Introduction
Tomato is the fourth vegetable more cultivated in the world with three millions of hectares, just behind rice, wheat, and soy (FAOSTAT, 2018). Nevertheless, the tomato crop is affected by several fungal diseases as Botrytis cinerea, Leveillula taurica, Alternaria solani, and F. oxysporum (Villasanti & Pantoja, 2013). F. oxysporum causes vascular wilt that is one of the most destructive diseases on tomato crop, and it can cause yield losses until of the 80 % (Marlatt et al., 1996; Hernández-Martínez et al., 2014; González et al., 2012). At present exist several methods to control the F. oxysporum; chemical control is the most used; nevertheless, several reports indicate that farmers use intensive applications of synthetics products, triggering on phytopathogenic microorganisms resistance (Bautista-Baños, 2006). For these reasons, at present, there is a great necessity to develop alternatives methods for the control of plant diseases (Jeong et al., 2017).
The plants from the Mexican semi-desert present a large number of phytochemicals with antifungal activity. Some of these plants are A. lechuguilla that exhibit the presence of steroidal saponins; C. illinoinensis with a high content of total phenolics compounds; that causes enzymatic inhibition by compound oxidation; J. dioica with a considerable amount of phytochemicals; L. tridentata with flavonoids, triterpenes, and triterpenoids; and L. graveolens that present phytochemical compounds like essential oils, iridoids, flavonoids and naphthoquinones (Blunden et al., 1980; Do Prado et al., 2009; García-Bores et al., 2017; Martínez et al., 2014; Martins et al. 2013).
The action mode of the phytochemical compounds present in plants are diverse, in case of terpenes and essential oils there is a membrane rupture by lipophilic compounds, the alkaloids intercalate their self with DNA, and the lectins and polypeptides create Ion channels in the microbial membrane or cause the competitive inhibition by adhesion of microbial proteins to the polysaccharide receptors from the host. (Masson, 1987; Cowan, 1999; Hernández-Lauzardo et al., 2007). In this sense, Jasso de Rodríguez et al. (2011) reported 100 % of inhibition with extracts from L. graveolens and A. lechuguilla on Rhizopus stolonifer. In the same way, the antifungal activity of L. tridentata was reported by Osorio et al. (2009) with extracts which presented fungicidal effect on the growth of Phytium sp., Colletotrichum coccodes, Colletotrichum truncatum, Alternaria alternata, Fusarium solani, and Rhizoctonia solani; and fungistatic impact on Fusarium verticilloides.
The objectives of this study were identified some phytochemicals compounds present in plant extracts from A. lechuguilla, C. illinoinensis, J. dioica, L. tridentata, and L. graveolens; and determinate the antifungal activity against F. oxysporum and their IC50 concentration of each plant.
Methods
Plant collection. The plant collection was in January 2016. This collect was in General Cepeda, Coahuila, México (25°21’40.55’’ N y 101°28’08.68’’ W). Samples stored in black plastic bags for their transfer at Laboratorio de Micología y Biotecnología from Universidad Autonoma Agraria Antonio Narro. Once in the laboratory, the samples washed with water and let it dry, then cut in small pieces around 1 cm. Samples placed in a drying stove to 60 °C until constant weight, finally each plant pulverized and sieve with a pore of 0.2 mm, this for the particle homogenization. Samplers stored in dark flasks to environment temperature (Castillo et al., 2010).
Plant extracts preparation. The plant extracts prepared following the Shami et al. (2013) methodology with some modifications. Water and ethanol used as solvents and two types of extracts developed, crudes, and concentrated. The first step was adding 14 g of the plant powder in 200 mL of solution, and then the flask was placed in a stirring grill during 72 h at 50°C (Jasso de Rodríguez et al., 2015). Lapsed 72 h, the obtained extract filtered with a filter paper Whatman No.1; after the purified extract separated in two parts, one of these parts was placed in the Eppendorf tube and stored to -20 °C, thus were obtained crude extracts. To get the concentrated extracts, the solvent was separated by rotary evaporation (IKA RV 10) to 150 RPM to 60 °C, after the rotary evaporation the extracts obtained were placed in a drying stove until the extracts presented constant weight and then were pulverized (Martins et al., 2013), the obtained powder from the pulverization stored to -20 °C. In Table 1, there are all the plant extracts.
