.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C. daviesii (Dillwyn) Stegenga | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Colaconema sp. | 12.9 | 0.6 | - | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | |
| Taenioma perpusillum (J. Agardh) J. Agardh | 0.02 | 0.1 | - | - | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Melanothamnus sp. | - | - | - | - | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Polysiphonia confusa Hollenberg | - | - | - | - | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| P. pacifica Hollenberg* | - | - | - | - | - | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| P. subtilissima Montagne | - | - | - | - | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Lithophyllum sp. | 12.9 | 3.1 | 7.9 | 8.9 | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pneophyllum fragile | - | - | - | - | - | - | 0.03 | 0.2 | 1.1 | 0.42 | - | - | - | - | - | 1.9 | - | - | - | - | - | - | - | - | 0.07 | 1.9 |
| Kützing | ||||||||||||||||||||||||||
| Lithothamnion sp. | - | - | - | - | - | - | - | - | - | - | 12.3 | 8.0 | 3.1 | 8.4 | 8.3 | - | - | - | - | - | - | - | - | - | - | - |
| Gelidium pusillum (Stackhouse) Le Jolis | 0.02 | 0.1 | - | - | 16.4 | - | 0.03 | - | - | - | - | 0.1 | - | - | - | 1.9 | - | - | - | - | - | - | - | - | - | - |
| Rhodymenia sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.9 | - | - | - | - | - | - | - | - | - | - | |
| Richness for phylum | 4 | 4 | 1 | 4 | 10 | 1 | 6 | 3 | 3 | 1 | 2 | 5 | 2 | 2 | 2 | 6 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 2 |
| Ochrophyta- phaeophyceae | ||||||||||||||||||||||||||
| Myrionema strangulans Greville | 0.2 | 0.04 | - | 0.002 | - | - | - | 0.003 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Streblonema sp | - | - | - | - | - | - | - | - | - | - | - | 0.1 | - | - | - | 1.9 | - | - | - | - | - | - | - | - | - | - |
| Ectocarpus sp. | 0.02 | 0.06 | - | - | - | - | - | - | - | - | - | - | - | |||||||||||||
| Feldmannia sp. | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Hapalospongidion gelatinosum Saunders | - | - | - | - | 16.3 | - | - | - | 0.3 | 0.5 | - | 0.1 | - | 0.03 | - | - | - | - | - | - | - | - | - | - | - | - |
| Richness for phylum | 2 | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlorophyta | ||||||||||||||||||||||||||
| Derbesia marina | - | - | - | - | - | - | - | - | 10.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| (Lyngbye) Solier | ||||||||||||||||||||||||||
| Cladophora columbiana Collins | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | - | - | - | - | - | - |
| Cladophora laetevirens Kützing* | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cladophora graminea Collins** | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6.0 | - | - | - |
| Cladophora microcladioides Collins | - | - | - | - | - | - | - | - | - | - | 4.9 | - | - | - | - | - | - | - | - | 4.5 | - | 0.1 | 6.0 | - | - | - |
| Cladophora sakaii | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6.0 | - | - | - |
| I.A. Abbott | ||||||||||||||||||||||||||
