Introduction
Coastal lagoons, in Mexico, are aquatic systems noted for their floral and faunal biodiversity, which increase their economic importance in terms of fisheries, industry, trade and recreation (Alcocer, 2007). Coastal lagoons have internal hydrological variability that depends on land connections and influences from other freshwater bodies. These salinities range from hyperhaline to oligohaline, depending on their size, location and season. Salinity in coastal lagoons is a physicochemical parameter that helps to maintain their hydrological variability, biological diversity and ecology (Herrera-Silveira, 2006).
Unfortunately, the ecological equilibrium in coastal lagoons are heavily affected by various factors such as: a) seasonal variability (rain and winds off-season), b) geomorphological and hydrological characteristics of the area, c) ocean acidification, d) atmospheric nitrogen deposition, and e) waste from nearby human activities that provide pollutants and high nutrient concentrations. Among the latter are primarily orthophosphates, nitrogen (nitrate and nitrite) and silica, substances unlikely to be flushed into the ocean, resulting in higher concentrations of these compounds, especially in enclosed coastal systems or those having little circulation (Contreras et al., 1997; La Barre et al., 2014).
Accompanying aquatic nutrient availability is an increased variability of physicochemical parameters and trophic status (Kormas et al., 2001; Sosnovsky and Quirós, 2006). Variations in nutrient concentrations cause different trophic states in coastal lagoons during seasons. The different trophic states are produced mainly by: effect of winds, water contributions and denitrification processes (López-Cortés, 2003). This promotes algal blooms including harmful algal blooms (HABs) that are toxic to aquatic organisms and humans (Contreras et al.,1996; Shaw et al., 2003). These phenomena have a negative impact in many regions of the world on public or animal health, plus adversely affecting the environment. The effects of harmful algal blooms are as follows: 1) massive mortality of marine, cultivated or wild organisms; 2) impairing coastal economic activities; 3) alteration of the landscape, 4) effects on public health (toxic syndromes: paralytic, diarrheal, amnesic and cyclogenic) (García-Mendoza et al., 2016).
The presence of Pseudo-nitzschia sp. also was recorded, it represents a wide distribution in marine and coastal waters. This genus includes several species of algae. Some of these have the property of producing a neurotoxin called domoic acid. More than forty species have been reported and about fourteen of them are domoic acid producers (Lim et al., 2012; Lim et al., 2013). This toxin causes amnesic poisoning when consuming contaminated bivalve molluscs. It is noteworthy to mention that potentially toxic Pseudo-nitzschia species such as Pseudo-nitzschia calliantha, P. cuspidata, P. delicatissima, P. pseudodelicatissima, P. pungens, P.brasiliana (Parsons et al., 2012) have been recorded in the coastal area of Veracruz.
The phytoplankton communities in the coastal systems are mainly composed of diatoms and dinoflagellates, these are energy producers at the beginning of the trophic chain (Orduña-Medrano, 2012). They also promote the sustainability of fisheries in coastal environments, and contribute to the nutrient dynamics and bio-geochemical cycles in these areas (Ceballos-Corona, 2006; Waters, 2007). Availability of nutrients, such as orthophosphate, is related to the discharge from anthropogenic activities in the ecosystem. This compound is involved in the eutrophication of coastal lagoons by reducing oxygen at deeper levels of the water column via the decomposition of organic matter. Nitrates and nitrites in coastal systems can influence the formation of blooms of nitrogen-fixing cyanobacteria (Aubriot et al., 2005).
The Gulf Coast of Veracruz has a wide variety of environments. The lagoon system of Mandinga is an estuarine system formed by fishery resources of great economic and ecological value. It is one of the leading producers of oysters Crassostrea virginica (Gmelin) in the Gulf of Mexico (INEGI, 2015). Considering that the socio-economic activities carried out by the population close to the lagoon system are mainly: tourism, agriculture, commerce and fishing (Lara-Dominguez et al., 2009; Aldasoro, 2015). The Mandinga lagoon system generates an important source of income from the oyster resource, oyster extraction, being that the exploitation of these resources represents the main productive activity of the surrounding population, in addition to complementing other fish products such as: flake, crab and shrimp. In which, a maximum income of up to $ 2,575.00 Mexican pesos per week is earned during the dry and rainy season (Navarrete-Rodriguez et al., 2015).