Table 1 Mexican desert plant extracts used against F. oxysporum strain.
| Plant extracts | |||
|---|---|---|---|
| Aqueous | Aqueous | ||
| Crudes | Crudes | Crudes | Crudes |
| A. lechuguilla leaves (AlLAC) | A. lechuguilla leaves (AlLAP) | A. lechuguilla leaves (AlLEC) | A. lechuguilla leaves (AlLEP) |
| A. lechuguilla roots (AlRAC) | A. lechuguilla roots (AlRAP) | A. lechuguilla roots (AlREC) | A. lechuguilla roots (AlREP) |
| C. illinoinensis husk (CiHAC) | C. illinoinensis husk (CiHAP) | C. illinoinensis husk (CiHEC) | C. illinoinensis husk (CiHEP) |
| J. dioica stem (JdSAC) | J. dioica stem (JdSAP) | J. dioica stem (JdSEC) | ** |
| J. dioica roots (JdRAC) | J. dioica roots (JdRAP) | J. dioica roots (JdREC) | J. dioica roots (JdREP) |
| L. tridentata leaves (LtLAC) | L. tridentata leaves (LtLAP) | L. tridentata leaves (LtLEC) | L. tridentata leaves (LtLEP) |
| L. graveolens leaves (LgLAC) | L. graveolens leaves (LgLAP) | L. graveolens leaves (LgLEC) | L. graveolens leaves (LgLEP) |
| L. graveolens steams (LgSAC) | L. graveolens steams (LgSAP) | L. graveolens steams (LgSEC) | L. graveolens steams (LgSEP) |
** = It was not determined.
Phytochemical analysis of plant extracts. The phytochemicals identification used qualitative techniques, in these techniques the presence or absence of phytochemicals were determinate by colorimetry where the extract reacted and change of color; for these tests concentrated extracts prepared to 1000 mg/L; the identified phytochemicals were alkaloids (Dragendorff and Sonnenschein reaction), cyanogenic glycosides (Grignard reaction), reducing sugars (Feeling and Benedict reaction), saponins (Liberman Bouchnard reaction), tannins (Jelly and FeCl3), quinones (ammonium hydroxide and Bontrager reaction), coumarins (Erlich reaction and ammonium hydroxide), purines and carotenoids (Sahgal et al., 2009; Usman et al., 2009).
Antifungal activity extract in the microdilution plate method
F. oxysporum strain. The F. oxysporum strain isolated from tomato plants provided from the microbiological collection of Laboratorio de Micología y Biotecnología belongs to the Departamento de Parasitología from Universidad Autonoma Agraria Antonio Narro located in Saltillo, Coahuila, México; the F. oxysporum strain identified with the code FoC1, with the access key in GenBank: KU533843.1.
Antifungal activity tests of the extracts against F. oxysporum in vitro. Microplates used, all wells filled with 100 μL of Sabouraud liquid medium. The antifungal test starts from column number four with 100 μL of the extracts; 100 μL of mixture took and added into the next column, thus successively until column 12. Getting concentrations from 1000 to 3.9 mg/L; this procedure performed for all the extracts using each row for each extract; next step added 2,3,5-Triphenyl tetrazolium chloride as growth indicator; finally, a solution of spores from F. oxysporum added in all wells except in column number one, the plate incubated to 28 °C during 48 h, the absorbance lecture performed at 490 nm. For test three repetitions completed, the inhibition percentage, calculated in the same way by Moreno-Limon et al. (2011) formulas adopted:
Statistical analysis. Probit analysis performed to determine the inhibitory concentration to 50% (IC50) of each extract. With the results obtained, variance analysis performed with the inhibitory concentrations; in the study, the treatments evaluated with three repetitions and a Tukey’s range tests performed (p<0.05).
