Introducción
Alumina is one of the most widely used materials as support in heterogeneous catalysis, adsorbent, electronic, abrasives, reinforcement of ceramic composites for its high strength, corrosion resistance, low thermal conductivity, and good electrical insulation due to several attractive features (Glorias et al., 2014; Yang, 2011, Jiratova et al., 1986). It is inexpensive and available in many different qualities making it suitable in a wide range of different chemical reactions. Furthermore, its synthesis is simple and economic. Though mainly used as support, alumina has also some catalytic activities by its own, this due to its acidity (Ancheyta et al., 2005). For specific applications, the surface area, the pore size, pore volume, thermal and mechanical stability of the support it has a large impact on the catalytic effect. Several Authors have emphasized that γ-Al2O3 with high pore volume may improve the adsorption performance if used as an adsorbent, and loading capacity if applied as a catalyst support, to minimize diffusion and transport influences (Xu et al., 2016, Stanislaus et al., 2002). The alumina is a material that still stands in limelight owing to its extensive application.
The used aluminum oxides exist in a variety of metastable structures which are usually called transitions alumina such as η, χ, κ, θ, λ, δ and γ, as well as the stable α-Al2O3 phase. Especially, the variety of γ-alumina is widely appreciated due to its excellent properties as surface chemical properties that can be either acidic or basic depending on the transition alumina structure and the degree of hydration and hydroxylation of the surface, which is a major attraction from catalytic/adsorption point of view (Sinfontes et al., 2014; Zhang et al., 2016).
Nowadays, there are many materials as aluminum sources to synthesis and obtain Al2O3, for example Al(NO3)3, Al2(SO4)3, NH4Al(SO4)2, AlCl3, NaAlO2, amoung others. The mesoporous γ-Al2O3 synthesized with the above aluminum sources has excellent properties (Zhang et al., 2016). However, these aluminum sources are expensive, because of that; it is difficult to realize the industrialization with these ways. However, aluminum sulfate with the presence of impurities can be attractive, because of its low cost. The presence of impurities can influence in alumina quality and consequently affect the oxide morphology and/or physicochemical properties. Interestingly, even a tiny amount of impurities in the Al2O3 structure, can exert a strong effect on acidity, including structural defects. On other hand, a small amount of impurity could be detrimental for the performance. However, not all impurities are harmful; there are impurities that may present advantages benefiting the reaction and/or application. Among the impurities contained in alumina powders we can find: Na, Si, Fe, Ca, Mg, etc. Moreover, depending upon the synthesis method, alumina can contain anion impurities from the precursors used in the synthesis as for xample Cl− ions, NO3- or (SO4)2−, something which is accepted by most of the people working in the field (Mishra et al., 2002).
Herein, we describe the synthesis and comparison of physic-chemicals features through several characterization techniques of two alumina materials prepared in similar conditions. One of them synthetized from an aqueous solution of aluminum sulfate analytical grade (Al2O3-AR) and a second alumina synthetized from aluminum sulfate technical grade with presence of impurities (Al2O3-TG), both alumina obtained by hydrolysis-precipitation route. We obtained materials with relatively high surface areas, with pore sizes and high pore volumes. Emphasis was put on the impurities of aluminum precursor and on the physic-chemical properties of the Al2O3. This paper contributes to a basic understanding of the purity of the aluminum salt and how it influences on textural characteristics of mesoporous materials with the presence of 0.33 % wt. of Mg. This fundamental knowledge could be exploited in the design of new materials.
Materials and Method
Materials
In the present study, the starting materials to obtain hydrated alumina (Al2O3) were Al2(SO4)3•18H2O with a purity of 95% (by weight, wt.) technical grade (TG), Alfa-Omega chemical, S. A. and an Al2(SO4)3•18H2O with a purity of 99.98% (wt.), analytical reagent (AR), CTR Scientific, S.A., deionized water (Karal S. A.) and anhydrous NH3 gas (Praxair, 99.98% (wt.)) as precipitant. Then, the impurities of both materials of aluminum sulfate are described (Table 1).
