To ensure a year-round supply of high-quality feed in the livestock activity, producers have sought techniques that make it possible to utilize the surplus herbage mass produced during the rainy period. In this scenario, the production and use of hay from grasses of the genus Panicum could be a very important alternative in the feeding of animals during the dry period of the year.
The haymaking process consists of harvesting, drying, baling, and storing forage plants1, which are steps that can be performed manually or mechanically. To be considered ‘hay’, the forage should have 10 to 15 % moisture, which allows for adequate storage conditions and prevents the occurrence of deterioration processes and losses2.
Hay can be made from any forage plant, but some characteristics render some plants more suitable for hay making than others, how elevated forage yielding potential, adequate nutritional quality, presence of thin stems, and high leaf percentage. Another interesting feature of forage plant is tolerance to frequent harvests, since, the harvest interval can influence their regrowth potential and persistence3.
The BRS Tamani cultivar released by EMBRAPA-Brazil in 2015 with the aim of improving the nutritional value of pasture and forage production in tropical regions4, may be a viable forage option for hay production. It has thin leaves, a high forage-yielding potential, elevated ground cover ability, and high nutritional value, in addition to being resistant to pests and diseases5.
However, there is little scientific data on its use as hay, especially regarding its production and nutritional potential. Therefore, research should be carried out with the objective of obtaining data that identify the morphogenetic characteristics of the BRS Tamani cultivar, relating its physiological behavior and productive and nutritional potential so that the needs of the animals are met.
Besides that, this paper as a pilot study might not be statistically representative for the reason that there was a short period of study, although, they will provide an interesting insight into the morphogenetic characteristics and productive and nutritional potential of the Panicum maximum cv. BRS Tamani grass. The present study objective was to evaluate the potential of Panicum maximum cv. BRS Tamani at different ages of sprouting (49, 63, 77 and 91 d) for the production of hay during the rainy season.
The experiment took place in the Forage Crops Section of the Farm School, located in Terenos - MS, Brazil (20°26’34.31’’S, 54°50’27.86’’ W, 530.7 m asl), and at the Laboratory of Applied Animal Nutrition and Forage Crops, Faculty of Veterinary Medicine and Animal Science, at the Federal University of Mato Grosso do Sul (UFMS). The experimental period was October 2015 to April 2016. Monthly precipitation and minimum, mean, and maximum temperature data during the experimental period were collected at the Center for Weather, Climate, and Water Resource Monitoring of Mato Grosso do Sul (CEMTEC) (Figure 1).
Soil samples were harvested from the 0 to 20 cm layer to determine its fertility before the experimental beds were implemented. The following results were obtained: pH (CaCl2): 5.31; P: 4.52 mg dm-3; organic matter: 35.34 g dm-3; K: 0.20 cmol dm-3; Ca: 7.35 cmol dm-3; Mg: 1.20 cmol dm-3; Ca + Mg: 8.55 cmol dm-3; Al: 0.00 cmol dm-3; H + Al: 5.18 cmol dm-3; CEC: 13.93 cmol dm-3; base saturation: 628.1 g kg-1. Dolomitic limestone was applied in the amount of 1.2 t ha-1 (PRNT: 800.0 g kg-1). Prior to sowing, 100 kg ha-1 of P2O5 and 60 kg ha-1 of K2O were applied. After sowing, 100 kg ha-1 of N were applied in the form of urea.
The grass Panicum maximum cv. BRS Tamani was sown in November 2015 and was established in a total area of 36 m2 in October 2015. The sowing rate was 4 kg of viable seeds for hectare and the area was divided into sixteen 9 m2-experimental plots. In December 2015, a uniformity cut was made in all plots at 10 cm-stubble to start the study this was following by application of 50 kg ha-1 of N in the form of urea.
Treatments consisted of four regrowth ages (49, 63, 77, and 91 d) of harvest evaluated at rainy season. After every evaluation finished, were cut the plants in all the experimental plots to a 10 cm of residual herbage. A randomized-complete-block experimental design was adopted, with four replicates, with 16 experimental units.
The variance analysis was performed considering a randomized block design with four replicates, and orthogonal decomposition of the sum of treatment squares into linear and quadratic effects of different regrowth ages to probe the best fit of the model.
The model used was Yij = μ + Ri + eij, in which μ is the general average; Ri is the fixed treatment i, i = 1… 4, and eij is the experimental error associated with each observation Yij.