Due to the previously mentioned, this study assessed the abundance and distribution of diatoms and dinoflagellates, and their relationship with aquatic physicochemical parameters in the lagoon system of Mandinga, during dry and North winds season.
Material and Methods
Our research was conducted in the lagoon system of Mandinga, which has a surface area of 3,250 ha and is located between 18°58’N, 96°07’W and 19°06’N, 96°01’W (Figure 1) in the state of Veracruz, Mexico. The system is associated with the Atoyac and Jamapa river basins connecting the Sierra Madre Oriental and coastal plains of the Gulf of Mexico. The system is separated from the sea by a barrier of sand dunes, although being connected to the Jamapa River estuary in the Gulf of Mexico, near Boca del Río.
The lagoon system of Mandinga has a North-South orientation, while the Gulf coastline is oriented Northeast to Southeast. Together, form the Punta de Antón Lizardo. The lagoon system is a low energy, tropical environment with an average temperature of 27 °C and salinity levels (18 psu) stay low due to its indirect connection to the sea, but are sufficient to host the sea grass species Ruppia maritima L. (now known to be cryptic species complex). Oxygen levels are high and can reach saturation in some areas of the system due to North winds and photosynthetic activity. Quartz and some iron-magnesium minerals, as well as biota typical of low energy areas are present (Castañeda & Contreras, 1993; INEGI, 2015).
The lagoon system of Mandinga is characterized by the establishment of oyster banks, and its collection is an important economic activity in the area (Arias De León, 2014). Its fish fauna consists of marine, estuarine and freshwater species, from different trophic levels (carnivores, herbivores and detritivores); euryhaline species predominate during the rainy season (Gómez-Sánchez, 2013; Castán-Aquino, 2013).
Water and phytoplankton sampling
Sample collection was performed monthly during North winds and dry seasons (November 2011 to April 2012). Five sampling sites were considered (Table 1) among which, four oyster banks and a site near the sea were located, as a reference, because they are marine organisms. Physicochemical parameters, such as dissolved oxygen, temperature, and salinity, were recorded in situ using an YSI 6600 Multi-parameter, Water Quality Probe. During the process, water was collected using a 3L Van Dorn bottle at a depth of 30 cm. The volume of each sample was 1L and was used for quantification of phytoplankton, which was fixed in situ using 0.5 to 1 mL of lugol acetate solution per 100 mL (Ferrario et al., 1995). Samples for analysis of phosphates (PO4 -3), nitrates (NO3-), silicates (SiO2) and chlorophyll-a were collected in 500 mlL polyethylene bottles.
Table 1 Geographic coordinates of the sampling sites.
| Site | N Latitude | W Longitude |
|---|---|---|
| S1 | 19°06.223ʹ | 096°05.798ʹ |
| *S2 | 19°03.385ʹ | 096°06.489ʹ |
| *S3 | 19°02.824ʹ | 096°05.159ʹ |
| *S4 | 19°02.923ʹ | 096°04.687ʹ |
| *S5 | 19°02.923ʹ | 096°04.265ʹ |
*Oyster bank
Phytoplankton samples were collected to identify complex species by towing a 60-µm mesh net and 250 mL collection tube behind a boat with an outboard motor for 5 minutes as it maneuvered slowly in circles. Samples were fixed with a final solution of 4 % formalin for later identification (Ferrario et al., 1995; Band-Schmidt et al., 2011) and transported to the Laboratorio de Investigación de Recursos Acuáticos (LIRA), at the Instituto Tecnológico de Boca del Río.
Laboratory analysis
Chlorophyll-a determination was performed using the techniques of Jeffrey and Humphrey (1975), water samples were filtered in the laboratory, using Whatman GF/C Glass microfiber filters, Grade GF/C of 47 mm, placed in 90% acetone and refrigerated at 4° C for 24 hours for extraction, after this, samples were read in a Thermo Scientific Genesys 20 spectrophotometer. The nutrients PO4 -3, NO3 - and SiO2 were determined using the colorimetric method CHEMets® Water Test Kit: K-8510, K-6904 and K-9010 respectively. Phytoplankton sample processing was performed directly by observation of a subsample and preliminary identification mainly of genus of diatoms and dinoflagellates was made using rapid recognition of taxonomic characteristics. Identification of phytoplankton was based on published keys (Hallegraeff, 1995; Esqueda-Lara & Hernández-Becerril, 2010). The observations were made using a binocular optical microscope Carl Zeiss Axiostar 47190 and a 10x objective. The total cell count for each phytoplankton sample was performed using a Sedgewick Rafter Counting Chamber (1 mL). Phytoplankton abundance was reported, according to the number of cells per liter (cel L-1). The analysis focused primarily on the diatoms and dinoflagellates as the predominant groups contributing to primary productivity.