Table 1. Chemical composition wt. % by atomic adsorption of Al2(SO4)3-TG, Alfa omega, S.A., and Al2(SO4)3-AR, CTR Scientific, aluminium precursors.
Synthesis of pseudoboehmite
The flow chart of the preparation procedure is depicted in Figure 1. Al2(SO4)3•18H2O TG and/or AR were dissolved into deionized water. The Al2(SO4)3•18H2O technical grade was filtered for removal of insoluble impurities. Precursors were prepared by dropping saturated solution of aluminum sulfate into a solution of water and ammonia gas under rigorous magnetic stirring at temperature of 50-70 ºC. The precipitated solution was filtered; the precipitated of pseudoboehmite was rinsed with distilled water three times respectively, and then dried at 110 °C for 12 h. The phase change of pseudoboehmite to γ-Al2O3 was performed by thermal treatment at 500 °C for 7 h. The materials were labeled as follows: Al2(SO4)3•18H2O Technical Grade (Al2O3-TG) and Al2(SO4)3•18H2O Analytical Reagent (Al2O3-AR).
Materials characterization
Thermal analysis TGA-DTA
The thermal stability of the samples was carried by Thermogravimetric and Differential thermal analysis (TGA/DTGA-DTA) was performed from 30 ºC to 1000 °C in a Thermogravimetric analyzer SDT Q600 V20.5 Build 15 under air flow (100 mL / min) at a heating rate of 10 ºC/min.
N2 physisorption
The textural properties were determined by N2 adsorption (Micromeritics, ASAP 2010). The samples were degassed at 200 ºC for 3 h under vacuum. Nitrogen adsorption isotherms were measured at liquid N2 temperature (77 K) and N2 pressures ranging from 10-6 to 1.0 P/P0. Surface area was calculated according to Brunauer-Emmett-Teller (BET) method and the pore size distribution was obtained according to the Barret-Joyner-Halenda (BJH) method.
X-ray diffraction (XRD)
The crystalline properties of the samples were determined at room temperature by using X-ray powder diffraction. A siemens D-500 diffractometer equipped with a Cu Kα radiation anode was used for these measurements under the following conditions: sweep of 10-80º at an angle 2θ, with applied voltage of 30 kV and current of 20 mA.
FT-IR analysis
The vibrational spectroscopy measurements were performed at room temperature by Fourier transform infrared (FTIR) spectroscopy on a Bruker Model 27 spectrometer through KBr (99%) disk method in a frequency range of 4000 - 400 cm-1.
Results and discussion
TEM/energy-dispersive X-ray (EDAX) analysis
The surface morphology of Al2O3-TG and Al2O3-AR samples is studied by TEM and the corresponding micrographs are presented in Figure 2. It can be seen that the products obtained represent typical TEM images of Al2O3 powders. They exhibit disordered morphology nanofibrillar with a length of about 20-100 nm for the Al2O3-TG and 20-80 nm for Al2O3-AR. The products obtained in our experiment are similar as those reported in the literature (Zhang et al., 2016). The development tendency of alumina is to be a more thermodynamically favored crystal (Nuru et al., 2015; Yu et al., 2012). TEM images indicate alumina products have better dispersion performance for Al2O3-TG, which indicate alumina product obtained has good tendency to be a thermodynamic favored crystal due to presence of the Mg as impurity. Figure 2(c-d) shows the secondary particles size of the obtained products. The size varied from 0.45 μm to 0.85 μm for Al2O3-TG and from 0.35 μm to 0.65 μm for Al2O3-AR, observing a larger size for the sample Al2O3-TG, this is attributed to larger sizes of fibers that tend to form agglomerates or higher particles.
