Introduction
High-mountain lakes (HMLs) are located in remote areas, in basins with cold climates, sparse vegetation, and thin soils; these conditions are usually associated with low pH values and very low mineralization, and nutrient concentrations (Battarbee et al., 2002; Granados et al., 2006). Because of these characteristics, HMLs are very sensitive ecosystems, constituting natural sensors of environmental changes (Catalán et al., 2002; Koinig et al., 2002). Despite their remoteness, human influence is possible due to the effects of global warming, airborne particles, acid deposition, and in some cases also increase in erosion associated with human activities in their basins. As in other lakes, HML sediments represent valuable archives where biological and geochemical indicators can be preserved (Caballero et al., 2020; Catalán et al., 2002; Ndayishimiye et al., 2020), keeping a historical record of past conditions. The study of short sedimentary sequences from HMLs can provide an overview of their recent history which may allow identifying whether they show signs of human-induced changes or if they represent relatively unaltered reference sites. These studies are relevant for developing sound management and preservation programs of these frequently pristine ecosystems.
There are only 2 high-mountain lakes in central Mexico, El Sol and La Luna, both inside the crater of the Nevado de Toluca volcano, at 4,200 m asl. Despite their proximity (600 m apart), each lake has distinctive limnologic characteristics and biological communities (e.g., diatoms, cladocerans, rotifers, benthic invertebrates), with the smaller and shallower Lake La Luna having a lower pH, mineralization, and nutrient levels than El Sol (Caballero, 1996; Dimas-Flores et al., 2008; Oseguera et al., 2016). Previous works showed evidence of human- induced alterations in these lakes. Cladoceran analysis in the sediments from Lake La Luna showed a short-term disappearance of planktonic Daphnia associated with failed fish (rainbow trout) introduction to this lake around 1940 to 1950 (Caballero et al., 2020; Cuna et al., 2014), while in Lake El Sol fish introduction was successful and trout are currently present in the lake. Furthermore, organic carbon burial rates have increased in both lakes since the 1950s, and particularly after 2000 in Lake El Sol, most likely associated with higher airborne particle deposition (Alcocer et al., 2020). Field data also point to an increase in electric conductivity and water turbidity in Lake El Sol in the last ~20 years and in both lakes a reduction in water levels associated with an increase in temperature and pH values (Alcocer et al., 2020, 2021).
Testate amoebae have proved to be sensitive bioindicators of organic matter concentration in sediments, trophic status, pH, and dissolved oxygen concentration (Charqueño-Celis et al., 2019; Dallimore et al., 2000; Escobar et al., 2008; Sigala et al., 2018). In addition, they have a good preservation potential under low pH environments (Swindles & Roe, 2007). The present work documents the testate amoebae species richness and diversity in the surface sediments from 2 tropical HMLs, El Sol and La Luna, as well as in core sediments recovered from both lakes. This investigation expands the limited knowledge on the ecological distribution of these organisms in tropical latitudes and to the best of our knowledge is the first report on the testate amoebae assemblages preserved in sediments from lakes at elevations higher than the local tree line. Our hypothesis is that there will be differences in the testate amoebae species composition and diversity present in each of the studied lakes because of their distinctive limnologic characteristics, with a lower species diversity in the more acidic Lake La Luna. We also expected to record changes in the testate amoebae species preserved in the sediments from both lakes during the last 2 decades as a response to the recent, human-induced changes recorded in these lakes.
Materials and methods
Lakes El Sol and La Luna are at 4,200 m asl, inside the crater of the Nevado de Toluca volcano (Fig. 1), which is part of a natural protected area established in 1936 (Toscana & Granados, 2015). The climate is cold with a mean annual temperature of 3.8 °C and extreme daily temperatures in the range of -9 °C to 19 °C (SMN, 2021). Rainfall is concentrated from May to September, with an annual average of 1,213 mm yr-1. The vegetation inside the crater is alpine meadow as it lies above the local tree line (ca. 4,000 m asl). It must be noted that these tropical HMLs are located at much higher elevations than their temperate counterparts.