Statistical analysis
To achieve normality, all the data were transformed using a logarithmic function (log2 X + 1) (Aké-Castillo, 2015), and the Levene test was used to check for homoscedasticity of variances. The software program STATISTICA version 7 (StatSoft, 2016), a Kruskal-Wallis nonparametric analysis of variance, a parametric F-test, a parametric analysis of variance (ANOVA), and the Tukey test were used to search for significant differences among physicochemical parameters of the water during sampling months, November 2011 to April 2012, in sampling sites. The relationship between water physicochemical parameters and phytoplankton were evaluated using a multivariate Canonical Correspondence Analysis in an Addinsoft ® XLSTAT version 2015.
Results and Discussion
Monthly mean values of the physicochemical parameters
Maximum mean temperatures were recorded in March (29.1 ± 0.8 °C) and April, 2012 (28.0 ± 0.5 °C), and minimum mean temperatures were observed in November, 2011 (21.9 ± 0.7°C). The analysis of variance showed temporal variation between months. The temperature recorded in January, March and April were different from the rest of the samplings (F = 76.9, p ≤ 0.05) (Table 2). The results obtained show the maximum temperatures recorded are similar to those reported by Martínez Del Rosario (2011) during the dry season (March and April), as well as those reported by Zaballa-Carranza (1982) during spring (April) (28.12 °C), and winter (February) (25.27 °C).
Table 2 Results from analyses of variance of monthly means for the water physicochemical parameters from November 2011 to April 2012, in the lagoon system of Mandinga, Veracruz, Mexico.
| Physicochemical Parameters | Monthly Means | ANOVA/p | |||||
|---|---|---|---|---|---|---|---|
| November | December | January | February | March | April | ||
| T (°C) | 21.9±0.7a | 25.2±0.4b | 27.0±0.9c | 24.1±0.3d | 29.1±0.8c | 28.0± 0.5c | F= 76.9/0.00001 |
| S (psu) | 20.6±3.6a | 22.9±2.9a | 25.3±1.9a | 28.5±0.8b | 30.8±1.1c | 31.0±2.2c | F= 15.20/ 0.000001 |
| DO (mg L-1) | 9.3±1.3a | 7.14±0.6b | 7.2±0.5b | 8.8±0.7ac | 8.3±1.8abc | 6.7±0.5bc | F= 5.29/ 0.002 |
| NO3 (µM) | 3.9±4.4a | 0.8±1.8a | 2.4±0.9a | 4.0±0.0ab | 20.3±44.9ab | 0.2±0.4ac | *KW-H = 12.18/0.03 |
| PO4 (µM) | 1.3±1.4a | 1.3±0.7a | 0.8±0.3a | 2.9±4.2a | 1.0±0.0a | 2.3±2.2a | F = 1.09/ 0.39 |
| SiO2 (µM) | 256.2±25.2a | 222.9±58.3a | 232.9±62.2a | 193.0±59.5a | 149.7±40.7ab | 119.8±24.6bc | F = 6.83/ 0.0004 |
| Clorophyll-a (mg m-3) | 9.9±2.5a | 7.9±4.1a | 11.7±13.7a | 9.3±3.5ab | 10.3±7.6ab | 14.6±5.6bc | F = 6.78/ 0.0005 |
*KW-H= Kruskal Wallis non-parametric test. Different letters indicate significant differences.
Maximum mean salinity was recorded in March and April, 2012 (30.8 ± 1.1 and 31.0 ± 2.2 psu, respectively), while minimum mean salinity was recorded in November, 2012 (20.6 ± 3.6 psu). Salinity showed differences in the first three months of sampling with respect to the rest, the latter presented the highest (F = 15.20, p ≤ 0.05) (Table 2) The variation of temperature and salinity during the last months of sampling is associated with seasonal change, and that the lagoons system of Mandinga is shallow (1-8 m in depth), is permanently connected to the sea, and is influenced by freshwater inputs from the Jamapa and Arroyo Hondo rivers (Ruíz-Barreiro, 2012).