| Cladophora sericea | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Kützing | ||||||||||||||||||||||||||
| Chaetomorpha californica Holden & Setchell* | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | - | - | - | - | - | - |
| Chaetomorpha linum (O.F. Müller) Kützing* | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | - | 0.1 | - | - | - | - |
| Chaetomorpha nodosa Kützing*** | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Chaetomorpha antennina (Bory) Kützing | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.7 | - | - | - |
| Siphonocladus sp. | - | - | - | - | - | - | 0.03 | 5.2 | 1.07 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 | - | - |
| Urospora penicilliformis (Roth) Areschoug*** | - | - | - | - | - | - | - | - | 10.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Urospora wormskioldii (Mertens ex Hornemann) Rosenvinge** | - | - | - | - | - | - | - | - | 10.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ulothrix flacca | - | - | - | - | 16.3 | - | - | - | 10.9 | - | 4.9 | - | 3.7 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| (Dillwyn) Thuret* | ||||||||||||||||||||||||||
| Ulva intestinalis | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Linnaeus | ||||||||||||||||||||||||||
| Ulva flexuosa | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Wulfen | ||||||||||||||||||||||||||
| Richness for phylum | 0 | 0 | 0 | 0 | 6 | 0 | 2 | 1 | 5 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 2 | 4 | 1 | 0 | 0 |
| Cyanobacteria | ||||||||||||||||||||||||||
| Blennothrix lyngbyacea (Kützing ex Gomont) Anagnostidis & Komárek | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - |
| Lyngbya majuscula | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.06 | - | - | - | - | - | - | - | - |
| Gomont | ||||||||||||||||||||||||||
| Lyngbya sp. | 0.02 | 0.1 | - | - | 16.3 | 0.03 | - | - | 10.9 | - | 4.9 | 0.1 | - | 3.5 | - | - | 0.01 | - | - | - | - | 0.1 | - | - | - | - |
| Spirulina sp. | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Microcystis sp. | - | - | - | - | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Calothrix sp. | - | - | - | - | - | - | - | - | - | - | - | 0.14 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Richness for phylum | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total richness | 7 | 7 | 1 | 5 | 21 | 2 | 8 | 5 | 10 | 2 | 5 | 9 | 3 | 4 | 2 | 7 | 4 | 3 | 1 | 5 | 1 | 4 | 6 | 1 | 1 | 2 |
The size of C. articulatus was significantly correlated with algal species richness (distLM: pseudo F = 4.28, p = 0.046), and accounted for 15.15% of the total variation (Fig. 2). This graph shows 3 different groupings (valves I, and VIII, valves II-VII, and suture I-VII) (Fig. 3). The PERMANOVA showed significant differences among every group, except for the belt. The extreme valves (VI and VIII) were significantly different from the middle valves (VII-VII) and from the sutures (Table 2).
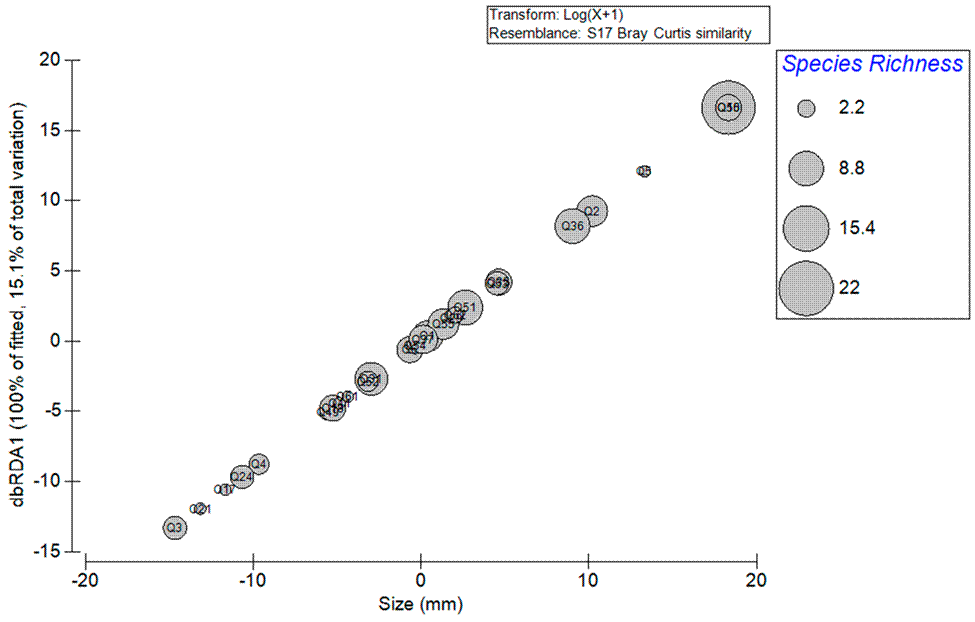
Figure 2 Distance-based redundancy analysis plot (dbRDA) of the relation of the C. articulatus sizes (mm) of collected specimens and the algae species richness as epibionts in the chiton shell.