Energy dispersive X-ray (EDAX) data of both samples are provided in Table 2. The punctual analysis (EDAX) shows Al and O were detected on surfaces, respectively. The determination of other present chemical elements as N and S are attributed to residues generated during the synthesis process. While than the presence of Mg is attributed as an impurity present in the low purity aluminum sulfate. The Mg was only possible to be observed since a micro-area analysis was performed. However, the assumption in this paper is that the trace element is homogenously distributed on the matrix and the limit is expressed as an atomic fraction, this attributing that the Na and Fe were removed during the synthesis process.
Thermogravimetric and differential thermal (TGA/DTGA-DTA) analysis
TGA/DTGA and DTA analysis of Al2O3-TG and Al2O3-AR are shown in Figure 3. Two endothermic peaks are observed in the DTA curve at 85 and 500 °C, the position of which varies with the type of the purity of aluminum salts that was used. The first peak is assigned to elimination of adsorbed water. At 500 °C pseudoboehmite decomposition to the γ-phase is produced; this involves dehydroxylation/dehydration [2AlO(OH)→γ− Al2O3 + H2O]. The derivative of thermogravimetric analysis DTGA and DTA shows a peak centred at 85 ºC indicating a thermal decomposition for Al2O3-TG. The weak broad DTGA and sharp DTA peaks around 500 ºC imply a transformation phase. These facts are confirmed by the thermogravimetric study, which shows a complete weight loss (17%) at 500 °C, this is consistent with the reported in the literature (Urretavizcaya et al., 1998). The Al2O3-AR shows two endothermic peaks, which are observed in the DTA curve at 60 and 500 °C. The first peak is assigned to elimination of adsorbed water. At 500 °C pseudoboehmite decomposition to the γ phase is produced. These facts are confirmed by the thermogravimetric study, which shows a complete weight loss (15%) at 500 °C.
The endothermic peak observed in the DTA curve at 950 °C, Figure 3b, which is not accompanied by a weight loss (TGA), which is corroborated by DTGA, is attributed to θ-Al2O3 formation according to the reported in the literature (Jan et al., 2014). Thermal transformations
Powder X-ray diffraction (XRD)
The high-angle XRD patterns of Al2O3-TG and Al2O3-AR samples are shown in Figure 4. The X-ray diffraction pattern was performed of 10 - 80º at an angle 2θ. The results of the XRD patterns wide angle shows seven weak diffraction peaks, those are observed at 2θ= 19.2, 31.0, 36.6, 39.3, 46, 61.5, and 67º, which can be indexed as the (111), (220), (311), (222), (400), (511), and (440) reflections of γ-alumina according to JCPDS card: 100425 (Cheng et al., 2006; Jan et al., 2014). When the precursors are calcined at 500 ºC, there are no obvious peaks of others phases in XRD pattern indicating that the products structure is amorphous.
On other hand, a disorder in the structure can be observed. This disorder is reflected in the unusual broadening of some X-ray diffraction peaks for the Al2O3-TG material in the region 2θ=20-48°. This is attributed to a strong faulting on the (111), (110), and (100) in the spinel-like structure of these materials, which occur as individual and/or interconnected defects on different plane families (Sakashita et al., 2001). According to the literature, this non spinel model is more stable than spinel type structures (Wolverton et al., 2000). The XRD results confirm the TG-DTA analyses indicating that the Al2O3-TG is more stable that Al2O3-AR. However, in this work the structural defect is attributed to the presence of Mg. This is due to that excess alumina introduced a substitution defect on the tetrahedral magnesium site and an aluminum vacancy or magnesium vacancy is formed to compensate the positive charge excess, as reported in the literature (Yuji et al., 2006).