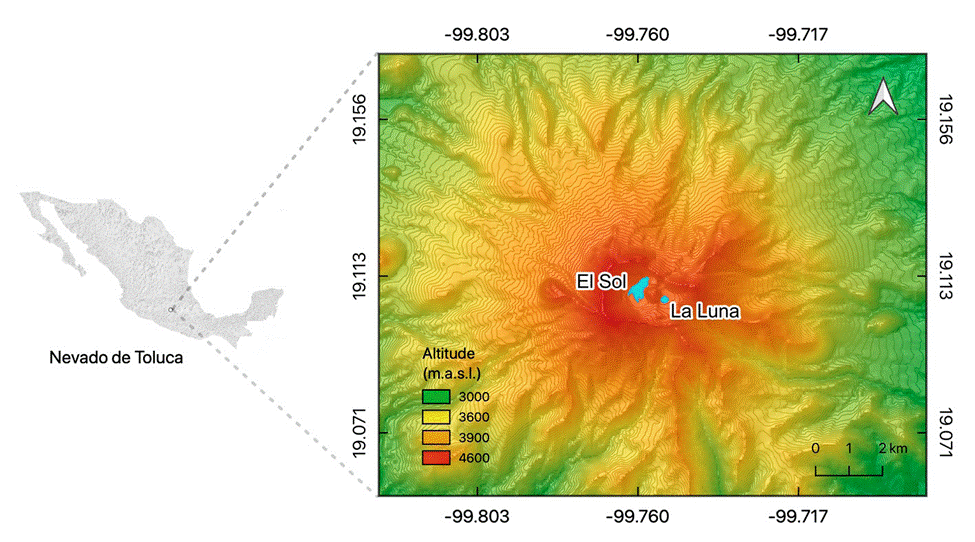
Figure 1 Location map of the lakes in the Nevado de Toluca crater: El Sol and La Luna, central Mexico.
The lakes are shallow (< 15 m), cold (< 12°C), transparent, with low alkalinity, and low mineralization (K25 < 50 µS cm-1). Lake La Luna is smaller, shallower (< 10 m), more acidic (pH < 6), and has a lower trophic level (ultraoligotrophic) than El Sol (oligotrophic). Despite their high elevation, their surfaces do not freeze during winter, and their mixing regime is discontinuous (El Sol) or continuous (La Luna) warm polymictic (Alcocer et al., 2021).
Water column physico-chemical measurements and surface sediment samples were obtained in March, June, and September 2019. Physico-chemical variables were recorded along the water column (discrete readings every meter) at the deepest part of each lake with a calibrated multiparameter water-quality data logger (Hydrolab DS4 & DS5). These measurements included temperature (± 0.10 °C accuracy, 0.01 °C resolution), dissolved oxygen (± 0.2 mg L−1 accuracy, 0.01 mg L−1 resolution), electric conductivity (K25 1.0 mS cm−1 accuracy, 0.0001 resolution), and pH (0.2 accuracy, 0.01 resolution).
Surface sediment samples were collected with an Eckman dredge (0.0225 m2). A total of 4 replicates from each lake were collected per visit at the deepest (> average depth) zone of both lakes, about 50% of the bottom area in Lake El Sol and 60% in Lake La Luna. The topmost sediments from the dredge (~1 cm) were transferred with a plastic spatula to 250 ml plastic jars and preserved with ethanol (70% v/v).
Additionally, 2 short sediment cores were recovered at the central area from each lake in 2013 using a Uwitec gravity corer. The cores were sampled every 1 cm for 210Pb and 137Cs activities, organic carbon concentration, grain size distribution, Fe concentrations, and microfossils analyses. Mass accumulation rates, sediment grain size distribution, and organic carbon concentrations from these cores were published by Alcocer et al. (2020) and will be used in this paper for comparison with the testate amoebae data. The age models from both cores were also published by Alcocer et al. (2020) and are used here to establish an estimated age for the core sediment samples (Table 1).
Table 1 Estimated ages based on 210Pb and 137Cs chronologies of core sediment samples from lakes El Sol and La Luna, Nevado de Toluca, central Mexico (based on Alcocer et al., 2020).
| Depth (cm) | El Sol | La Luna |
|---|---|---|
| 1 | 2012 ± 0.2 | 2012 ± 0.2 |
| 2 | 2010 ± 0.3 | 2008 ± 0.4 |
| 3 | 2006 ± 0.4 | 2003 ± 0.5 |
| 4 | 2002 ± 0.6 | 1998 ± 0.6 |
| 5 | 1996 ± 0.7 | 1993 ± 0.7 |
| 6 | 1990 ± 0.9 | 1987 ± 0.9 |
| 7 | 1981 ± 1.0 | 1981 ± 0.9 |
| 8 | 1971 ± 1.3 | 1975 ± 1.0 |
| 9 | 1958 ± 1.6 | 1968 ± 1.1 |
| 10 | 1938 ± 2.2 | 1959 ± 1.3 |
Core and surface sediment samples (2 to 3 cm3) were freeze-dried and homogenized using an agate mortar at the laboratory. The Fe percentage in the samples was determined by X-Ray fluorescence (XRF) using a portable NITON XL3t analyzer. This terrigenous element is used as an indicator of increased sediment input to the lakes (Roy et al., 2014).