Maximum mean dissolved oxygen levels occurred in November 2011, and February 2012 (9.3 ± 1.3 mg L-1 and 8.8 ± 0.7 mg L-1, respectively), and a minimum mean of 6.7 ± 0.5 mg L-1 in April 2012. Analysis of variance (ANOVA) allowed observing significant differences during the month of April with respect to November and February F = 5.29, p = 0.05 (Table 2).
Values of dissolved oxygen obtained during the study are similar to the average value of 4.5 mg L-1 reported by Contreras & Castañeda (2004). These variations are due to North winds and evaporation, characteristics of the seasonal climate in the area (Zaballa-Carranza, 1982; Martínez Del Rosario, 2011). Bornn & Ruíz-Zamites (1982) reported an even lower mean concentration of 1.73 mg L-1 in April, 1982; due to the increase of organic matter in suspension and biochemical demand caused by dredging in the system.
A maximum mean concentration of chlorophyll-a was 14.6 ± 5.6 mg m-³ in April 2012 and a minimum level of 7.9 ± 4.1 mg m-³ occurred in December 2011. Analysis of variance showed variations in the concentrations of silicates and chlorophyll-a during November, February and April (F = 6.83, p ≤ 0.05; F=6.78, p ≤ 0.05) (Table 2). The chlorophyll-a in the lagoons system was consistent with Barreiro-Güemes & Aguirre-León (1999), who reported mean values of 8.7 and 14 mg m-3 of chlorophyll-a in areas with greater influence of the tide, greater turbulence and less transparency. While, in November 2011 and April 2012 (Table 2), a minimum mean for silicates was 119.8 ± 24.6 µM and the maximum mean was 256.2 ± 25.2 µM. The silicate concentration is inversely proportional to that of chlorophyll-a, where values obtained were not considered limiting within the system (Bornn & Ruíz-Zamites, 1982).
Maximum monthly means for nitrates were recorded in February and March, 2012, with 4.0±0.0 and 20.3±44.9 µM, and a minimum mean of 0.2 ± 0.4 µM in April, 2012. Nitrogen compounds showed significant differences in January, February and April with respect to the rest of the samples (W = 12.18, p ≤ 0.05, Table 2).
The variability in nitrate concentrations that were recorded during the study may be due to climatic or extemporaneous factors, which caused turbulence in the bottom, mobilizing and incorporating to the water column the nitrogen accumulated in the sediment. For Kennish & Perl (2010), nitrogen and other nutrients precipitate and tend to accumulate in the bottom attached to the sediment where the biogeochemical interactions take place.
Maximum mean of phosphate was 2.9 ± 4.2 µM in February, 2012, and a minimum mean (0.8 ± 0.3) µM was observed in January, 2012. The analysis of variance allowed recognizing significant differences in physicochemical parameters, with the exception of phosphates (F = 1.09, p = 0.39) (Table 2). November, 2011, and March, 2012, were transition periods between the windy and rainy seasons, where many nutrients were transported (Contreras et al., 1996). Rodríguez (2008) found that nitrogen compounds are inversely proportional to temperature, and total phosphate is greater during the warmer months. Bornn & Ruíz-Zamites (1982) determined that nutrients are not a limiting factor in phytoplankton growth. Such compounds settle out of the water column to eventually be incorporated by benthic organisms, and have their greatest concentrations in coastal sites.
Monthly averages of water physicochemical parameters in sampling sites
A maximum mean temperature was 29.9 °C during March 2012, at site S4. A minimum mean occurred in November 2011, at site S2 (20.8 °C) (Figure 2). A maximum mean salinity predominantly occurred in site S1, as it was the closest site to the ocean. However, in April 2012, a maximum mean occurred at site S2 (33.9 psu), and a minimum mean (17.9 psu) at site S5 in November 2011 (Figure 3). The maximum level of dissolved oxygen was 10.9 mg L-1 at site S4 during March 2012, and a minimum during April 2012, (5.8 mg L-1) at site S3 (Figure 4). A maximum mean for chlorophyll-a was 35.1 mg m-³, which occurred at site S5 during January 2012, and a minimum mean (0.63 mg m-³) occurred during December 2011, at site S1 (Figure 5).