Adsorption/desorption analysis
The nitrogen adsorption isotherms and BJH pore size distribution of samples calcined at 500 ºC are shown in Figure 5. It is seen that the two materials exhibit the shape of type-IV Isotherm curves according to the IUPAC classification (Sing et al., 1985; Jan et al., 2014), which is characteristic of mesoporous materials. These materials show irregular shape isotherms with hysteresis loop type E and indicate that the pores in the material have an inkwell-type shape. Existence of the hysteresis loops is ascribed to the capillary condensation of N2 gas occurring in the pores (Zhang et al., 2016). Characterizations of powders obtained at 500 ºC and values reported in the literature by different authors from different routes of synthesis were shown in Table 3. Surface area and pore volume values of the materials were higher for the sample Al2O3-TG that for Al2O3-AR. Due to that exhibit nanofiber with a higher length, as it is shown in TEM images, it is attributed to the presence of Mg in the structure of the Al2O3-TG. A predominance of mesopores that could be of interest for heterogenous catalysis and the adsorption area is observed. In adittion, the intercrossing degree and disorder among the fibers (interfibrillar porosity) as well as their length may cause the non-uniform pore size distributions.
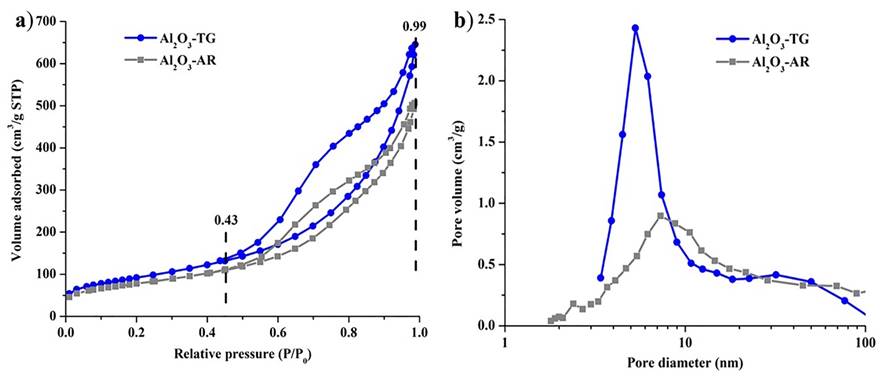
Figure 5 a) N2 adsorption-desorption isotherm and b) pore size distribution curve of Al2O3-TG and Al2O3-AR.
Table 3 Results of the properties textural of samples obtained and reported by different synthesis routes.
| Samples | SSAa | Vapb | Dapc | References |
|---|---|---|---|---|
| γ-Al2O3-TG (Hydrolysis-precipitation) | 311 | 0.86 | 11.6 | This work |
| γ-Al2O3-AR (Hydrolysis-precipitation) | 272 | 0.76 | 10.8 | This work |
| γ-Al2O3 (hydrolysis method) | 183 | 0.4 | 10 | Sifontes et al., 2014 |
| γ-Al2O3 (hydrothermal route) | 248 | 0.30 | 4.3 | Guzman et al., 2005 |
| γ-Al2O3 (CONDEA commercial) | 220 | - | 5.9 | Del Angel et al., 2005 |
| γ-Al2O3 (Template method) | 328.9 | 0.81 | 12.0 | Renuka et al., 2016 |
| γ-Al2O3 (precipitation/digestión) | 220 | 0.48 | 7.0 | Potdar et al., 2007 |
| γ-Al2O3 (dual-templating route) | 320 | 0.60 | 7.0 | Sandeep et al., 2015 |
| Mesoporuos Alumina (double hydrolysis method) | 335.5 | 0.60 | 7.1 | Benjing et al., 2017 |
| γ-Al2O3 (sol-gel method) | 370 | 0.52 | 6.0 | Rudina et al., 2012 |
| γ-Al2O3 (sol-gel hydrolysis method) | 236 | 0.4 | 7.0 | Wei et al., 2016 |
| γ-Al2O3 commercial (J&K chemical) | 331 | - | 5.8 | Zongbo et al., 2016 |
| γ-Al2O3 (Solid state Method) | 220 | 0.49 | 6.1 | Soodeh et al., 2016 |
| γ-Al2O3 (coprecipitation method) | 346.4 | 1.0 | 10.2 | Zhang et al., 2016 |
| γ-Al2O3 (Reverse precipitation method) | 240 | 0.90 | 7.6 | Wu et al., 2012 |
| γ-Al2O3 (coprecipitation/sol-gel method) | 264 | 0.30 | 5.1 | Mohammed et al., 2012 |
a Specific surface area (m2/g)
b Average pore volume (cm3/g)
c Average pore diameter (nm)
The comparative values obtained and reported by different authors from several synthetic pathways are similar and in some cases higher. This textural behavior is typical of materials where condensation occurs mainly between particles. This is related to grain size. After the calcination there is a considerable increase in the values of specific areas caused by dehy-droxylation leading to the formation of γ-Al2O3. It is seen that the synthesized material from an inexpensive aluminum precursor with impurity presence make larger nanofibers presenting very attractive textural properties obtained by a simple and economical synthesis method.