For testate amoebae analysis, 2 cm3 of wet sediment were collected from the surface sediment samples and also every 2 cm on the top 10 cm of the cores from each lake. These were the sections of each core that were dated and represent ~75 years in Lake El Sol and ~55 in Lake La Luna. All samples were sieved through a 53-μm screen to retain the amoebae tests. Each sieved sample was subdivided into aliquots of the same volume, which were examined until reaching a minimum of 150 tests (Payne & Mitchell, 2009). Samples were observed in a Petri dish at 64 x to 100 x with a stereomicroscope (Olympus SZX and AmScope Zoom). Some amoebae tests were extracted with a fine brush and kept on semi-permanent slides with glycerin as reference material, and some of these tests were also observed under a scanning electron microscope (JEOL NeoScope JCM-600). Specialized taxonomic keys were used for their identification (Kumar & Dalby, 1998; Lee et al., 2000; Siemensma, 2021).
Lacustrine arcellacean species can display ample ecophenotypically controlled morphological variability and it is an accepted practice to assign informal infra- subspecific ‘strain’ names to these ecophenotypes, largely to avoid the possible description of unjustified new species (Patterson & Kumar, 2000). Although the International Code of Zoological Nomenclature stipulates that infra- subspecific-level designations have no taxonomic status (ICZN, 1999), in this work we use these ‘strain’ names as they are useful for distinguishing environmentally significant populations in lacustrine environments (Patterson et al., 2013).
Species richness (S) and Shannon diversity indices (H) transformed with the natural logarithm were calculated using Past software (Hammer et al., 2001). Kruskal-Wallis pairwise tests were done also using Past to establish statistical differences between physico-chemical variables of the lakes. Also, a detrended correspondence analysis (DCA) was performed including the surface and core sediments samples, to identify groups of samples with similar species composition (Correa-Metrio et al., 2014). The analysis was done using R statistical software (R Core Team, 2014) and the package vegan (Oksanen et al., 2013).
Results
Water column conditions were relatively uniform in both lakes, therefore only average values with their standard deviations for March, June, and September 2019 are presented in table 2. Lake El Sol showed consistently higher pH, dissolved oxygen, and K25 ranges (p < 0.001) compared to Lake La Luna in the 3 sampling periods (Table 2, Fig. 2).
Table 2 Limnological variables from lakes El Sol and La Luna measured in March, June and September 2019. Values correspond to water column averages and standard deviations (n = 12 in El Sol and n = 9 in La Luna). * Denotes statistical difference in Kruskal-Wallis pairwise comparisons between El Sol and La Luna values (p < 0.001).
