Introduction
Thermal stress occurs when body heat gains or losses are not balanced by energy exchanged with the environment, requiring additional energy expenditure for animals to maintain their thermoneutral zone (Bohler et al. 2021). This leads to detrimental effects on their health, growth, reproduction, and productivity, impacting their performance and overall fitness (Guthery et al. 2001, Archambault et al. 2014, Mesa et al. 2002, Xie et al. 2017, Pattinson et al. 2020, Bohler et al. 2021, Cunnigham 2021, Mckechnie et al. 2021, Fathima et al. 2022). Temperature and potential thermal stress can fluctuate spatially and temporally, and may depend on factors such as the time of the day, climate, relative humidity, and animal species (Kingsolver et al. 2013, Santillán et al. 2018, Rodrigues et al. 2022, Sudyka et al. 2023).
Birds are endothermic homeotherms that can regulate their body temperature to maintain a particular temperature range regardless of the ambient temperature (Bicego et al. 2007). However, altricial birds have young that are underdeveloped and highly dependent on parents during the first days after hatching, and lack strategies to maintain body temperature independent of environmental temperature in the early stages of development (Dawson and Evans 1957, Choi and Bakken 1990, Visser 1998, Baarendse et al. 2007). In this sense, young altricial nestlings behave thermally like ectotherms and rely on parental care as an external source of heat (Ricklefs 1984). The age of endothermy, when altricial nestlings have developed thermal strategies enabling them to produce heat and thermoregulate, is species-specific (Morton and Carey 1971, Dunn 1975, Visser and Ricklefs 1993), and develops as nestlings gain mass allowing them to increase their metabolic rate when ambient temperature is below neutral temperature (O’Connor 1975, Visser 1998, Nagy 2005). This implies that prior to the onset of endothermy, adequate thermoregulation in the nest can have implications for nestling fitness as nestlings are unable to regulate their body temperature during early stages of development.
Hence, the thermal environment inside the nest could be key for altricial nestlings at early stages of development when they are more susceptible to thermal stress, for which the use of tree cavities can beneficial. Predation has been proposed as the main selection force driving the evolution of the use of different nest types (Vanadzina et al. 2024). However, the thermal benefits of cavity nests may outweigh the benefits provided against predation compared to open-nests (Martin et al. 2017). In particular, cavity nests may buffer temperature fluctuations in extreme environments, and provide a constant thermal environment regardless of outside temperatures (McComb and Noble 1981, Rhodes et al. 2009).
Urban areas represent extreme environments with modified microclimates that produce the heat island effect, where cities are often warmer that adjacent natural areas (Arnfield 2003). The frequency of heatwaves and number of hot days are also increasing in urban areas (Mishra et al. 2015). Nest-boxes are frequently used in cities to compensate for a low availability of natural cavities for birds (Blewett and Marzluff 2005, Harper et al. 2005, LaMontagne et al. 2015). However, nest boxes may be of low thermal quality due to their thinner walls compared to natural cavities (Maziarz et al. 2017, Strain et al. 2020, Sudyka et al. 2023), negatively impacting fitness (Ardia 2013, Sudyka et al. 2022). Given the significant impact of thermal stress on the health and survival of nestlings (Andreasson et al. 2018, Corregidor-Castro and Jones 2021, Arct et al. 2022), understanding the thermal environment of nest-boxes in cities can provide insights on the potential impact of nest provision on thermal stress in urban conditions.
Our understanding of the temperatures experienced by altricial nestlings during early development is limited, particularly for cavity-nesting species in urban environments with limited nesting opportunities, where nest-boxes may frequently be used. Understanding the development of thermoregulation capabilities of nestlings, and how this may fluctuate with dial temperature variations can elucidate whether nestlings experience thermal stress during their early development. The Bewick's Wren (Thryomanes bewickii) is an altricial passerine bird that is resident from Canada southwards to central Mexico, occupying a variety of natural and urban environments throughout its range (Kennedy and White 2020). Therefore, nestlings of the Bewick's Wren may face a variety of thermal environments that could present thermal challenges, putting nestlings at thermal stress, outside of their thermoneutral zone. We aimed to evaluate the thermal ecology of Bewick’s Wren nestlings during the early stages of development to determine whether they were at thermal stress, with body temperature above or below the internal nest temperature, when using nest-boxes in an urban setting.