Results
Identified phytochemicals in aqueous and ethanolic plant extracts. The identified phytochemicals from ethanolic extracts are in Table 2; from A. lechuguilla both leaves and roots were identified compounds like carbohydrates, reducing sugars, saponins, and tannins; in the case of C. illinoinensis husk extracts alkaloids, flavonoids, cyanogenic glycosides, reducing sugars, saponins, tannins, quinones, coumarins, purines and carotenoids presented; for J. dioica root extract observed saponins, tannins, quinones, and purines; while L. tridentata extract presented alkaloids, flavonoids, cyanogenic glycosides, reducing sugars, saponins, tannins, quinones, coumarins, and carotenoids; finally in L. graveolens extracts both leaves and steam alkaloids, flavonoids, reducing sugars, saponins, tannins, quinones, coumarins, and carotenoids identified.
Table 2 Identified phytochemicals in concentrated ethanolic extracts.
| Extract | A | C | F | GC | AZ | S | T | Q | Cu | P | Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | Az1 | Az2 | S1 | S2 | T1 | T2 | T3 | T4 | Q1 | Q2 | Q3 | |||||||
| AlLAP | - | + | - | - | - | - | - | - | + | + | - | + | - | + | + | + | + | - | - | + | - |
| AlRAP | - | - | - | - | - | - | - | - | + | + | - | - | - | + | + | + | + | - | - | - | - |
| CiHAP | - | + | - | - | - | + | + | + | + | + | - | + | + | - | + | + | + | + | + | + | + |
| JdSAP | - | + | - | - | - | - | + | + | + | - | - | - | + | - | + | - | + | - | + | - | - |
| JdRAP | - | + | - | - | - | - | - | - | + | + | - | - | - | + | + | + | + | - | + | + | - |
| LtLAP | - | - | - | - | - | + | + | + | + | + | - | + | + | - | + | + | + | + | + | + | + |
| LgLAP | - | + | + | + | + | + | - | - | + | - | + | + | + | - | + | - | + | - | - | - | - |
| LgSAP. | - | + | - | + | + | + | - | + | + | - | + | - | + | - | + | - | + | - | - | + | - |
+ = Phytochemical present; - = Phytochemical no present; A = Alkaloids; C = Carbohydrates; F = Flavonoids; GC = Cyanogenic glycosides; AZ = Reducing sugars; S = Saponins; T = Tannins; Q = Quinones; Cu = Coumarins; P = Purines; Ca = Carotenoids; F1 = Flavonones; F2 = Flavones; F3 = Flavononas; F4 = Chalcones; Az1 = Reducing sugars Fehling reaction; Az2 = Reducing sugars Benedict reaction; S1 = Triterpenoids; S2 = Steroidal; T1 = Jelly; T2 = Derivatives Gallic Acid; T3 = Catechol Derivatives; T4 = Phenols; Q1 = Anthraquinones; Q2 = Benzoquinones; Q3 = Antronas. ** = It was not determined.
Phytochemicals detected from aqueous extracts presented in Table 3. From A. lechuguilla, both leaves and roots presented the same compounds as reducing sugars, saponins, tannins, and quinones, only carbohydrates, and purines were different compounds being present in leaves, for C. illinoinensis husk identified all the phytochemicals listed in Table 3 except alkaloids.
Table 3 Identified phytochemicals in concentrated aqueous extracts.
| Extract | A | C | F | GC | AZ | S | T | Q | Cu | P | Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | Az1 | Az2 | S1 | S2 | T1 | T2 | T3 | T4 | Q1 | Q2 | Q3 | |||||||
| AlLEP | - | + | - | - | - | - | - | + | - | + | - | - | - | + | + | - | - | - | - | - | - |
| AlREP | - | - | - | - | - | - | - | - | + | + | - | - | - | + | + | - | - | - | - | - | - |
| CiHEP | + | - | - | - | - | + | + | + | + | + | - | + | + | - | + | + | + | + | + | + | + |
| JdSEP | ** | ||||||||||||||||||||
| JdREP | - | - | - | - | - | - | - | - | - | + | - | - | - | + | + | + | - | - | - | + | - |
| LtLEP | + | - | + | + | + | + | + | + | + | - | + | + | - | + | + | + | + | + | - | + | |
| LgLEP | + | - | + | + | + | + | - | - | + | - | + | + | + | - | + | + | + | - | + | - | - |
| LgSEP | - | - | + | + | + | + | - | - | + | + | - | - | + | - | + | - | + | - | + | - | + |
+ = Phytochemical present; - = Phytochemical no present; A = Alkaloids; C = Carbohydrates; F = Flavonoids; GC = Cyanogenic glycosides; AZ = Reducing sugars; S = Saponins; T = Tannins; Q = Quinones; Cu = Coumarins; P = Purines; Ca = Carotenoids; F1 = Flavonones; F2 = Flavones; F3 = Flavononas, F4= Chalcones, Az1 = Reducing sugars Fehling reaction; Az2 = Reducing sugars Benedict reaction; S1 = Triterpenoids; S2 = Steroidal; T1 = Jelly; T2 = Derivatives Gallic Acid; T3 = Catechol Derivatives; T4 = Phenols; Q1 = Anthraquinones; Q2 = Benzoquinones; Q3 = Antronas. ** = It was not determined.