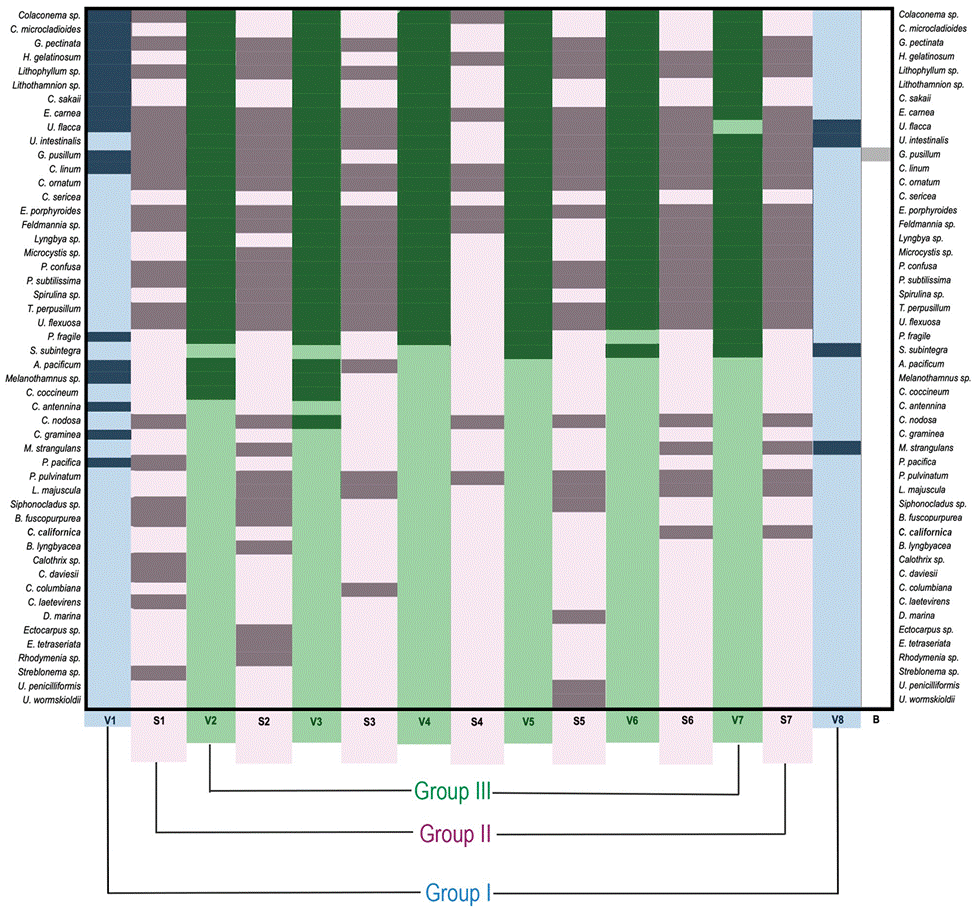
Figure 3 Graph illustrating the algae species distribution on C. articulatus valves (V1-V8) group I in blue, and sutures (S1-S7) group II in purple and the belt (B) group III in green.
Table 2 PERMANOVA of the algae species in C. articulatus shell. Significant values are marked with an*.
| Unique | |||
|---|---|---|---|
| Groups | t | P(perm) | perms |
| Valves I and VIII, Valves II-VII | 3.4258 | 0.039* | 28 |
| Valves I and VIII, Sutures I-VII | 2.697 | 0.024* | 36 |
| Valves I and VIIII, Belt | 2.1942 | 0.308 | 3 |
| Valves II-VII, Sutures I-VII | 2.6291 | 0.001* | 747 |
| Valves II-VII, Belt | 16.879 | 0.139 | 6 |
| Sutures I-VII, Belt | 3.2735 | 0.122 | 8 |
Eleven species were recorded in the valves; 17 species in the sutures; and 22 species in both zones. In the shell only Gelidium pusillum (Stackhouse) Le Jolis was recorded; still, this species was also encountered in the valves and suture. In valve III and in the suture located between the II and III valves, the biggest number of epibionts were found, counting each 27 algal epibionts. The valves of the shell that presented the smaller number of species were valve I, counting 17 epibionts species, and valve VII with only 10 species. Also, the suture between valves IV and V recorded fewer algal epibionts (Fig. 3). Five functional algae groups were recognized: microscopic (12%), filamentous (68%), crust (12%), foliose (4%), and filamentous corticated algae (4%); the PERMANOVA test showed no significant differences (p > 0.05) among them (Table 3).
Table 3 Main PERMANOVA test of the functional algae groups in the chitons shell.
| Source | df. | SS | MS | Pseudo-F | P(perm) | Unique perms |
|---|---|---|---|---|---|---|
| Functional algae group | 4 | 16,533 | 4,133.2 | 10.986 | 0.297 | 998 |
| Res | 20 | 75,245 | 3,762.3 | |||
| Total | 24 | 91,778 |
The most abundant functional algal group registered in Chiton articulatus was the filamentous one. On El Palmar beach, the functional algal groups associated with C. articulatus were filamentous (64%), crust and microscopic with 14% each, lastly, foliose and filamentous corticated algae with 4%. Additionally, in the Municipal pier, only filamentous algae (68%) and crust algae (32%) were recorded.