Methods
Study site
We conducted the study in 2019, at three sites in Morelia city, in the state of Michoacán, Mexico. The study sites were located at: 1) Universidad Latina de América (19°41'50.02"N, 101°14'7.86"O) west of the city center; 2) Universidad Michoacana de San Nicolás de Hidalgo (101°14'7.86"O, 101°12'5.87"O) south of the city center; and 3) Instituto Tecnológico del Valle de Morelia (19°44'55.39"N, 101° 9'51.64"O) on the northern outskirts of the city. Morelia city has an extension of 67.2 km2 (IMPLAN 2022), with agriculture and some pasturelands around the outskirts of the city. The average annual temperature is 18.2°C, and mean total annual rainfall is 803.6 mm, where 75% of rainfall occurs from June to September, followed by a drought from October to May (IMPLAN 2022).
We also identified a nest of the species in a cavity of a Casuarina eqisetifolia tree at Instituto Tecnológico del Valle de Morelia, on the outskirts of the city. We conducted observations at the nest during 12 to 20 February 2024, for a total of 240 mins, to obtain data on the length of time parents were off the nest for food provisioning. When observations were taken, nestlings were about midway through development.
Nest-boxes
At each of the three study sites, we placed 30 nest-boxes, which were attached with an eyebolt on either a tree branch or the main trunk. Nest-boxes were rectangular, made of 1.5 cm width plywood, and measured 14.5 cm depth x 12 cm width. The front of the nest-box measured 25 cm high, with a 5 cm diameter entrance hole, while the back of the nest-box measured 29 cm high, so that the roof of the nest-box was inclined allowing rainfall runoff and to reduce the likelihood of flooding. We placed nest-boxes on trees from mid-February 2018, so that breeding pairs of the Bewick’s Wren could become accustomed to their use. We checked nest-boxes weekly to register the onset of nesting activity, which was determined when nesting material was observed within the nest-box. Once we determined that a nest-box was in use by a nesting pair, we checked the nest every other day to register the laying and hatch-date. Therefore, we could determine the nestling’s age within two days of accuracy. The study was conducted under the Mexican Secretary for the Environment permit SGPA7DGVS/03994/17.
Nestling age of endothermy
To determine nestling' age of endothermy, we exposed 14 nestlings from 8 nests to a thermal challenge at 5 days of age and on each nest visit thereafter (McCafferty et al. 2015). For the thermal challenge, we inserted a thermocouple probe inside the chicks’ cloaca, which was attached to an Omega HH501AJK thermometer. Each nestling was then individually placed inside an insulin portable refrigerator for 5 mins at a constant temperature of 7°C. The thermocouple probe allowed us to register the chicks’ core temperature at the beginning and at the end of the thermal challenge. We then calculated the endothermy index (H) for each individual (Visser 1998) using: H = (Tf - Tc)/(Ts - Tc), where Tf is the nestling’s temperature at the end of the 5 min challenge, Tc is the refrigerator temperature, and Ts is the nestling’s initial temperature (Ricklefs 1984). The endothermy index provided an estimation of the nestlings’ ability to maintain their core temperature within an optimal threshold. A completely endothermic bird would have a value of H = 1, while values close to 0 would indicate a poikilotherm bird that is unable to regulate body temperature, and has a temperature closer to that of the environment. Following Visser (1998), we considered a nestling to have reached the age of endothermy when it registered H ≥ 0.8.
Estimation of thermoregulatory set-point range (Tset)
The thermoregulatory set point (Tset) is defined as the range of preferred temperature along a gradient that enables individuals to maintain a given body temperature, and can also be considered the thermoneutral zone (Hertz et al. 1993). Therefore, to determine the thermoregulatory set point (Tset) of nestlings, we took the cloacal body temperature (Tb) of five nestlings from three nests (2 broods = 1 nestling, 1 brood = 3 nestlings), at each nest inspection from hatching until they fledged. We then obtained Tset 25% - 75% as the temperature range that included 50% of all Tb measurements, corresponding to the second and third quartiles of all nestling body temperatures. In addition, given that thermoregulatory capabilities vary by age in altricial birds, we divided the entire developmental period in two stages: early- (hatching to the day before age of endothermy) and late-nestling (age of endothermy to fledging), and obtained Tset 25% - 75% for both developmental phases separately and combined.