| Month | Temperature (°C) | pH | Dissolved oxygen (mg L-1) | K25 (µS cm-1) | |
|---|---|---|---|---|---|
| Lake El Sol | March | 9.6 ± 0.3 | 8.6 ± 0.0* | 7.8 ± 0.2* | 42.4 ± 1.9* |
| June | 10.3 ± 0.1* | 7.8 ± 0.1* | 7.6 ± 0.3* | 44.5 ± 0.2* | |
| September | 12.3 ± 0.1* | 7.4 ± 0.2* | 8.0 ± 0.3* | 45.5 ± 1.4* | |
| Lake La Luna | March | 9.7 ± 0.9 | 5.0 ± 0.3* | 7.1 ± 0.1* | 11.7 ± 0.5* |
| June | 9.4 ± 0.0* | 5.2 ± 0.3* | 6.5 ± 0.0* | 12.5 ± 0.1* | |
| September | 11.4 ± 0.0* | 4.9 ± 0.0* | 7.1 ± 0.1* | 10.8 ± 0.0* |
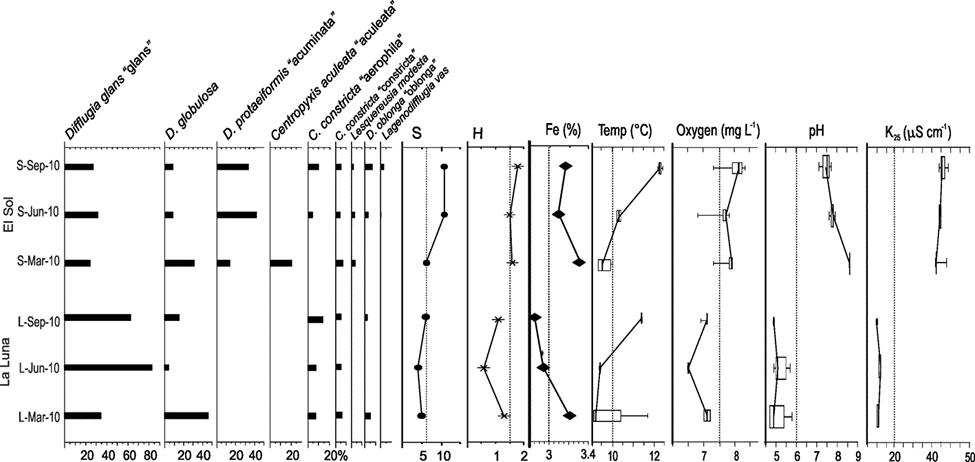
Figure. 2 Main testate amoebae taxa (> 3%), species richness (S), Shannon diversity (H) and Fe concentrations in surface sediment samples from lakes El Sol (top) and La Luna (bottom), Nevado de Toluca, central Mexico. The graph also shows boxplots of water column parameters: temperature (Temp.), oxygen concentrations, pH and electric conductivity (K25).
A total of 18 taxa were found distributed in 7 genera (Table 3), some of which are illustrated in figure 3. One taxon was identified only to the genus level, 7 taxa to species level, and 10 taxa were assigned to a strain. Of the 18 identified taxa, 6 were common to both lakes, 11 were exclusively present in lake El Sol, and 1 was exclusive to Lake La Luna (Bullinularia). Except for Pontigulasia compressa, which represents the first report for Mexico, all the other species had been previously reported for the country (Charqueño-Celis et al., 2019; Sigala et al., 2016, 2018).
Table 3 Testate amoebae taxa present in surface sediment (SS) and core sediment (CS) samples from lakes El Sol and La Luna, Nevado de Toluca, Mexico.
| Code | Taxa | El Sol | La Luna | ||
|---|---|---|---|---|---|
| SS | CS | SS | CS | ||
| ADE | Arcella discoides Ehrenberg, 1843 | x | x | ||
| BUL | Bullinularia Deflandre, 1953 | x | |||
| CAA | Centropyxis aculeata Ehrenberg, 1832 strain “aculeata” | x | x | ||
| CAD | Centropyxis aculeata Ehrenberg, 1832 strain “discoides” | x | |||
| CCA | Centropyxis constricta Ehrenberg, 1843 strain “aerophila” | x | x | x | x |
| CCC | Centropyxis constricta Ehrenberg, 1843 strain “constricta” | x | x | x | |
| CCS | Centropyxis constricta Ehrenberg, 1843 strain “spinosa” | x | x | x | |