The SIMPER analysis showed that the species with the higher contribution in both localities was Grania pectinata with 10.51%. This same species contributed 33.7% in El Palmar beach and 57.26% in the Municipal pier. The species that best contributed to characterizing the epibionts in Chiton articulatus in the Ixtapa-Zihuatanejo region are listed in table 4. The SIMPER analysis showed that the algal epibionts are different for both localities (average dissimilarity = 88.27), sharing only 2 species (Grania pectinata and Pneophyllum fragile) (Table 4). However, the main PERMANOVA test for the locality factor did not show significant differences between the 2 sites (gl = 1 t = 1.2901 p = 0.061, perms = 994).
Table 4 Species with the highest percentage contribution to coverage by month.
| January | May | ||
| Especies | % of contribution | Species | % of contribution |
| Lithophyllum sp. | 89.59 | Grania pectinata | 39.73 |
| Colaconema sp. | 4.61 | Erythrotrichia pulvinata | 23.21 |
| July | November | ||
| Grania pectinata | 53.53 | Grania pectinata | 42.29 |
| Siphonocladus sp. | 33.80 | Lithothamnion sp. | 36.53 |
In the PCO analysis, the clearest separation was due to the sampling month (Fig. 4). A clear separation in January and November was observed, even though the separation in the graph was not clear, the percentage of crust algae recorded in both months was higher in comparison to May and July, where the filamentous algae was the dominant functional group. Lithophyllum sp., Lyngbya sp., Lithothamnion, Myrionema strangulans Greville, Ectocarpus sp., Grania pectinata, Pneophyllum fragile, and Bangia fuscopurpurea (Dillwyn) Lyngbye, best explain the differences among the sampled chitons (Fig. 4). The PCO1 explained 22.4% of the total variation and the PCO2 17.2%, both accounting for 39.57% of the cumulative variation.
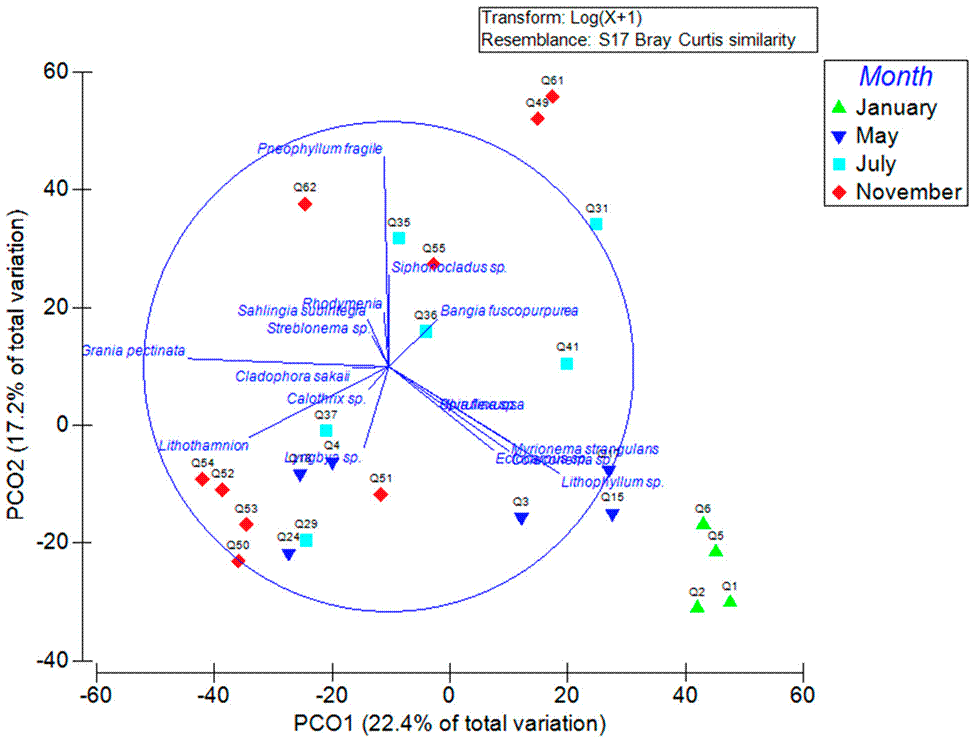
Figure 4 Principal components ordination (PCO) of the chitons sampled with algae epibionts on their shell plotted by month. Vectors represent the algae coverage (only the dominant species were plotted).