The significance of effects was analyzed by the Tukey test, at α=0.05, using PROC MIXED (Statistical Analysis Systems - SAS, version 9.1, SAS Institute, Inc. Cary, NC, USA).
To evaluate the morphogenetic and structural variables, five tillers representative of each plot were chosen, identified with a colored thread, and evaluated during the entire regrowth period of each age. The length of the marked tillers was measured every 7 d with a centimeter-graduated ruler. The lengths of stem (from the soil to the last leaf with a fully expanded ligule), leaf (measured from the expanded ligule to the extremity of the blade), and leaf under elongation (measured from the ligule of the last expanded leaf until the end of the blade) were measured in the tiller. Leaf appearance rate (LAR), leaf elongation rate (LER), stem elongation rate (SER), leaf senescence rate (LSR), phyllochron, number of live leaves per tiller (NLL), leaf lifespan (LL), and final leaf length (FLL) were calculated as proposed by Lemaire and Chapman6.
To quantify the herbage mass, forage samples were collected from each plot using a 1 m2 square frame and harvested at 10 cm from the soil surface, at random. After harvesting, the sample collected from within each frame was taken to the laboratory to be manually separated into the following morphological components: leaf (leaf blades), stem (stems + leaf sheaths), and senescent material. These were then dried at 55 ºC in a forced-air oven until reaching a constant weight for the determination of the dry weight and further laboratory analyses.
After the herbage mass was collected, the hays were made for the evaluation of the remaining forage from the entire experimental bed. The green (fresh) forage was chopped and weighed and then spread on the floor of a covered shed for drying. Upon reaching the haying point, the material was baled manually and weighed again. Four bales of hay were made per regrowth age and stored for 30 d in an appropriate shed. Subsequently, a 0.5 kg sample of each bale was collected, dried in an oven at 55 ºC, and then analysed in the laboratory.
Samples were ground through to 1 mm particles for the analysis of chemical composition of the morphological components of the plant (leaf and stem) and of the hay. The concentrations of dry matter (DM), organic matter (OM), and crude protein (CP) were determined as described in the AOAC7. Neutral detergent fibre (NDF) content was measured as proposed by Van Soest8, without a heat stable amylase and not corrected for ash. Acid detergent fibre (ADF) content was measured as proposed by Van Soest9 without corrected for ash. Lignin [lignin (sa)] was determined by solubilization of cellulose with sulphuric acid. Lastly, the in vitro digestibility of DM and NDF was evaluated according to the recommendations of Silva10, using the technique described by Tilley and Terry11 adapted to an artificial rumen developed by ANKON®, as described by Holden12.
Leaf appearance rate (Figure 2A), NLL (Figure 2D) and LER (Figure 2E) decreased linearly (P<0.05) as the regrowth age increased. The phyllochron increased linearly (Figure 2B), though. Stem elongation rate (Figure 2F) and LL (Figure 2C) showed a quadratic response (P<0.05), with maximum values of 0.80 cm tiller-1 day-1 (at 91 d) and 40.30 d (at 77 d), respectively. Despite the FLL (43.33 cm) and LSR (1.95 cm tiller-1 d-1) were not influenced (P>0.05) by the regrowth ages. These results can be explained by as a tiller ages, it gradually loses vigor, and this effect has great impacts on its morphogenetic and structural characteristics13. The higher LAR observed at the earlier ages can be related to the higher photosynthetic efficiency of younger tillers at those ages in relation to that observed in the older tillers found at the greater regrowth ages13. In the same way, LAR might have been reduced due to the changes occurring in LER and SER, because, LAR is affected by two factors, when these vary in the tiller: leaf elongation rate and stem length14.