| DEL | Difflugia elegans Penard, 1890 | x | x | ||
| DFR | Difflugia fragosa Hempel, 1898 | x | |||
| DGG | Difflugia glans Penard 1902 strain “glans” | x | x | x | x |
| DGL | Difflugia globulosa Dujardin, 1837 | x | x | x | x |
| DOO | Difflugia oblonga Ehrenberg, 1832 strain “oblonga” | x | x | x | x |
| DPA | Difflugia protaeiformis Lamarck, 1816 strain “acuminata” | x | x | ||
| DPM | Difflugia protaeiformis Lamarck, 1816 strain “amphoralis” | x | |||
| DUE | Difflugia urceolata Carter, 1864 strain “elongata” | x | x | ||
| LVA | Lagenodifflugia vas Leidy, 1874 | x | |||
| LMO | Lesquereusia modesta Rhumbler, 1895 | x | x | ||
| PCO | Pontigulasia compressa Carter, 1864 | x | |||
| Species richness | 13 | 16 | 6 | 5 | |
| Total species richness per lake | 17 | 7 |
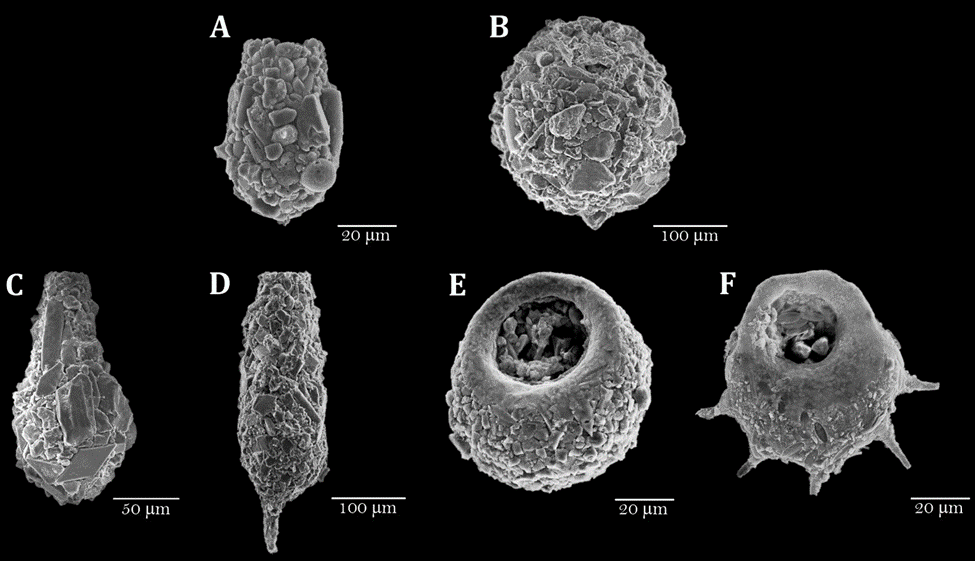
Figure 3 Scanning electron microscopy images of selected species of testate amoebae in the sediments from lakes El Sol and La Luna, Nevado de Toluca, central Mexico. A, Difflugia glans strain “glans”; B, Difflugia globulosa; C, Difflugia oblonga strain “oblonga”; D, Difflugia protaeiformis strain “acuminata”; E, Centropyxis constricta strain “aerophila”; F, Centropyxis constricta strain “spinosa”.
In Lake El Sol the species richness was 17 taxa; 12 were recorded in surface and core sediments, 1 (Lagenodifflugia vas) was present only in surface sediments, and 4 were present only in core sediments. Species richness in surface sediment samples from El Sol was 13 (range 6 to 11), with Shannon index values ranging from 1.55 to 1.81 (Fig. 2). In core sediment samples from El Sol species richness was 16 (range 8 to 10), with Shannon index values from 1.22 to 1.69 (Fig. 4a).
Species richness in Lake La Luna was 7 taxa; 4 were present in both surface and core sediments, 2 only in surface sediments, and 1 only in core sediments. Species richness in surface sediment samples from La Luna was 6 (range 4 to 6), with Shannon index values ranging from 0.64 to 1.25 (Fig. 2). In core sediment samples from Lake La Luna species richness was 5 (range 2 to 5); Shannon index values ranged from 0.02 to 1.26, with the lowest values (< 0.5) in the 2 most recent core samples (~1998, ~2008) (Fig. 4b).