Figure 2 Mean monthly water temperatures, from November 2011 to April 2012, in the lagoon system of Mandinga, Veracruz, Mexico.

Figure 3 Mean monthly water salinity, from November 2011 to April 2012, in the lagoon system of Mandinga, Veracruz, Mexico.

Figure 4 Mean monthly values for dissolved oxygen in the water column, from November 2011 to April 2012, in the lagoon system of Mandinga, Veracruz, Mexico.

Figure 5 Mean monthly for chlorophyll-a in the water column, from system of Mandinga, Veracruz, Mexico.
According to La Barre et al. (2014), the variability of physicochemical characteristics of water in coastal systems is due to climate change, rainfall events, off-season winds, and contributions from industrial and household nutrients. The latter can contribute to eutrophication of the environment, resulting in reduced dissolved oxygen and high concentrations of nitrates, phosphates and silicates (Álvarez-Gongóra et al., 2012) (e.g. site S1 in February, 2012). In general, the shallow coastal systems have short hydraulic residence times, which allow a renovation in a few days, entering well-oxygenated seawater. In addition, other factors such as primary production, the mechanical action of wind and the lack of barriers that prevent its action, also favor the incorporation of oxygen from the atmosphere (López-Monroy et al., 2017).
Maximum mean concentrations of nitrates and phosphates were 100.7 and 10.5 µM during March (S5) and February 2012 (S1), respectively (Table 3). Concentrations observed in site S5 can be explained by increased tourist and commercial activities in the area. There is also influence from sewage discharge from the population of Mandinga, which has no drainage system. The lowest means for nitrates were detected during December, 2011, March and April, 2012. However, phosphates were at their lowest concentrations during January 2012, followed by consecutive increases during February, March and April 2012. A maximum mean for silicates was recorded during January 2012 (332.8 µM) at site S1, and the minimum mean (83.2 µM) at site S4 during April 2012. Chlorophyll-a concentrations increased during March and April, 2012.
Table 3 Mean monthly for nutrient concentrations in the water column, from November 2011 to April 2012, in the lagoon system of Mandinga, Veracruz, Mexico.
| Months/Sampled sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nutrients | November | December | ||||||||
| µM | S1 | S2 | S3 | S4 | S5 | S1 | S2 | S3 | S4 | S5 |
| NO3 | 9.3 | 8.0 | < 0.3 | < 0.3 | 2.0 | 4.1 | < 0.3 | < 0.3 | < 0.3 | < 0.3 |
| PO4 | < 0.2 | 3.8 | < 0.2 | 1.0 | < 0.2 | 2.7 | < 0.2 | < 0.2 | < 0.2 | 1.0 |
| SiO2 | 282.8 | 282.8 | 232.9 | 249.6 | 232.9 | 249.6 | 249.6 | 199.6 | 282.8 | 133.1 |
| January | February | |||||||||
| NO3 | 4.03 | 2.0 | 2.0 | 2.0 | 2.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| PO4 | 0.2 | < 0.2 | < 0.2 | 1.0 | < 0.2 | 10.5 | 1.0 | 1.0 | 1.0 | 1.0 |
| SiO2 | 332.8 | 183.0 | 183.0 | 249.6 | 216.3 | 299.5 | 166.4 | 166.4 | 166.4 | 166.4 |
| March | April | |||||||||
| NO3 | < 0.3 | < 0.3 | 1.0 | < 0.3 | 100.7 | < 0.3 | 1 | < 0.3 | < 0.3 | < 0.3 |
| PO4 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 6.3 | 2.1 | 1.0 | 1.0 | 1.0 |
| SiO2 | 83.2 | 149.7 | 149.7 | 183.0 | 183.0 | 149.7 | 133.1 | 116.4 | 83.2 | 116.4 |
In the phytoplankton analysis, thirty-one genera of microalgae and sixteen species were identified, classifying four dinoflagellates and twenty-seven diatoms. The keys that correspond to these genera and species are in the last column of Table 4.