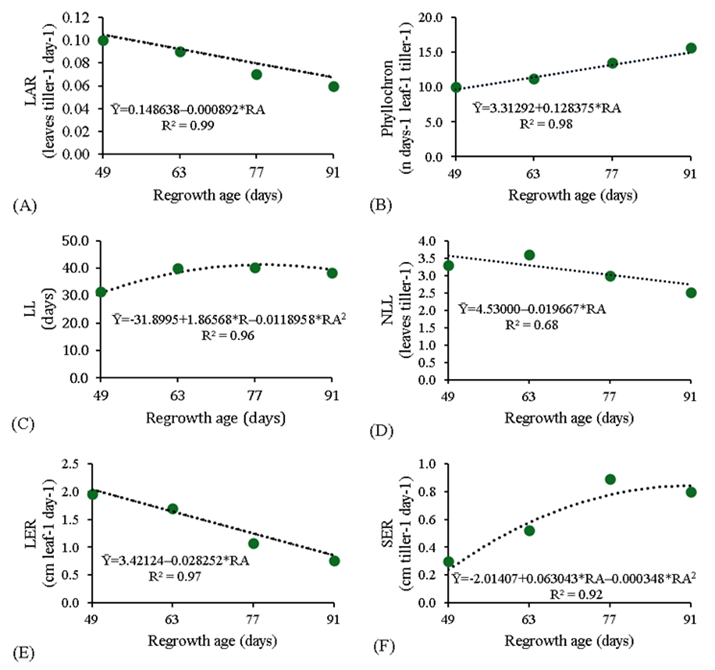
Figure 2 Leaf appearance rate (LAR); leaf lifespan (LL); number of live leaves per tiller (NLL); leaf elongation rate (LER); stem elongation rate (SER) of Panicum maximum cv. BRS Tamani at different regrowth ages (RA)
The regrowth age increased the elongation time of new leaves, then, this affect the phyllochron positively, since the SER (Figure 2F) and the proportion of stem (Figure 3C) rose with ages. Which can be explained by some researchers, which observed that increasing phyllochrons as a plant ages are due to the longer time necessary for the leaf to cover the distance between the apical meristem and the extremity of the stem, which is longer at longer regrowth ages15.
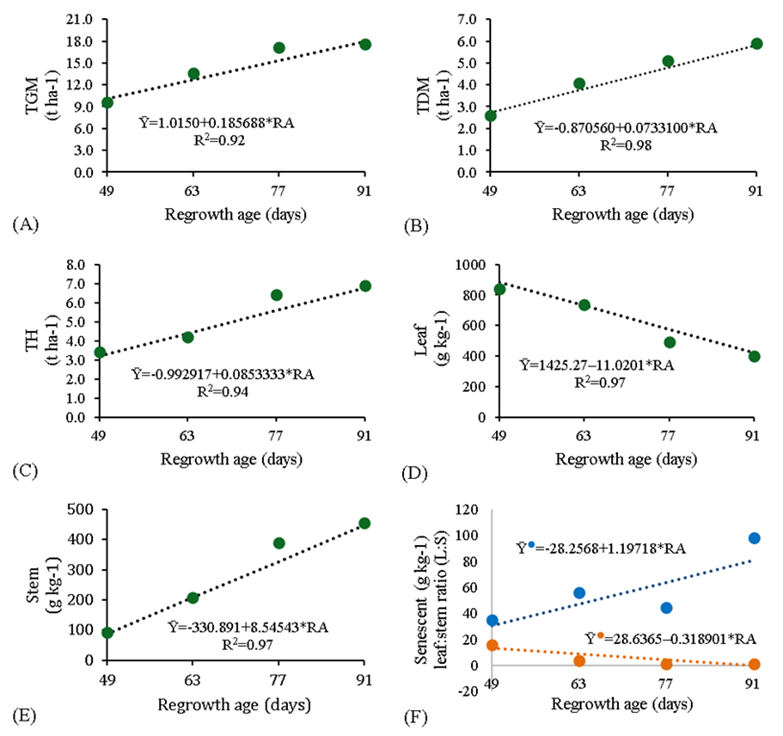
Figure 3 Total green mass (TGM), total dry mass (TDM), and total hay (TH), proportions of leaves, stems, and senescent material (Ῡ•) and leaf:stem ratio (Ῡ•; L:S) in Panicum maximum cv. BRS Tamani at different regrowth ages (RA)
The increasing LL up to 77 d may have been a consequence of the adaptation of the forage plant when it reduced its tiller renewal, as observed by the lower LAR and LER and higher SER (Figure 2A, E, F), leaves already existing remained alive for a longer time, though. In an experiment with Tanzania grass, the plant adapts when leaf-tissue renewal is low, which allows the leaves to remain alive longer16. Thus, older tillers are characterized by lower LAR, which lead to lower NLL and longer LL as compared with younger tillers17.