Fourier transforms infrared spectroscopy (FT-IR) analysis
The FTIR spectra for the different products obtained, is shown in Figure 6. The results of the sinthesized materials are consistent with the reported in literature (Riad et al., 2007). In the FT-IR patterns, the broad band at 3200-3700 cm−1 its characteristic of -OH stretching vibration that is bonded to Al3+, and the band at 1638 cm−1 corresponding to physisorbed water are observed. Meanwhile the peak at 1470 cm-1 was also due to water deformation vibrations (Boumaza et al., 2009). Band appearing at 1100 cm-1 is typically for γ-alumina due to Al-O vibration mode (Xu et al., 2017). The valley between 1000 cm−1 and 435 cm−1 confirms the γ-form, and the one at 873 cm−1 is assigned to the bending vibrations of Al-O bond. Al-O-Al bond in the gamma phase of alumina generates the band at 670 cm−1 (Cheng et al., 2006). These results are consistent with the XRD analysis where the g-phase was identified.
The peaks in the region of 500 - 750 cm-1 are assigned to AlVI, whereas the shoulder at 750 and the line at 890 cm-1 are assigned to AlIV. Thus, γ-Al2O3 phase contains both tetrahedral and octahedral coordination (Renuka et al., 2012). In this sense, it is possible to observe more intense vibrations and slightly more widened in the material Al2O3-AR, reason why a greater content of the tetrahedral coordination assuming a greater acidity is attributed. Several Authors have observed that the presence of Mg decreases the weak Brønsted acidity and increases the basicity of the alumina (Zhang et al., 2016). Then that product obtained of Al2O3-TG presents lower content of tetrahedral coordination.
Effect of salt’s purity
The results clearly show that the presence of Mg into the Al crystal lattice change the acid and basic properties of this oxide. It is interesting to verify that the values of the surface area (Table 3) change when comparing Al2O3-TG with Al2O3-AR. In recent years the use of different grades of aluminum salts have become important areas of research and development due to their importance as precursors for aluminum oxides. In some applications, such as advanced manufacturing alumina-based ceramics, and high-purity aluminium oxides compounds are required. Here tolerable concentrations of impurities are rather low and an analytical control became a limiting step of the technology (Koksal et al., 2002). Other applications that required high-purity aluminum oxides are pharmaceuticals products, refractories, electronics, among others (Mishra, 2002). However, there are applications where the presence of impurities can benefits the application or reaction, as mentioned earlier. This study determined that a small amount of impurity in precursor salt can modify the physical properties of alumina compared with conventional γ-alumina. In addition it, exhibit attractive properties for several applications as the process of adsorption and catalytic, in addition to a low cost.
Conclusion
The material of γ-Al2O3 nanofibrillar synthesized by hydrolysis-precipitation method presented physicochemical properties attract as possible catalytic support and adsorbent material. The results showed that 0.33 wt. % of Mg as impurity was sufficient to generate structural defects and decreased the acidity in the Al2O3-TG material. However, the presence of Mg extended the fibrillar chain of the alumina besides giving greater thermal stability and greater textural properties. Hence from precursors of aluminum with presence of impurities can be obtained attractive materials for several applications.











 nueva página del texto (beta)
nueva página del texto (beta)