Comparison of ambient and operative temperature (Te)
We further compared the potential thermal environment offered in the interior and exterior of nest-boxes with that experienced by biophysical models of nestlings. We characterized the potential thermal environment by measuring ambient temperature both inside and outside the nest-box. Temperature readings were stored in a custom-made Arduino nano based datalogger that was programed to register the temperature every 15 mins. This datalogger has several sensors that can register simultaneously readings from independent sources. Two of those sensors were used to obtain simultaneously temperature readings from inside and outside the nest-box.
In addition, we obtained the operative temperature (Te) for the thermal environment experienced by nestlings in nest-boxes. Operative temperature is the temperature of an inanimate object that does not thermoregulate, with zero heat capacity, and has the same size as the animal exposed to the same thermal environment (Bakken 1992). Thereby, operative temperature may be a close representation of the temperature experienced by an ectotherm, and enabled us to take into consideration the potential heat exchange due to radiation, convection, and conduction (Shine and Kearney 2001) of a non-endothermy nestling when parents were off the nest. Using a Ultimaker 2+ 3D printer, we created 3D Acrylonitrile Butadiene Styrene (ABS) biophysical models that imitated the size, shape, posture, and heat capacity of the target organism (Hertz 1992a, 1992b, Shine and Kearney 2001). Although Cooper-made physical models have been used in the past, ABS physical models have proved to be an equally reliable device (Watson and Francis 2015). Biophysical models were 3D printed with a hole in the ventral region, where we inserted an additional sensor of the thermal custom-made datalogger, thereby obtaining operative temperature simultaneously with potential ambient temperature. We built 3D physical models in two sizes according to developmental stage: (a) early-stage model representing individuals that were ≤ the age of endothermy, and (b) late-stage model, with the size of individuals ~14 days, when endothermic nestlings are ready to fledge. We placed 3D physical models in three randomly selected nest-boxes that had previously been used by Bewick’s Wrens: two nest-boxes at Universidad Latina de America, and one at Instituto Tecnológico del Valle de Morelia. Models were placed within nest-boxes on top of the nest-cup after nestlings had fledged as adults of the Bewick Wren usually remove objects from an active nest. We maintained dataloggers and models within nest-boxes for three days to obtain a range of measurements simulating the temperatures experienced by non-endothermic nestlings.
Thermoregulation indices
We obtained the difference in temperature inside and outside the nest-box (ΔT°) recorded by sensors on dataloggers, and the coefficient of variation (CV). We determined operative temperature for three time periods: a) morning (08:00 to 13:59 hrs), b) afternoon (14:00 to 18:59 hrs), and c) night (19:00 to 07:59 hrs). We also obtained the mean body temperature of nestlings (Tb) in both the early- and late-development stages. Based on these indices, we would consider nestlings to be under thermal stress if their thermoneutral zone, estimated from the second and third quartiles of all nestling body temperatures (Tset 25% - 75%), was above or below ambient or operative temperatures, indicating additional energy expenditure to maintain the thermoneutral zone. We also determined the period of the day when temperature inside the nest-box was not within the thermoneutral range of the second and third quartiles of nestling body temperatures (Tset 25% - 75%), to determine the time of day or night when nestlings could experience thermal stress. Results are expressed as means and standard deviations.