Number of live leaves is a genetically defined variable whose value is relatively constant. However, this variable may be changed depending on climatic conditions and pasture management18, which might have caused the variations observed in this study with the advancing regrowth ages. Another explanation is that the tiller entered in reproductive stage after 63 d of regrowth and it carried nutrients in older leaves to panicle, consequently, older leaves died and decreased the NLL with rose the regrowth ages.
Leaf elongation rate declines as a result of increased competition for photoassimilates as the plant grows older, which leads to the appearance of new tillers or inflorescences19. In young tillers, in order to attain high growth rates from the capture of resources, the plant increases its leaf elongation rate. Older tillers, in turn, depend on strategies to preserve these resources17 reducing these rates so as to minimize lesions caused by stressful conditions20.
The greater stem elongation demonstrated by SER is related to the increasing growth of the plant that was observed with time, since the competition for quantity and quality of light increases as the plant develops and recovers its leaf area. This culminates in greater stem elongation as a plant’s attempt to place the leaf blades in the upper part of the canopy, which receives a significant amount of light16. Another likely explanation for the increased stem elongation is the plant’s transition from vegetative to reproductive stage, because during the experimental period, was observed that cv. BRS Tamani evolved to the reproductive stage at 63 d of regrowth.
The proportion of leaves (Figure 3D) and the leaf: stem ratio (L:S; Figure 3F) declined linearly (P<0.05) with the higher regrowth ages, while the opposite response was seen for the proportions of stem (Figure 3E) and senescent material (Figure 3F). These responses can be explained by the appearance of the panicle with the advance of maturity, which was observed after 63 d of regrowth, since the grass reduces the leaf size and prioritizes the growth of stems to expose the inflorescence. When plants enter the reproduction period, the leaf blade percentage declines immediately, even in pastures under constant management, which consequently elevates the proportion of stems21.
At the higher regrowth ages the proportion of senescence material (Figure 3F) was also higher, which is due to the gradual increase in competition for light and in stem elongation. In this regard, the quantity and quality of light that reaches the canopy decline lead to morphophysiological alterations in the plant20. In this way, the leaves located near the base and that are shaded accelerate the senescence process18.
The decreasing L:S (Figure 3F) is a result of the increasing proportion of stem (Figure 3C) and decreasing proportion of leaves (Figure 3D) observed with the progression of regrowth days. Decreasing L:S are often related to the aging process of a forage plant22. The lower L:S found at 91 d of regrowth, it was 0.90, which characterizes this regrowth age as inadequate for hay making for cv. BRS Tamani, because there was more proportions of stem to leaves and greater senescent material than others regrowth ages.
Total green mass (TGM; Figure 3A), total dry mass (TDM; Figure 3B) and total hay (TH; Figure 3C) rose linearly (P<0.05) with the regrowth age. Total green mass, TGM, and TH increased by 0.18, 0.07, and 0.08 t ha-1 with the regrowth age, respectively. The increased of all production can be explained by greater stem elongation and proportion of stem and dead material, observed in Figure 2E, 2F and Figure 3F. While the leaf production declined by 11.02 g kg-1 with every day of regrowth (Figure 3D).
In the leaf, the DM, NDF, ADF, and lignin (sa) contents increased linearly (P<0.05) as regrowth ages were greater (Table 1). Nonetheless, the opposite is true for CP content in leaf. The DM, ADF and Lignin (sa) content of the stem and the hay increased linearly (P<0.05) with the regrowth ages. Nevertheless, the CP in stem and hay decreased (P<0.05) as regrowth ages were greater.