Corroborating the PCO results the pair-wise PERMANOVA analysis for months showed significant differences (p < 0.05) between January and all the other months (May, July and November), also, between the May and November. Lastly, July was not significantly different (p > 0.05) from May and November (Table 5).
Table 5 Pair-wise PERMANOVA test of the algae epibionts on the chitons sampled by factor month. Significant values are marked with *.
| Groups | t | P(perm) | Unique perms |
|---|---|---|---|
| January, May | 17.159 | 0.009* | 209 |
| January, July | 19.533 | 0.004* | 208 |
| January, November | 25.343 | 0.005* | 572 |
| May, July | 11.565 | 0.159 | 415 |
| May, November | 15.558 | 0.018* | 911 |
| July, November | 12.997 | 0.085 | 901 |
Discussion
In the study area, juvenile stages were the most abundant chitons sampled (92%) and only 8% represented adult specimens. The examined chitons in the present contribution recorded smaller lengths (15.8 mm to 60 mm) than those reported in previous studies. For instance, Keen (1971) recorded C. articulatus with lengths up to 100 mm. Also, Ávila-Poveda (2013) in Oaxaca, recorded length sizes of chitons from 40 to 70 mm; whereas Holguín-Quiñones & Michel-Morfin (2002) in the Socorro Island registered length sizes ranging from 29 to 108 mm. It is extremely vital to mention the decrease in size of this species, since in the Tropical Mexican Pacific C. articulatus is a commercially important species. More importantly, the knowledge given on specimen sizes helps increase the data that is usually scarce for the species and contributes to new features for a commercially exploited species where data is limited and unofficial. For example, the number of organisms extracted by fishermen. It is known that C. articulatus is offered as an exotic food along the coast of Guerrero; therefore, the information presented in this study is extremely valuable as a first approach to understand the status of juvenile populations of this chiton species. This is also relevant since usually the organisms collected for food are mostly juvenile and the uncontrolled fisheries common in the state of Guerrero could mean an important loss in this chiton population.
The richness of red algae, followed by green algae and in lesser numbers the brown algae recorded in this work was similar to that encountered by Pedroche & Sentíes (2003) and Mateo-Cid & Mendoza-González (2012) in Guerrero. Pedroche and Sentíes (2003) identified 103 species of Rhodophyta, 30 of Chlorophyta, and 24 species of Ochrophyta while Mateo-Cid and Mendoza-González (2012) identified 93 species of Rhodophyta, 28 of Chlorophyta and 25 of Heterokontophyta. In this PPPstudy, the same pattern of species richness for the 3 main algae groups was found (22 Rhodophyta, 17 Chlorophyta, and 5 Ochrophyta-Phaeophyceae). According to Quiroz-González et al. (2020) from the 50 taxa of algae present in this study, 12 species represented new records for flora of algae from Guerrero, 3 species for the Mexican tropical Pacific, as well as 3 species for the Mexican Pacific.
The epiphytism and the epibiosis are important variables in the study of the structure of marine benthic communities (Menezes de Széchy & Faria de Sá, 2008; Montañés et al., 2003). Mendoza-González et al. (2011) mentioned that because of their usually small size epibionts are inconspicuous organisms in floral inventories Also, it is more frequent to find algae studies focused on substrates such as other algae or marine grasses (epiphytism), leaving aside studies regarding animals as substrates. This could be the main reason why focusing on neglected substrates increases considerably the specific richness as observed by Quiroz-González et al. (2020), indicating that usually the algae diversity has been underestimated.