Statistical analysis
All variables followed a log normal error distribution except for nestling body temperature, which followed a normal error distribution, hence we performed Linear Mixed Models (LMM). We performed five LMM to evaluate the factors influencing ambient and nestling temperatures. To determine whether the type of environment influenced the potential thermal environment available for nestlings, we carried out LMM using ambient temperatures measured by the custom-made dataloggers as the response variable. The fixed effect of type of environment was considered with two levels: i) inside and ii) outside the nest-box. Furthermore, to evaluate whether period of the day could explain variations in ambient temperatures, we constructed two additional LMM, one with the response variable of temperature inside the nest-box, and the second model considering outside temperature. In both these models, we grouped temperature readings by period of the day, with three levels, i) morning, ii) afternoon, and iii) night, as the fixed effect. To assess the influence of nestling development on body temperature, we constructed a fourth LMM using nestling body temperatures from cloaca measurements. For this model, nestling developmental stage of i) early- and ii) late-stage nestling was considered as a fixed effect. Finally, to determine the influence of nestling developmental stage on operative temperatures in the nest-box, we constructed a fifth LMM with operative temperatures with the 3D-printed models as the response variable. Model developmental stage was considered as the fixed effect, with two levels: small, and large.
To account for the lack of independence in multiple temperature readings taken continuously, we considered the nest-box as the random effect in models, except for the nestling body temperature model, for which nestling identity was used as random effect. We used the nlme package for R (Pinheiro et al. 2015) for normal distribution dataset, and the lme4 package (Bates et al. 2014) for the log normal distribution datasets. Finally, we performed a Pearsons test to evaluate the association between temperature inside the nest-box and the outside temperature.
RESULTS
Nestling thermoregulatory set-point range and endothermy age
Although Linear Mixed Models indicated that nestling body temperature did not vary significantly with developmental stage, early-stage nestlings had on average a slightly lower 35.2 ± 2.6°C body temperature (range: 30.1 - 38.3°C, n = 8) than late-stage nestlings (36.6 ± 1.6°C, range: 32.2 - 38.4°C, n =8). Nestling body temperature for the entire development period ranged from 31.1°C to 40.9°C, with a 50% quartile of nestling body temperatures that ranged from 35.9°C to 38.4°C for the entire nestling development period. By comparison, early-stage nestlings had a lower 50% quartile of body temperatures from 35.1°C to 37.4°C, while for late-stage nestlings this was between 36.9°C and 38.6°C. Nestlings reached age of endothermy at a mean 9 ± 2.3 days after hatching (range: 7 - 13 days, n = 7 nestlings). In the natural cavity, Bewick’s Wren parents were off the nest for 10.4 ± 6 mins, and up to 20 mins, foraging for food.
Ambient temperatures
Linear Mixed Models demonstrated that the type of environment influenced ambient temperature (Estimate = 0.03 ± 0.01 (SE), 95% CI = 0.02 - 0.04; t = 5.25, P < 0.001), with temperature inside the nest-box being higher (18.3°C ± 3.3°C, range: 12.0 - 25.2°C, CV = 18.1%) than the outside ambient temperature (17.8 ± 3.9°C, range: 10.4 - 28.3°C, CV = 21.8%). Temperature inside the nest-box was significantly correlated with ambient temperature outside the nest-box (r = 0.95, P < 0.001), but the inside-outside temperature difference (ΔT°) was on average 0.5 ± 1.2°C. This indicates that overall, the nest-box maintained a slightly warmer internal ambient temperature. Nevertheless, the interior of the nest-box was cooler than the exterior at 16:45 hrs (ΔT°= -4°C), indicating that nest-boxes could provide more benign thermal environments when extreme temperatures occur at key times of the day, such as early afternoon.
Timing of extreme ambient temperatures
Extreme high ambient temperatures were recorded during the afternoon (Fig. 1), with the highest ambient temperature of 25.0°C - 25.2°C inside the nest-box between 16:00 and 18:00 hrs, and the highest temperatures of 25.8°C - 28.3°C outside the nest-box recorded between 15:00 and 16:45 hrs. The lowest temperatures both inside and outside the nest-box occurred around day-break (Fig. 1). The lowest temperatures of 12.0°C - 12.2°C inside the nest-box occurred between 06:45 and 08:15 hrs, while temperatures of 10.4°C - 10.8°C outside the nest-box occurred between 06:00 and 07:45 hrs. This indicates that the timing of extreme temperature differs by type of environment, with lowest and highest temperatures occurring earlier in the day outside the nest-box compared to within the nest-box. This also demonstrates that nestlings face a wide range of temperatures in the nest-box during the day, with fluctuations of up to 13.2°C between the highest and the lowest temperatures recorded.