Table 1 Chemical composition of leaf, stem, and hay of Panicum maximum cv. BRS Tamani at different regrowth ages
| Ítem | Regrowth ages (days) | Regresion equations (r2) | SEM | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 49 | 63 | 77 | 91 | L* | C◊ | |||
| Leaf | ||||||||
| MS, g kg-1 | 280.1 | 303.5 | 299.1 | 349.5 | Y=209.483+1.35917*ER (0.80) | 0.907 | 0.0005 | 0.1827 |
| MO, g kg-1 MS | 930.7 | 918.0 | 919.3 | 921.8 | Y=922.45 | 0.302 | 0.2888 | 0.1621 |
| PC, g kg-1 MS | 84.0 | 62.1 | 62.2 | 61.1 | Y= 101.65-0.49*ER (0.64) | 0.323 | 0.0002 | 0.0039 |
| FDN, g kg-1 MS | 740.9 | 745.5 | 750.0 | 723.0 | Y=676.314+1.1145*ER (0.84) | 0.833 | 0.0001 | 0.4212 |
| FDA, g kg-1 MS | 481.0 | 487.7 | 534.1 | 549.8 | Y=434.156+0.9651*ER (0.68) | 0.709 | 0.0002 | 0.1519 |
| Lignin (sa), g kg-1 MS | 49.4 | 52.6 | 78.8 | 80.9 | Y=1.954+0.9107*ER (0.89) | 0.256 | 0.0001 | 0.4267 |
| Stem | ||||||||
| MS, g kg-1 | 207.1 | 251.2 | 257.5 | 295.7 | Y=121.281+1.81455*ER (0.94) | 0.985 | 0.0001 | 0.6161 |
| MO, g kg-1 MS | 919.0 | 923.4 | 939.2 | 939.9 | Y=930.38 | 0.503 | 0.0514 | 0.8268 |
| PC, g kg-1 MS | 42.6 | 44.0 | 25.5 | 21.9 | Y=72.5572-0.538897*ER (0.83) | 0.315 | 0.0001 | 0.2534 |
| FDN, g kg-1 MS | 798.9 | 803.8 | 801.9 | 804.0 | Y=802.15 | 1.522 | 0.1777 | 0.2330 |
| FDA, g kg-1 MS | 566.5 | 590.3 | 669.8 | 651.9 | Y=451.78+2.3978*ER (0.78) | 1.320 | 0.0001 | 0.0124 |
| Lignin (sa), g kg-1 MS | 47.6 | 58.3 | 109.6 | 108.1 | Y= -35.5+1.6629*ER (0.85) | 0.853 | 0.0001 | 0.0431 |
| Hay | ||||||||
| MS, g kg-1 | 881.7 | 875.9 | 847.2 | 852.8 | Y=920.197-0.7694*ER (0.77) | 0.458 | 0.0001 | 0.0521 |
| MO, g kg-1 MS | 917.9 | 920.2 | 921.5 | 882.8 | Y=910.60 | 1.149 | 0.2723 | 0.3296 |
| PC, g kg-1 MS | 81.2 | 79.5 | 63.6 | 47.6 | Y=124.436-0.7789*ER (0.92) | 0.456 | 0.0001 | 0.0982 |
| FDN, g kg-1 MS | 746.5 | 750.2 | 752.9 | 759.2 | Y=752.2 | 0.293 | 0.0953 | 0.8046 |
| FDA, g kg-1 MS | 519.8 | 531.1 | 556.5 | 567.7 | Y=462.039+1.1276*ER (0.97) | 0.614 | 0.0001 | 0.9753 |
| Lignin (sa), g kg-1 MS | 74.3 | 73.2 | 82.8 | 86.4 | Y=56.99335+0.3057*ER (0.85) | 0.187 | 0.0001 | 0.1878 |
DM= dry matter; OM= organic matter; CP= crude protein; NDF0: neutral detergent fiber; ADF= acid detergent fiber; RA= regrowth ages. SEM= standard error of the means; *Linear; ◊Quadratic.
Raising DM contents in the leaf and in the stem as the regrowth age did are due to the increasing amount of fibrous components in the cell wall (Table 1). However, the decreasing DM content in the hays resulting from the progression of regrowth time may be associated with the losses of leaf blades when the bales were made, besides the higher losses of moisture content in the forage at earlier ages, when stems are younger23. Despite these decreases in DM values, they were within the acceptable limits, which correspond to 10 to 15 % moisture, in which no losses or deterioration occur2.
As a forage plant grows older, its fibrous fraction roses (Table 1) for the reason that the development of supporting structures provided by the fibrous carbohydrates and lignin. The cell wall then thickens and is lignified, due mainly to the increasing quantity and thickness of stem24. The opposite is true to crude protein concentrations declined as the regrowth age of cv. BRS Tamani advanced. This result may be related to the thickening of the cell wall observed at older ages, which may lead to a reduction of cell wall content, which includes protein and soluble carbohydrates. Another explanation for the lower CP content is that protein components complex to those of ADF, becoming the insoluble fraction of the forage plant25.