Some species of algae in this study have been recorded in other algal epibiont studies. Chaetomorpha linum (O.F.Müller) Kützing and Cladophora laetevirens (Dillwyn) Kützing were also recorded by Martins et al. (2014) as epibionts of the mollusk Patella aspera Röding in Argentina, both species are considered cosmopolitan. Several of those genera match with the genera recorded in this study (Lithothamnion, Gelidium, Polysiphonia, Calothrix, and Ulva). Martins et al. (2014) }Lithothamnion sp. and Gelidium sp. as the species with more coverage on the limpets. In the present contribution, Grania pectinata was the dominant species in the shells of the chitons, Lithothamnion sp. was the third species in numerical importance, and Gelidium sp. was registered in the 9th position. Another important aspect to consider is the existence of crust algae with a high number of specimens and in some cases covering the totality of the shell. Brandani et al. (1974) highlighted the value of encrusting coralline algae in the development of the epizoic community; these represent a pioneer state, this is the case of Lithothamnion sp. Regarding the epibionts recorded in other studies, Bretos and Chihuailaf (1990) pointed out that the limpet Fissurella pulchra G. B. Sowerby I, have algal epibionts such as Lithophyllum sp., Gelidium sp. and Acrochaetium sp., genera that were also recorded in the present contribution. On the other hand, O’Connor and Crowe (2007) when working with mussels in Ireland, recorded 8 species of algal epibionts, nevertheless a comparison with this study is irrelevant since the study areas are completely different. Connelly and Turner (2009), in Florida, recorded specimens of Ceratozona squalida (C. B. Adams), 27 species of epizoic algae, Chaetomorpha antennina (Bory) Kützing and Ulva flexuosa Wulfen were also recorded in this work. The genera shared among this last study and the present one, are: Lygnbya, Calothrix, Myrionema, Polysiphonia, and Cladophora.
The DistLM and dbRDA plots showed a significant positive relationship between chitons size and algal species richness; this same relationship was observed by Martins et al. (2014). In this study, chiton sizes were smaller in comparison to other chiton studies in the region. This may be one of the reasons a high percentage (58%) of the sampled chitons did not record epibionts on their shell. Harder (2009) mentioned that while chitons grow, they become more susceptible to host epibionts on their shells. These epibionts affect the valves of the mollusk, mainly because of the fixing structures in the interior of the valves and sutures, and their mobility is affected due to the additional weight of the epibionts.
The chitons’ behavior in the intertidal zone is sessile in comparison to that observed in other gastropods. For instance, the patelliforms are homing species, meaning they move throughout the intertidal zone and return to the refugee afterward. On the contrary, according to Otaiza and Santelices (1985) small sized chitons are usually encountered in low wave impact zones, and in high wave exposed zones adult chitons have been observed. Wahl (2008) reported that this could be a response to their feeding habits, since the low impact subtidal zones are where small size chitons find a favorable environment for feeding and protection, and it is this zone where the algae epibiotic association probably happens. Also, algae could benefit for this association since chitons may function as dispersers of thalli and reproductive structures.
In this study, a differential distribution of algal species on the shell of Chiton articulatus was found. Only epizoic algae were found on the valves and the species Gelidium pusillum was detected in the shells of the adult organisms. Gelidium pusillum has a compact thallus (corticated filament) and is resistant to wave impact; this facilitates its adhesion through its rhizoids to the spaces between the shell and the valves. It should be noted, that according to Wahl (2008), in C. articulatus the tegument is completely smooth, which makes it more difficult for the establishment of spores, hormogonia, thalli, or algal species fragments or propagules. We found that the mantle, the paleal cavity, and the foot were free of algae. This same was reported by Brandani et al. (1974) in their study of Plaxiphora aurata (Spalowsky) in Mar de Plata, Argentina. Higher species richness was registered in the middle valves (II-VII). Meanwhile, in the cephalic valve (I) and the anal valve (VIII), the lowest species richness was observed. This could be due to the size of the valves being bigger than the middle ones, therefore having a bigger adhesion area for the algae epibionts. Adding to this, Wahl (2008) explained that these 2 extreme valves have a bigger slope or inclination, somehow hindering the fixation of rhizoids and fixing disks of the algae. Regarding the algae encountered in the sutures of chitons, these were mainly filamentous. This could be owed to the protective space these sutures offer to the fixation of the algae, reducing the detachment by the effect of the waves.
The high species richness and the functional algal groups found on the valves of C. articulatus could be related to the chiton response to light, as mentioned by Chelazzi et al. (1988) C. articulatus is a nocturnal animal, therefore, this species could be benefiting from the algae epibiosis finding refuge from the typical solar incidence in the high intertidal zone, where adults chitons are commonly found. Wahl (2008, 2009) mentioned that the algal epibiosis helps the chiton camouflage from predators and helps them improve their mobility for feeding habits. The dominance of filamentous and crust algae in this study was also documented by Brandani et al. (1974), Bretos and Chihuailaf (1990), and Connelly and Turner (2009).