Temperatures by day period
LMM analysis indicated that ambient temperatures also varied significantly with period of the day, both inside and outside the nest-box (Table 1). In particular, the environment outside and inside the nest-box in the afternoon was skewed towards high temperatures (Fig. 2a), while the environment inside and outside the nest-box at night was skewed towards low temperatures (Fig. 2a). The distribution of temperatures was more homogeneous during the morning (Fig. 2). Tukey HSD pairwise comparisons showed that temperatures outside the nest-box differed significantly between all periods of the day (night-morning: q = -3.2, P < 0.001; morning-afternoon: q = -4.0, P < 0.001; night-afternoon: q = -7.2, P < 0.001). Mean temperatures outside the nest-box by day period were lowest at night (15.5 ± 2.4°C), followed by the morning (18.7 ± 3.4°C), and higher in the afternoon (22.7 ± 2.0°C).
Table 1 Results of Linear Mixed Models for temperature inside and outside of Bewick’s Wren nest-boxes in Morelia City, Mexico. Day period [afternoon] was considered as reference in models.
| Temperature inside nest box | Temperature outside nest box | ||||||
| Predictors | Estimate ± SE | Test value | 95% Confidence Interval | Estimate ± SE | Test value | 95% Confidence Interval | |
| Interval | Interval | ||||||
| (Intercept) | 3.12 ± 0.01 | t = 336.7, P < 0.001 | 3.10 - 3.14 | 3.12 ± 0.01 | t = 227.9, P < 0.001 | 3.10 - 3.15 | |
| Day phase [Morning] | -0.23 ± 0.01 | t = -31.6, P < 0.001 | -0.24 - -0.21 | -0.2 ± 0.01 | t = -26.2, P < 0.001 | -0.21 - -0.18 | |
| Day phase [Night] | -0.3 ± 0.01 | t = -48.9, P < 0.001 | -0.31 - -0.29 | -0.38 ± 0.01 | t = -56.7, P < 0.001 | -0.40 - -0.37 | |
| Random effects σ2 | 5.82 | 6.69 | |||||
| Observations | 2469 | 2469 | |||||
| Marginal R2 | 0.002 | 0.003 | |||||
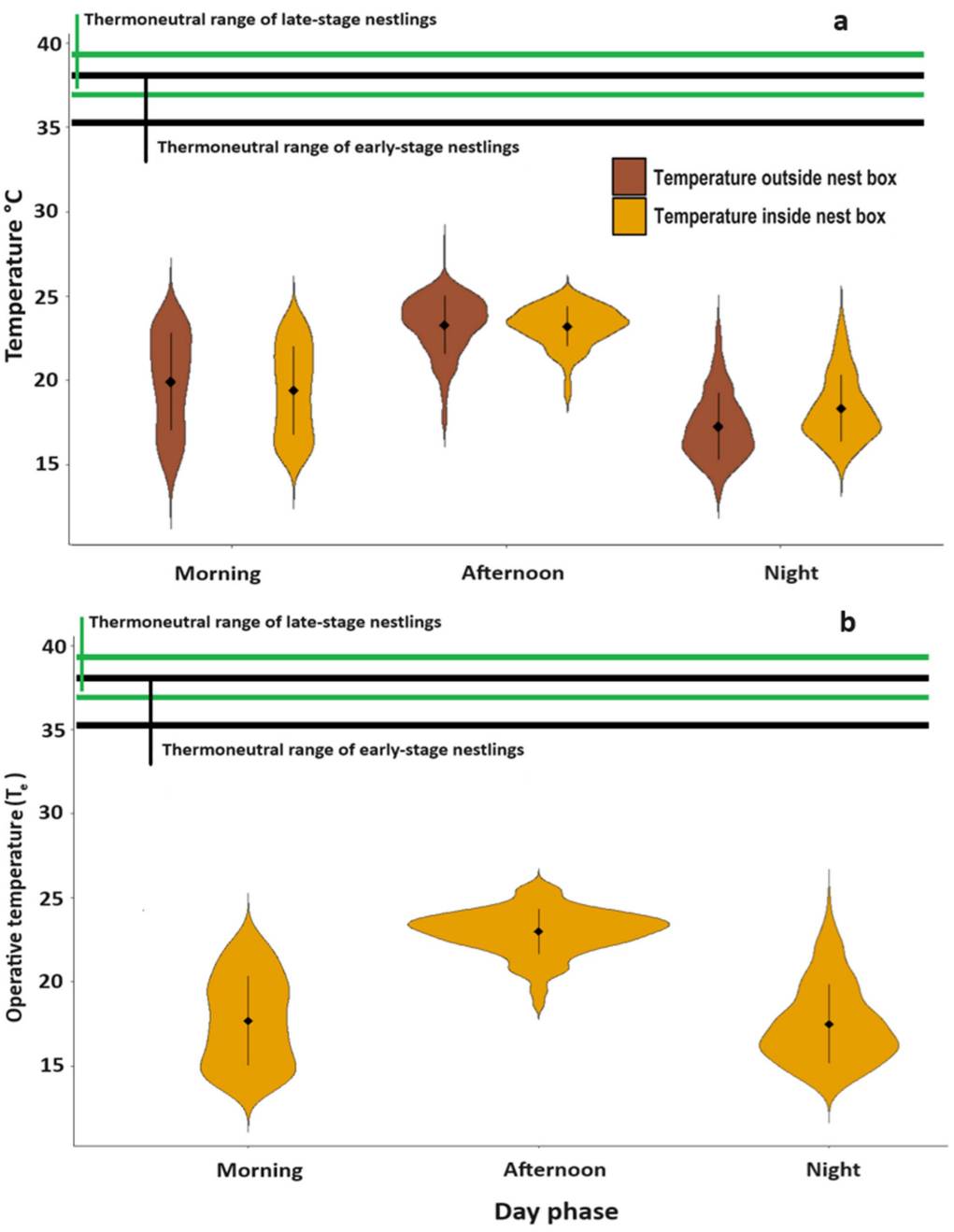