In the case of J. dioica observed in stems and roots carbohydrates, reducing sugars, saponins, tannins, quinones, and coumarins, while cyanogenic glycosides and purines were only in stems. L. tridentata presented all the phytochemicals except alkaloids and carbohydrates; finally, in L. graveolens leaves were observed carbohydrates, flavonoids, reducing sugars, saponins, tannins, and quinones, whereas in the stems were presented the same compounds that in leaves except for purines.
Antifungal activity tests of the extracts against F. oxysporum in vitro
Antifungal activity of aqueous plant extracts against F. oxysporum. In general, in aqueous extracts the antifungal activity was less compared with the ethanolic extracts; in the case of crude aqueous extracts, it can see in Fig. 1, that the inhibition percentage increased when the concentration increased, even so, the higher inhibition percentage reached it did not overcome the 50 %. The treatments with the better antifungal activity were AlLAC, JdSAC, and LgLAC; these treatments presented higher antifungal activity when they achieved to the concentration of 31.2 mg/L and kept the same inhibition percentage until the strength of 1000 mg/L with 46, 37, and 45 % respectively. In the concentrated aqueous extracts, it observed a higher inhibition percentage; nevertheless, it did not overcome the 60 %, presented in Fig. 2. In this case, found that the extracts with higher antifungal activity were AlRAP with 53 and LgSAP with 60 %, both to 1000 mg/L.
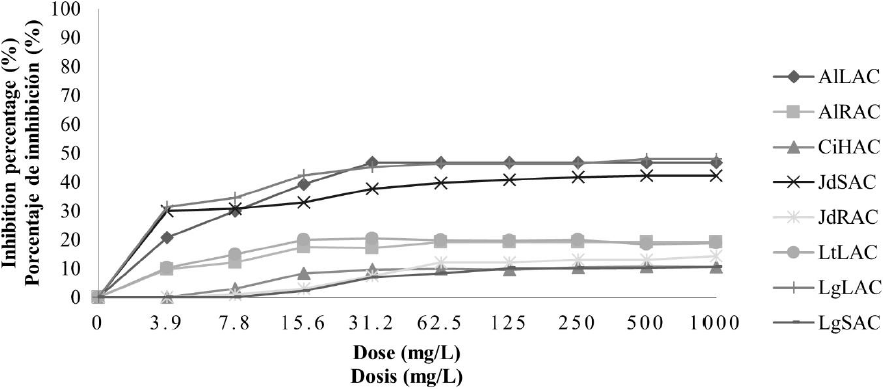
LtLAC: L. tridentata leaves. AlLAC: A. lechuguilla leaves. AlRAC: A. lechuguilla roots. JdRAC: J. dioica roots. JdSAC: J. dioica stem. CiHAC: C. illinoinensis husk. LgSAC: L. graveolens stem and LgLAC: L. graveolens leaves.
Fig. 1 Inhibition percentage of aqueous crude extracts on F. oxysporum.