Table 4 Phytoplankton abundance (cel L-1) from November 2011 to April 2012, in the lagoon system of Mandinga, Veracruz, Mexico.
| DIATOMS | |||||||
|---|---|---|---|---|---|---|---|
| Genera and species | Nov | Dec | Jan | Feb | Mar | Apr | Abbreviation |
| Amphora sp. | 1×103 | - | - | 3×103 | - | 1×103 | Amp_sp |
| Cylindrotheca closterium (Ehrenberg) Reiman & J.C. Lewin | 1.2×104 | 1.9×104 | 8×103 | - | - | - | Cylin_Clos |
| Coscinodiscus sp. | 2×103 | 1.6×104 | 6×103 | 4.1×104 | 2.7×103 | 7×103 | Cosc_sp |
| Diploneis sp. | 2.7×104 | 2.6×104 | 5×104 | 6×104 | 0 | 1×103 | Dipl_sp |
| Nitzschia sp. | 1.1×104 | 1.8×104 | 1.9×104 | 5×104 | 6×103 | 4×103 | Nitzs_sp |
| Gyrosigma sp. | 2.3×104 | 3.2×103 | 1.3×104 | - | - | 7×103 | Gyro_sp |
| Pseudo-nitzschia sp. | 6.7×104 | 5×104 | - | 2×103 | - | - | Pseudo_sp |
| Rhizosolenia setigera Brightwell | 5×103 | 3×103 | 3×103 | 1×103 | - | - | Rhizo_seti |
| Thalassionema nitzschioídes (Grunow) Mereschkowsky | 4×103 | 9×103 | - | - | - | - | Thala_nithz |
| Chaetoceros sp. | 8×103 | 4×103 | 1×103 | - | - | - | Chaet_sp |
| Navicula sp. | 1.1×104 | 2×104 | - | 6×103 | 4×103 | 1×103 | Navi_sp |
| Lithodesmium undulatum Ehrenberg | - | - | - | 4×103 | 1×103 | 1.1×104 | Litho_und |
| Skeletonema costatum (Greville) Cleve | - | - | 2×103 | 3×104 | - | - | Skele_cos |
| Tabellaría sp. | - | 1×103 | - | 2×103 | - | 1×103 | Tabe_sp |
| Grammatophora oceanica Ehrenberg | - | - | - | 3×103 | 4×103 | 1×103 | Gram_ocea |
| Actinoptychus senarius (Ehrenberg) Ehrenberg | - | 2×103 | - | - | 6×103 | - | Actino_sen |
| Actinoptychus sp. | 1×103 | - | - | - | - | - | Actino_sp |
| Asterionellopsis gracialis | 1×103 | 1×103 | - | - | - | - | Aster_gra |
| Leptocylindrus danicus Cleve | 2×103 | 3×103 | - | - | - | - | Lepto_dan |
| Entomoneis sp. | 4×103 | 6×103 | - | - | - | - | Ento_sp |
| Licmophora ehrenbergi (Kützing) Grunow | 1×103 | 1×103 | - | - | - | - | Licmo_ehr |
| Melossira moniliformis (O. F. Müller) C. Agardh | 1.2×104 | 7×103 | - | 1.6×104 | - | - | Melo_mon |
| Surirella sp. | 1×103 | 1×103 | - | - | - | - | Suri_sp |
| Thalassiosira sp. | - | 4×103 | - | - | - | 3×103 | Thala_sp |
| Guirnardia striata (Stolterforth) Hasle | 3×103 | 3×103 | - | - | - | - | Guir_stri |
| Biddulphia alternans (Bailey) Van Heurck | - | 1×103 | 5×103 | Biddul_alter | |||
| Bacteriastrum sp. | - | 1×103 | - | - | - | - | Bacter_sp |
| DINOFLAGELLATES | |||||||
| Protoperidinium oceanicum (Vanhöffen) Balech | - | - | 2×103 | 1.6×104 | 5×103 | 1×103 | Prot_ocea |
| Tripos furca (Ehrenberg) F. Gómez | 1×103 | 2×103 | 2×103 | 5.3×104 | 1×103 | 2×103 | Trip_fur |
| Prorocentrum micans Ehrenberg | 3×103 | - | 1×103 | 2×103 | 8×103 | 4×103 | Proro_mic |
| Sccrippsiella sp. | - | - | - | 2×103 | 2×103 | 1×103 | Scrip_sp |
These types of dinoflagellates/diatoms associated with algal blooms have been recorded in different coastal systems, such as: Tripos furca (Ehrenberg) F. Gómez; Prorocentrum mican Ehrenberg; Asterionellopsis gracialis (Castracane) Round; Cylindrotheca closterium (Ehrenberg) Reimann & J.C. Lewin; Skeletonema costatum (Greville) Cleve; Thalassiosira spp. and Chaetoceros spp. (Guerra-Martínez & Lara-Villa, 1996; Orellana-Cepeda et al., 2002; Gárate-Lizarraga et al., 2007; Nuñez-Vázquez et al., 2008; Muciño-Márquez et al., 2011b; Aké-Castillo et al., 2014; Calvo-Vargas et al., 2016).