Wahl (2008) mentioned that the longevity of the host has to be enough to allow the algae epibionts to complete their life cycle. There are longevity records for other species such as, Chiton tuberculatus Linnaeus. Comfort (1957) mentioned that this species can live up to 12 years. Another example is Stenoplax magdalenensis (Hinds), which according to Comfort (1957), can live for 3 to 4 years. For C. articulatus there are no studies regarding its life cycle, but considering the other species something similar could be expected. This longevity explains the survival of the algae, since it allows the entire algae life cycle to take place, therefore annual and perennial algae were recorded on the chiton shell in C. articulatus.
The filamentous functional group, according to López et al. (2017), is related to the sedimentation rates since they have the capacity to retain sediments and tolerate adverse conditions that not many algae could survive. Mateo-Cid and Mendoza-González (2012) presented records for the Mexican Tropical Pacific for the first time for Myrionema strangulans. However, these authors only registered this species as epiphyte of Padina durvillae Bory and did not point out that it is an epizoic species. Another difference is that the former work recorded Myrionema strangulans only in dry season, while in the present contribution only in the rainy season was recorded. Most of the species identified in this paper have been recorded on rocks or growing on other algae. A lot of them are strict epiphytes. The present contribution widens the knowledge of the substrates that these algae could be exploding in marine communities. The seasonal fluctuations encountered agree with the patterns recorded in other algal studies from similar localities in Guerrero, Nayarit, Jalisco, Colima and Michoacán (Mateo-Cid & Mendoza-González 1991, 1992; Mendoza-González & Mateo-Cid, 1998; Mendoza-González et al., 2011).
In this study, significant differences were found among the sampling months; January clearly separated in the PCO analysis. The species that were exclusive for January were Acrochaetium pacificum Kylin, Colaconema coccineum (K.M. Drew) P.W.Gabrielson and Ectocarpus sp. and the most abundant algae coverage during this month were from Lithophyllum sp. and Myrionema strangulans. In November, 5 exclusive species were recorded (Calothrix sp., Lithothamnion sp., Rhodymenia sp., Polysiphonia pacifica Hollenberg, and Streblonema sp.), this could be due to the seasonality, November was the only dry season month in which chitons were collected, and the rest of the algae were shared in both sampling seasons (dry and rainy season). Mateo-Cid and Mendoza-González (2012) have mentioned that annual algae dominate in the dry season and diminish or even disappear in the rainy season. This could be the reason for the differences encountered for the months of January and November both being dry months in comparison to the rainy months (May and July). Also, Mateo-Cid et al. (2011) y Mateo-Cid and Mendoza-González (2012) have observed in some Mexican Tropical Pacific localities that perennial algae species from the families: Rhodomelaceae, Ceramiaceae, Achrochaetaceae, Cladophoraceae, Delesseriaceae, Ulvaceae and Ectocarpaceae, dominate in the rainy season, meanwhile in the dry season families as Corallinaceae and Sargassaceae prevail. This could be the reason in the dry season (January and November) the crust functional group dominated in the shell of the chitons and the filamentous group recorded higher algae coverage in the rainy months. According to Mateo-Cid and Mendoza-González (2012) algae families such as Colaconemataceae and Cordariaceae have commonly annual algae species present with their reproductive peak in the dry season. This could explain the presence of species like Erythrotrichia carnea (Dillwyn) J.Agardh and Myrionema strangulans, as well as the genera Colaconema sp. and Lithophyllum sp. in chitons (Q1, Q2, Q5 y Q6) observed in the PCO analysis.
It is important to mention and highlight the scarcity of studies regarding algal epibionts in communities of marine invertebrates, even though the importance that clearly the epibionts algae have on the chitons of rocky intertidal communities. Also, most of the studies are up to 30 years old and mainly in temperate areas; this emphasizes the importance of new data in other environments, mainly tropical ones since these latitudes are known to be scarce in studies regarding marine topics.
We conclude that chitons, specifically C. articulatus, offers a substrate for the colonization of epibiont algae. Studies that explore new substrates are of vital importance, since here lies the opportunity to find new taxa or new records that are not usual in floristic inventories. These works can contribute to increasing the richness of algae in a locality and their monitoring will allow us to understand the ecological interactions that these taxa have with algal species in the Mexican tropical Pacific.











 nueva página del texto (beta)
nueva página del texto (beta)