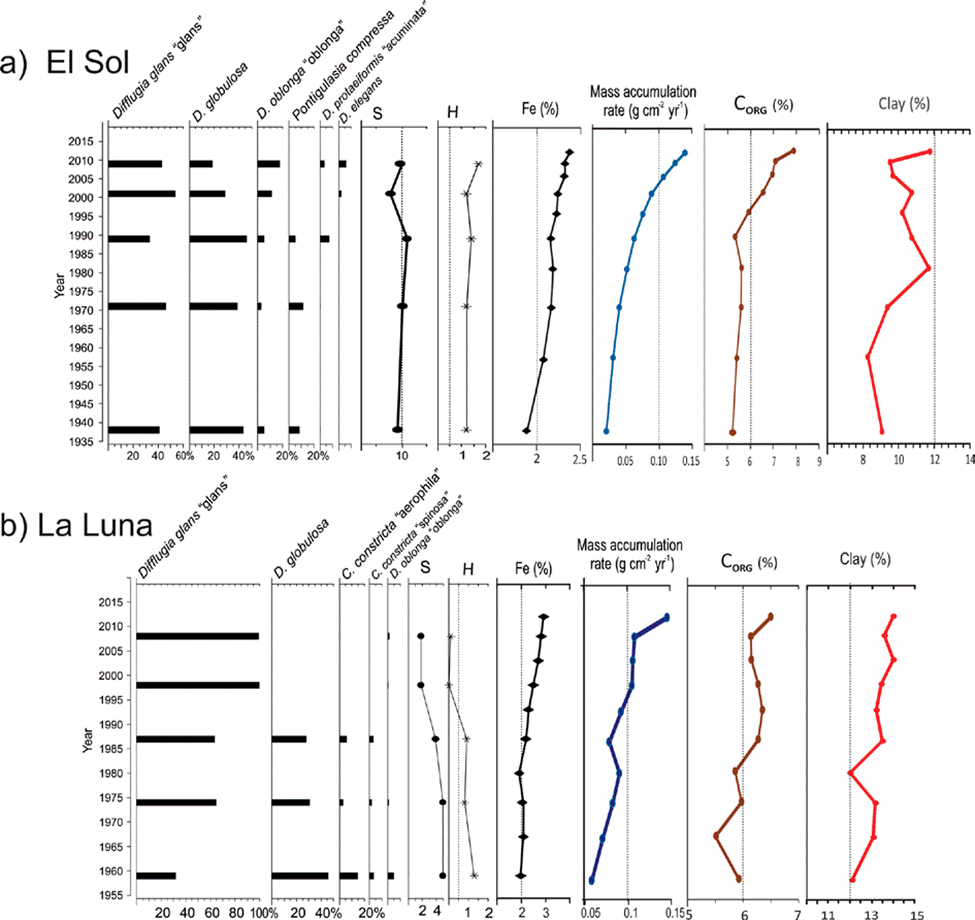
Figure 4 Main testate amoebae taxa (> 3%), species richness (S), Shannon diversity (H), and Fe concentrations is sediment cores from lakes El Sol (4a) and La Luna (4b) Nevado de Toluca, central Mexico. The graphs also show mass accumulation rates, organic carbon (Corg) and clay content (Clay) in the core sediments from both lakes (taken from Alcocer et al., 2020).
The most abundant species in the surface sediment samples from Lake El Sol were D. glans “glans”, D. globulosa, and Difflugia protaeiformis “acuminata”, with lower abundances of Centropyxis aculeata “aculeata”, Centropyxis constricta “aerophila”, Centropyxis constricta “constricta”, and Difflugia oblonga “oblonga” (Fig. 2). The most abundant species in the surface sediment samples from Lake La Luna were Difflugia glans “glans” and Difflugia globulosa, with lower abundances of Centropyxis constricta “aerophila” and Centropyxis constricta “constricta” (Fig. 2).
In the core sediment samples from Lake El Sol, the 2 taxa that shared the highest abundances were D. glans “glans” and D. globulosa (Fig. 4a). The 3 bottom samples (1938, 1971, 1990) had equal proportions of these 2 species, and low abundances (< 20 %) of P. compressa, while the top 2 samples (2001, 2009) showed higher proportions of D. glans “glans”, lower abundances of D. globulosa, and low abundances (< 15%) of D. oblonga “oblonga” and D. elegans. The most abundant testate amoebae taxa along the core sequence from Lake La Luna were also D. glans “glans” and D. globulosa (Fig 4b); however, in the 2 more recent samples (1998 and 2008) D. glans “glans” accounted for > 90% of the tests.
The Fe concentrations in the core sediments from Lake El Sol ranged from 1.9 to 2.4%, with an increasing trend from the bottom, particularly since 1996 (Fig. 4a), and in surface sediments they ranged from 3.1 to 3.3% (Fig. 2). In the core sediments from Lake La Luna, Fe concentrations ranged from 2.5 to 3.0%, with an increasing trend since 1987 (Fig. 4b), and in surface sediments they ranged from 2.9 to 3.2% (Fig. 2). The increases in Fe concentrations in the more recent core sediments from both lakes mirror the trends previously observed in mass accumulation rates, organic carbon content, and clay proportions (Fig. 4).