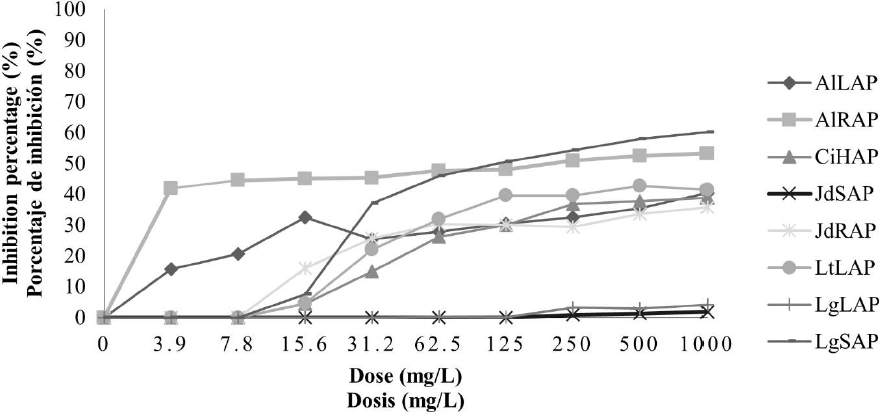
LtLAP: L. tridentata leaves. AlLAP: A. lechuguilla leaves. AlRAP: A. lechuguilla roots. JdRAP: J. dioica roots. JdSAP: J. dioica stem. CiHAP: C. illinoinensis husk. LgLAP: L. graveolens leaves and LgSAP: L. graveolens stem.
Fig. 2 Inhibition percentage of concentrated aqueous extracts on F. oxysporum.
Antifungal activity of plant ethanolic extracts against F. oxysporum. In the case of the ethanolic extracts, in crude extracts and concentrated extracts, it was observed an increase in antifungal activity. In Fig. 3 it is found that all ethanolic crude extracts except LtLEC extract, presented inhibition percentage higher to 90 % in the concentration of 500 mg/L, nevertheless the treatments CiHEC, LgLEC and LgSEC were the best because there was inhibition of the pathogen to 100 % in the concentration of 250 mg/L. About concentrated ethanolic extracts, in Fig. 4, it is observed that the extracts LgSEP, AlLEP, AlREP, and LgLEP inhibited the pathogen development to 100 % to concentrations of 125 mg/L, 250 mg/L and 500 mg/L respectively.
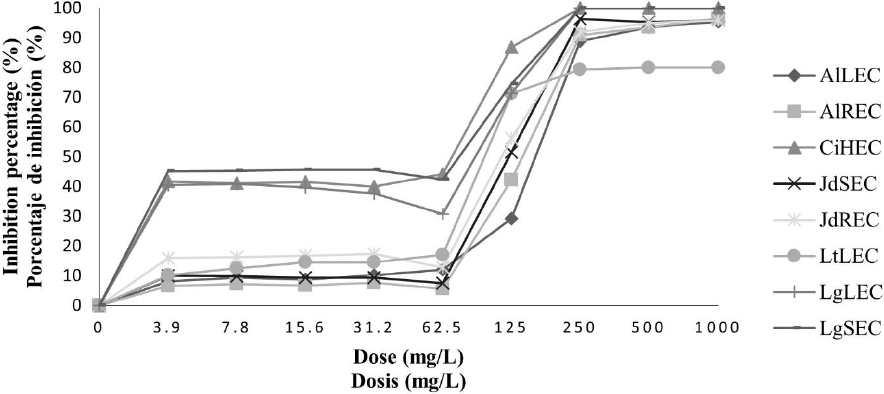
LtLEC: L. tridentata leaves. AlLEC: A. lechuguilla leaves. AlREC: A. lechuguilla roots. JdREC: J. dioica roots. JdSEC: J. dioica stem. CiHEC: C. illinoinensis husk. LgLEC: L. graveolens leaves and LgSEC: L. graveolens stem.
Fig. 3 Inhibition percentage of ethanolic crude extracts on F. oxysporum.