The lower CP observed in hays at older regrowth ages may be associated with a lower L:S; i.e., a larger proportion of stems and a smaller proportion of leaves (Figure 3). Along with this factor, the CP content of leaf and stem declined as the regrowth age advanced (Table 1). The minimum CP level of the diet should be considered 70 g kg-1, and values below that may compromise animal performance, since the development of rumen microorganisms and digestibility would be negatively affected26. Therefore, to prevent the utilization of CP from being restricted, cv. BRS Tamani should be used from 49 to 55 d of regrowth, during which period the CP content of the material would be higher than the minimum necessary (80.88 to 71.13 g kg-1).
The in vitro digestibility of dry matter (IVDMD) and neutral detergent fiber (IVNDFD) decreased linearly (P<0.05) in the leaf, the stem and in the hay as the regrowth days were greater (Table 2). The lowest values of these two components were in the stem. In the hay, the lowest values of the respective components were 568.03 and 483.69 g kg-1, both observed at 91 d of regrowth age.
Table 2 In vitro digestibility of leaf, stem, and hay of Panicum maximum cv. BRS Tamani at different regrowth ages
| Item | Regrowth ages (days) | Regression equations (r2) | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| 49 | 63 | 77 | 91 | L* | Q◊ | |||
| Leaf | ||||||||
| IVDMD, g kg-1 DM | 649.9 | 617.4 | 611.5 | 588.7 | Y=708.383-1.2622*RA (0.94) | 0.550 | <0.0001 | 0.6026 |
| IVNDFD, g kg-1 DM | 610.2 | 565.0 | 539.0 | 515.8 | Y=702.951-1.9743*RA (0.90) | 1.034 | <0.0001 | 0.2827 |
| Stem | ||||||||
| IVDMD, g kg-1 DM | 554.1 | 485.0 | 445.1 | 431.8 | Y= 694.551-2.9193*RA (0.89) | 1.267 | <0.001 | 0.4781 |
| IVNDFD, g/kg DM | 509.0 | 432.0 | 378.5 | 345.2 | Y=746.255-4.6412*RA (0.91) | 1.654 | <0.001 | 0.3705 |
| Hay | ||||||||
| IVDMD, g kg-1 DM | 638.2 | 601.8 | 586.4 | 555.0 | Y=710.94-1.6959*RA (0.92) | 0.985 | <0.001 | 0.4135 |
| IVNDFD, g kg-1 DM | 598.8 | 557.1 | 520.7 | 489.8 | Y=719.647-2.4983*RA (0.97) | 1.103 | <0.001 | 0.5073 |
IVDMD= in vitro dry matter digestibility; IVNDFD= in vitro neutral detergent fiber Digestibility; RA= regrowth ages. SEM= standard error of the means; *Linear; ◊Quadratic.
The decreasing digestibility values (Table 2) detected with the advancing regrowth age are due to the reduction of fiber quality, since the lignin contents of the cell wall increased (Table 1). Athayde et al23 stated that the unfavorable effects of lignin are more pronounced in tropical grasses as their regrowth age progresses. This negative effect might have generated a barrier that blocks the microorganisms from adhering and promoting enzymatic hydrolysis27. Another factor that can be lead to a reduction in digestibility is an imbalance between nutrients. Vasconcelos28 submitted that one of the reasons for declines in rumen digestibility is nutritional imbalance, especially of energy (carbohydrates) and protein. Thus, the increasing fiber and decreasing CP contents resulting from the increasing regrowth period described in Table 1 reduce the fiber utilization in the rumen.
In view of the present results, BRS Tamani has potential for hay production. Although the later regrowth ages provided higher green matter, dry matter, and hay yields, they had a negative impact on the morphogenetic characteristics, nutritional values, and digestibility of the grass. Therefore, the regrowth ages of 49 and 63 d showed the best results for the production of hay with the best nutritional value without compromising the morphogenetic characteristics of the plant.
The Panicum maximum cv. BRS Tamani grass has the potential to produce 3.4 to 4.2 t ha-1 of hay in the regrowth interval of 49 to 63 d. When defoliated in this range, BRS Tamani hay presented better nutritive value and high proportion of leaves, which characterizes a gramine suitable for use in the form of hay in larger cut intervals aiming at higher productivity and nutritional quality. Above 63 d of regrowth, tillers from BRS Tamani progressed from vegetative to reproductive stage, that resulted in greater stem elongation rate and the nutrients decreased in the leaf and stem and come to seeds. Further studies should be undertaken focusing on the regrowth ages with more than a single year, so, it can evaluate the effect of the year under different environmental conditions.











 texto en
texto en 