In the DCA1 (eingenvalue = 0.273) vs. DCA 2 (eigenvalue = 0.152) biplot (Fig. 5), most of the samples from Lake El Sol are distributed on the positive side of DCA2 owing to their higher abundances of D. globulosa (DGL). The surface sediment samples from Lake El Sol form a group to the right of the diagram (positive side of DCA1) owing to the higher abundances of D. protaeiformis “acuminata” (DPA) while the 2 oldest cores samples from Lake El Sol (1938, 1971) separate to the top- left of the diagram due to the presence of P. compressa (PCO). On the other hand, the samples from Lake La Luna are distributed on the negative side of DCA2, owing to their generally higher abundances of D. glans “glans” (DGG); this is true particularly for the 2 most recent core samples (1998 and 2008) and for the surface sediment samples from June and September 2019, which group to the bottom-left of the biplot.
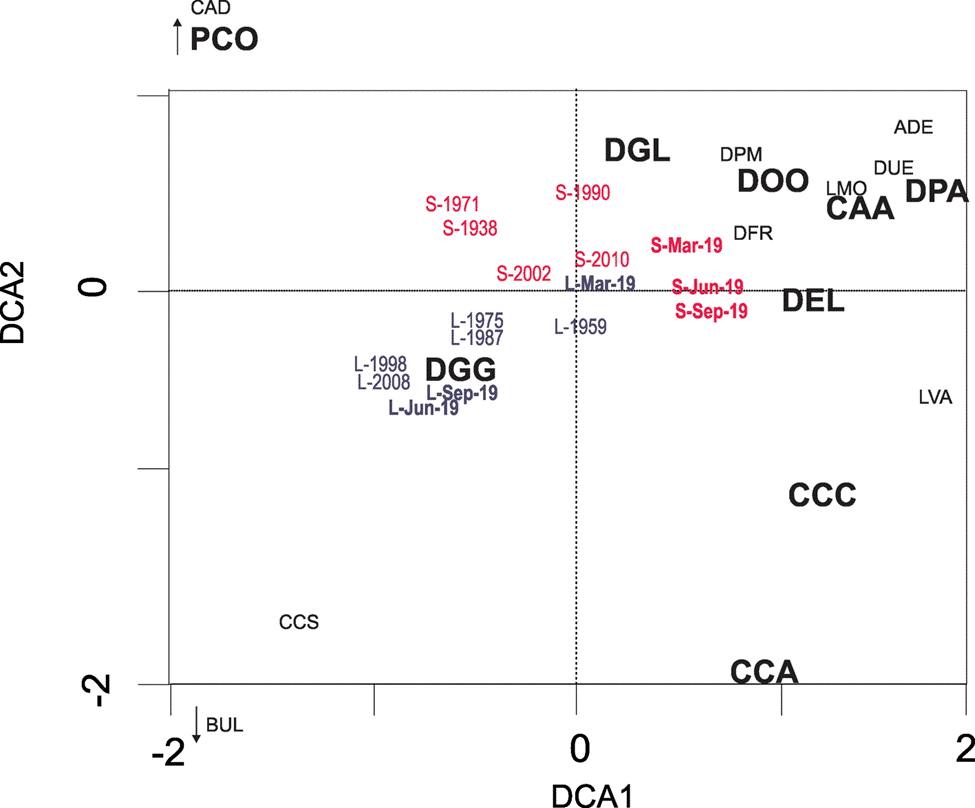
Figure 5 Detrended correspondence analysis biplot (DCA1 vs. DCA2) of testate amoebae taxa in surface and core sediment samples from lakes El Sol and La Luna, Nevado de Toluca, central Mexico. Samples from Lake El Sol in red and from Lake La Luna in gray, surface sediment samples from both lakes in bold. Species with abbreviations in smaller print were present in abundances < 3%. Species abbreviations are according to those in Table 3.
Discussion
In this work we found that the 2 most abundant testate amoebae taxa in the Nevado de Toluca lakes were D. glans “glans” and D. globulosa. However, we could not locate any testate amoebae studies from other lakes above the local tree line, so it was impossible to compare the faunas from the Nevado de Toluca lakes with others from similar ecosystems. Nevertheless, D. glans “glans” has been reported around the world mostly from oligotrophic ecosystems (Patterson et al., 2013), while D. globulosa (including all its synonyms Difflugia globularis, D. globula, D. globulus) has been associated with cold temperatures (Asioli et al., 1996; Trappeniers et al., 1999; Wall et al., 2010). These ecological affinities agree with their high abundance in the Nevado de Toluca lakes, which are characterized by low temperatures (< 12°C) and low nutrient levels (ultraoligotrophic and oligotrophic). Regarding the distribution of these species in other lakes in Mexico, they were present, but uncommon, in a survey that included 30 lakes in central Mexico (Sigala et al., 2018). Their low abundances in this survey suggest that they are not favored by the limnologic conditions of the lower elevation lakes in central Mexico, which were mostly mesotrophic to hypertrophic, had alkaline pH values, and higher mineralization (K25 > 100 mS cm-1) (Sigala et al., 2017), in contrast with the characteristics of the Nevado de Toluca lakes.