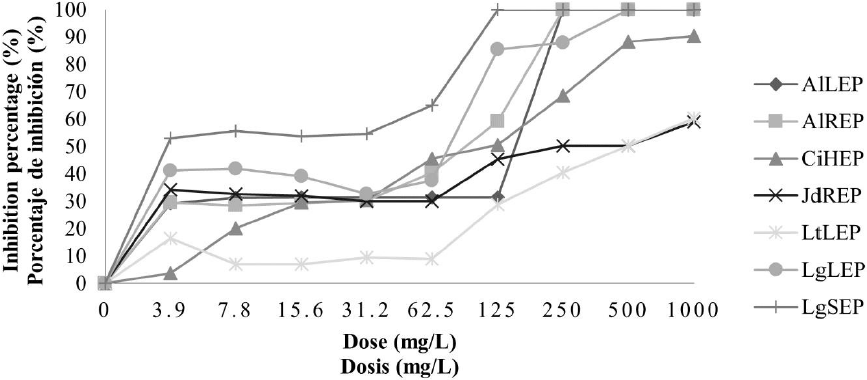
LtLEP: L. tridentata leaves. AlLEP: A. lechuguilla leaves. AlREP: A. lechuguilla roots. JdREP: J. dioica roots. CiHEP: C. illinoinensis husk. LgLEP: L. graveolens leaves and LgSEP: L. graveolens stem.
Fig. 4 Inhibition percentage of concentrated ethanolic extracts on F. oxysporum.
IC50 of plant extracts. The variance analysis showed that existed significant differences in the IC50 (Table 4). For aqueous crude extracts, the lower IC50 was by the extract LgLAC with 535.19 mg/L, and in concentrated aqueous extracts, the lower IC50 were by the extracts LgSAP and AlRAP with 218 and 203 mg/L respectively. The ethanolic extracts had better IC50 than the aqueous extracts; this observed in Table 4. In this case, the crude extracts with the lower IC50 were the extracts CiHEC and LgSEC with 18 and 16 mg/L, respectively. By the concentrated extracts, the best IC50 was by the extracts LgLEP and LgSEP with 22 and 8 mg/L, respectively.
Table 4 Inhibitory concentration to 50 % (IC50) of aqueous and ethanolic extracts for inhibition of F. oxysporum.
| Extract | IC50* | Extract | IC50 | Extract | IC50 | Extract |
|---|---|---|---|---|---|---|
| AILAC | 535.30±0.06g | A1LAP | 13109.00±0.06c | A1LEC | 115.00±0.12a | AILEP |
| A1RAC | 253613034.00 ± 0.03a | A1RAP | 2()3.74±0.06h | A1REC | 113.72±0.16b | AlREP |
| CiHAC | 9508417.00±0.03b | CiHAP | 983.5 l±0.09e | CiHEC | 18.03±0.19g | CiHEP |
| JdSAC | 7875.00±0.03f | JdSAP | 83361.00±0.14a | JdSEC | 92.00±0.20d | ** |
| JdRAC | 69721.00±0.04e | JdRAP | 1821.00±0.06d | JdREC | 75.00±0.20e | JdREP |
| LtLAC | 88075.00±0.02d | LtLAP | 673.55±0.09f | LtLEC | 108.50±0.21c | LtLEP |
| LgLAC | 535.19±0.03h | LgLAP | 53047.00±0.02b | LgLEC | 23.09±0.22f | LgLEP |
| LgSAC | 195805.00±0.07c | LgSAP | 218.91±0.31g | LgSEC | 16.24±0.15h | LgSEP |
Discussion
Because all the plants are natives from the Mexican semi-desert, the phytochemicals identified are similar; thus identified compounds in leaves and roots from A. lechuguilla coincide with the reported by Sidana et al. (2016) who reported 141 steroidal saponins of pharmacologic interest; about the tannins, Castillo et al. (2010) mentioned them in his work. C. illinoinensis husk, according to Bottari et al. (2017), contains compounds like tannins and flavonoids. Nevertheless, it also has been identified as the presence of quinones by Xiang-ming (2010), which is similar to the compounds identified in this work. Martínez et al. (2014) mention quinones, coumarins, flavonoids, saponins, tannins, carbohydrates, and reducing sugars for extracts from the root of J. dioica. The extracts from leaves of L. tridentata coinciding with Martins et al. (2012), who identified flavonoids, saponins, and cyanogenic glycosides. Finally in extracts from L. graveolens was identify tannins, coinciding with the report of Hernandez-Castillo et al. (2010); coumarins similar to the mentioned by Cruz et al. (2011); and flavonoids as flavonols and flavons being this results analogous to the obtained by Güereca et al. (2007). Many studies have revealed the efficacy of Mexican semi-desert plant extracts to inhibit the growth of phytopathogenic fungi; previously, several works have been reported these plants as a source of compounds with antifungal activity. Carvalho et al. (2011) tested aqueous extracts of Jatropha curcas, against Alternaria alternata and presented 46 % of control; Mendez et al. (2012) mentioned inhibition percentage of 60 % with aqueous extracts of L. graveolens and A. lechuguilla over different food bacteria. Also, these results are similar to obtained by Hernandez-Castillo et al. (2010), who reported 100 % of inhibition in R. solani with ethanolic extracts of C. illinoinensis, and coincide with the results of Jasso de Rodriguez et al. (2011) who used ethanolic extracts of A. lechuguilla and they observed 100 % of inhibition on C. gloeosporioides. Nevertheless, our results differ with the results reported by Mendez et al. (2012), who observed an inhibition percentage of 60 % on food bacteria using ethanolic extracts of L. graveolens and A. lechuguilla.