Figure 2 Violin plots depicting the frequency of temperatures registered by dataloggers in three periods of the day for a) ambient temperatures outside and inside the nest-box, and b) operative temperatures experienced by nestlings, as determined by 3D models, in the nest-box. The mean (diamond) with its standard deviation (line) are indicated inside the plot. Second and third interquartile range (Tset 25% - 75%) of body temperature is shown for early- (green line) and late-stage (black line) nestlings.
Likewise, pairwise comparisons showed that temperatures inside the nest-box differed significantly among periods of the day (night-morning: q = -1.3, P < 0.001, morning-afternoon: q = -4.6, P < 0.001, and night-afternoon: q = -5.8, P < 0.001). However, average temperatures inside the nest-box showed less variation than the exterior, being only slightly lower at night (16.8 ± 2.4°C) and in the morning (17.2 ± 2.8°C), and higher in the afternoon (22.5 ± 1.4°C).
Operative temperatures in nest-boxes
Operative temperatures (Te), representative of those experienced by nestlings in the nest-box environment without parental incubation, fluctuated throughout the day concomitantly with temperature both outside and inside the nest-box (Fig. 1). Likewise, operative temperatures by period of the day followed a similar pattern to that of ambient temperatures, with higher operative temperatures in the afternoon and lower operative temperatures at night and in the morning (Fig. 2b). Operative temperature differed significantly for late-developmental stage models (Estimate = 0.04 ± 0.01 (SE), 95% CI = 0.03 - 0.06; t = 5.6, P < 0.001), with a higher mean 19.2 ± 2.9°C compared to early-developmental stage models (mean: 18.8 ± 3.4°C). The 50% quartile of operative temperature was 16.12°C - 21.9°C, although early-stage models had a slightly lower 50% quartile operative temperature (15.6°C - 21.1°C) than late-stage models (16.5°C - 22.2°C). Overall, both early- and late-stage nestlings had higher body temperatures than ambient temperatures inside the nest-box, and the operative temperatures of models in the nest-box (Fig.1). Therefore, as operative temperature of models closely followed internal ambient temperature it is likely that in the absence of brooding adults, nestlings would experience thermal stress.