The testate amoebae assemblages from lakes El Sol and La Luna showed differences between both ecosystems in terms of species richness and Shannon diversity (p < 0.001). Species richness in Lake El Sol was nearly double (Savg = 9.4±1.7) that of La Luna (Save = 4.1±1.5), with 33% of the total identified taxa common to both lakes, 61% exclusive to El Sol and only 6% (one taxon) exclusive to La Luna. This shows a nested pattern in the testate amoebae species distribution between both lakes, as the species present in Lake La Luna are a subset of those present in Lake El Sol. A similar nested pattern was observed in the benthic macroinvertebrates from these lakes, with 8 taxa present in Lake El Sol of which only 2 were present in La Luna (Oseguera et al., 2016). However, in other biological groups, such as diatoms and rotifers, a turnover between dominant taxa rather than a nested pattern was observed (Caballero, 1996; Dimas et al., 2008).
The Shannon diversity index was also higher in Lake El Sol (Havg = 1.45±0.2) compared with Lake La Luna (Havg = 0.75±0.5). These values show that at La Luna the assemblages are not only formed by a lower number of species, but also that they have a lower equitability (higher dominance). The lower diversity in Lake La Luna has been attributed in previous works to its more extreme limnologic conditions (lower pH, lower mineralization, and lower nutrient levels), which can limit the development of some taxa (Oseguera et al., 2016). This is in accordance with the diversity-stability hypothesis (Pimm, 1984), which suggests that diversity will decrease as environmental variables reach extreme values, given that tolerant species will grow without competition, resulting in abundance patterns of heavy dominance of a few species (Tilman et al., 1997; Van Dam, 1982; Wassen et al., 2005). In particular for testate amoebae assemblages, it has been proposed that extreme or hostile environments will typically have Shannon index values < 0.5 (Patterson & Kumar, 2002). These very low Shannon index values were only recorded in the 2 most recent core samples from Lake La Luna (~1998, ~2008), where D. glans “glans” accounted for over 90% of the counts. These extremely low Shannon index values can be taken as an indication that during this period (~1998 to ~2008) the lake experienced even higher environmental stress, even though it is not clear which environmental variable could have been the stressor.
It is interesting to note that in both core sequences the 2 most recent samples show changes in the testate amoebae assemblages compared to the older core samples, but in Lake El Sol the surface sediment samples also have a distinctive assemblage. Even though D. glans “glans” and D. globulosa remain as the main taxa, in Lake El Sol the 2 most recent core samples (~2002, ~2010) showed higher proportions of D. oblonga “oblonga” and D. elegans, which in the surface sediment samples was replaced by D. protaeiformis “acuminata”. The reason for these changes is unclear; the higher abundance of D. oblonga “oblonga” and D. protaeiformis “acuminata”, which are species that in other lakes have been associated with high organic carbon concentrations in the sediments (Kihlman & Kauppila, 2009; McCarthy et al., 1995), suggests that these changes could be related with the increase in the organic carbon content in the sediments from this lake. The organic carbon content in the sediments from Lake El Sol nearly doubled since 1995, and was associated with higher Fe proportions, as well as higher clay percentages and mass accumulation rates that Alcocer et al. (2020) attributed to human induced wind-blown particles from the volcano slopes and from long distance transport from urban areas. The changes in the testate amoebae assemblages from the Nevado de Toluca lakes recorded in this work add to previous evidence of recent changes in the limnologic characteristics and the biotic communities from these lakes (Alcocer et al., 2021; Oseguera et al., 2016), which also point to more significant changes in Lake El Sol compared with La Luna. The changes in the biota of these lakes are warnings that urge us to improve the protective measurements for lakes El Sol and La Luna, not only at the local (crater) level but also with a more regional perspective.











 nueva página del texto (beta)
nueva página del texto (beta)