The phytochemical analysis showed that the extracts from these species have compounds with antifungal activity as alkaloids, flavonoids, tannins, and saponins. These compounds have different ways of effect on the pathogens; in the case of the alkaloids could be for the presence of nitrogen in their structure as amine or amide. By flavonoids presence, their composition of phenolic hydroxyls can penetrate the cellular membrane, so these hydroxyls combine, precipitate, and denature the protoplasmic proteins (Ruiton et al., 1998). From tannins form complexes with enzymes and other proteins provoking the inhibition of the enzymes, also they can inhibit the electrons transport through the membranes, and they can alter ions like iron and copper inhibiting the activity of some essential enzymes for microorganisms life (Scalbert & Williamson, 2000). The saponins form complexes with sterols, can affect proteins and membranes phospholipids (Stuardo & San Martin, 2008).
IC50 is the most widely used and informative measure of substance efficacy. It indicates how much a compound is needed to inhibit a biological process by half, thus providing a means of the potency of an antagonist substance in research. This measure is essential because a low IC50 indicates that a plant extract is an excellent candidate to control phytopathogenic fungi (Aykul & Martinez-Hackert, 2016). The results obtained in this work are comparable to reported by Caceres-Rueda de Leon et al. (2013) who observed an IC50 of 200 mg/L with aqueous extracts of L. graveolens on F. oxysporum, but differ with the report of Hernandez-Castillo et al. (2010) who reported IC50 of 1930 and 4340 mg/L with ethanolic extracts of L. graveolens and C. illinoinensis on R. solani.
The above result suggests that the low IC50 of L. graveolens could be due to the presence of compounds as thymol and carvacrol; these compounds are the most common volatile components in the Labiatae family, and distributed in plants as L. graveolens; these compounds are capable of inhibiting the growth of many fungus species (Chen et al., 2019). In the case of the A. lechuguilla extracts, the antifungal activity attributed to compounds like flavonoids, phenylpropanoids, and polyphenols (López‐Romero et al., 2018). Finally, the C. illinoinensis extract could be an essential source of non-polar compounds with antimicrobial activity, even though these extracts contain an unknown number of molecules (Cruz-Vega et al., 2008). Thus all compounds present in plant extracts award antifungal activity disrupting the cell membrane, affecting the mitochondrial function, arresting cell cycle processes at the S-phase, and provoking leakage of intercellular components due to the deterrent effect as in case of saponins (López-Romero et al., 2018).
Conclusion
The ethanolic plant extracts presented higher antifungal activity against F. oxysporum compared with the aqueous extracts, being the extracts of A. lechuguilla leaves and roots, C. illinoinensis husk and L. graveolens leaves and stem, the extracts with better antifungal activity. Therefore, it can be concluding that the solvent used to produce Mexican semi-desert plant extracts affects the phytochemical compounds presents in the extracts. For this reason, the use of plant extracts proposed as an alternative for control vascular wilt caused by F. oxysporum.











 nueva página del texto (beta)
nueva página del texto (beta)