Discussion
Our results demonstrated that ambient temperatures outside and inside nest-boxes were correlated, and that the interior of nest-boxes were only slightly warmer than the exterior at night and cooler in the day, although they did not reach the same extremes of high and low temperatures as the exterior. This suggests that the thermal benefits to nestlings offered by nest-boxes may be minimal (Maziarz et al. 2017), and require improvement by parental thermal buffering by brooding. Our results also demonstrated that the thermoneutral range of nestlings’ body temperature was higher than both the internal thermal environment and operative temperatures of models inside the nest-box, regardless of the developmental stage. Therefore, nestlings could suffer thermal stress when parents do not offer thermal buffering, such as when they are off the nest to forage. This may be critical for altricial birds during the first days after hatching, when nestlings are not fully able to thermoregulate.
We found that the thermoregulatory set-point of Bewick’s Wren nestlings was 20.1°C above the average temperature in the nest-box and 22.3°C above the operative temperature of models in the nest-box. This indicates that the interior of the nest-box could potentially be thermally challenging for early-stage nestlings, particularly during parental off-bouts, which may be for up to 20 mins on foraging trips, when chicks might experience thermal stress. Critical periods may occur early in the morning between 06:45 and 08:15 hrs, when nestlings may experience low 12.0°C - 12.2°C temperatures in the nest-box, with the risk of hypothermia. Parent birds may buffer high and low temperatures in the nest by either staying more time in the nest when ambient temperature is low, or reducing the time in the nest when temperature is high (Andreasson et al. 2018, DuRant et al. 2019). Therefore, parental brooding may be crucial in the early- developmental stage when nestlings are not fully endothermic, and hence parent birds may be off the nest for short periods of time at this stage. This suggests that the nest-box may be a thermally challenging environment for nestlings unless brooded by parents.
We found that nest-boxes used by Bewick’s Wrens in Morelia City were on average 0.5°C warmer than external temperatures. Nest-boxes in cold weather temperate areas are regularly reported to be warmer than the outside (McComb and Noble 1981, Wachob 1996), although other studies have recorded larger differences of around 20°C (Corregidor-Castro and Jones 2021). In colder temperate regions, a warmer nest-box not only enables longer foraging trips off the nest by females (Haftorn 1988), but also influences reproductive success (Wachob 1996), as it reduces the thermal stress at low temperatures. However, in a tropical environment we recorded a small temperature difference between the exterior and interior of nest-boxes.
Our results suggest that nest-boxes in a tropical urban setting may provide minimal thermal benefits for secondary cavity nesting birds. This contributes to the ongoing and inconclusive debate on the suitability of nest-boxes as an alternative to natural cavities. The provision of nest-boxes may increase the availability of nesting sites and number of breeding pairs (Corrigan et al. 2011), particularly where there may be limited availability of adequate cavities (Cockle et al. 2010). Nesting pairs may also have greater productivity of eggs and nestlings in nest-boxes (Robertson and Rendell 1990, Libois et al. 2012, Norris et al. 2018), possibly because nest-box design can incorporate dimensions to increase internal space and reduce access to competitors and predators. However, the benefits of nest-boxes for reproductive output may be species-specific (Purcell et al 1997), and they may foster a maladaptive habitat use (Mänd et al. 2005). Moreover, the few studies of thermal constraints on avian reproduction have been conducted in cooler temperate regions (Andreasson et al. 2020). Therefore, our results also shed light on the potential inadvertent consequences of the use of alternative human-made cavities for nesting birds in topical urban areas.
Notably, we have also documented the use of these nest-boxes for roosting at night by birds in Morelia city. Therefore, while nest-boxes may provide limited thermal benefits for breeding, they could provide safe places for roosting in urban areas. Furthermore, the fact that nest-boxes maintain warmer temperatures at night means that they may have a valid utility for roosting activities. Nevertheless, we emphasize the importance of promoting the presence of large, old trees in urban areas that can develop cavities for nesting. These tree-cavities can provide a more stable thermic and humid environment (Sudyka et al. 2023), offering cavity-nesting species with a safe nest-site, and a potentially better environment for raising their young.











 nueva página del texto (beta)
nueva página del texto (beta)