Phytoplankton samples from the lagoons system of Mandinga, during the study period, consisted mainly of benthic and planktonic diatoms and dinoflagellates taxa. This is consistent with Okolodkov & Blanco-Pérez (2011), who stated that the microalgal flora in lagoon systems of Veracruz are dominated by benthic diatoms in shallow areas and planktonic forms in coastal lagoons having strong influence from the ocean. The lagoons system of Mandinga has a semi-closed and shallow mouth to the ocean, directly influences primary productivity thus, hydrological characteristics are euryhaline, especially during the rainy season (Castán-Aquino, 2013; Gómez-Sánchez, 2013). As reported by Álvarez-Gongóra et al. (2012), who observed a greater variability in total phytoplankton abundance during the rainy season.
The diversity and ecology of phytoplankton along the coastal zone of Veracruz is influenced by higher densities of diatoms and dinoflagellates, although in the present study the concentrations of these microalgae were not greater than 6.7 x104 cells L-1 (Table 4) (Muciño-Márquez et al., 2011b; Rodríguez-Gómez et al., 2015). Their reduced concentration can be explained by the reportedly greater zooplankton abundance which directly influences primary productivity (Orduña-Medrano, 2012). According Contreras & Castañeda (2004), lagoons and estuaries along the Gulf of Mexico are characterized by the dominance of copepods. According to Contreras (2016), the community structure of zooplankton at the mouth of the Jamapa River is made up of eleven families of copepods and two families of cladocerans that dominate in North winds and rainy seasons, respectively.
Canonical Correspondence Analysis
Canonical Correspondence Analysis (CCA) revealed that the abundance and distribution of phytoplankton in the lagoons system of Mandinga accounted for 62.67 % of the variance (p = 0.0001) in physicochemical parameters (Table 5). Salinity (r = -0.553) best defined axis 1 followed by temperature (r = -0.455). These parameters alone explained 44.90 % of the variance. Muciño-Márquez et al. (2011a), also considered salinity as an important factor (r = 0.90) that promoted increases in total phytoplankton abundance and HAB forming species. The parameters defining axis 2 were silicates (r = -0.733) and dissolved oxygen (r = -0.598), and explained 17.76 % of the variation (Figure 6). For Álvarez-Gongóra et al. (2012), the axes that best explained 83 % of the variability were: Axis I, related to dissolved oxygen, silicates and chlorophyll-a and Axis II, to temperature, salinity, nitrates and phosphates.
Table 5 Restricted Inertia Canonical Correspondence Analysis (RICCA).
| Axis 1 | Axis 2 | Accumulated | |
|---|---|---|---|
| Actual Values | 0.426 | 0.168 | 0.594 |
| RICCA | 44.903 | 17.765 | 62.668 |
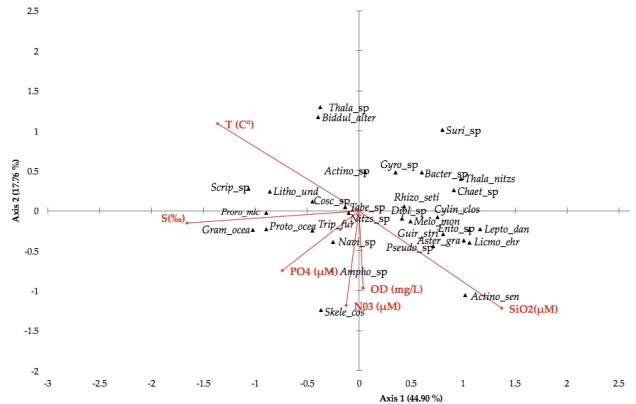
Figure 6 Canonical Correspondence Analysis (CAC): Ordination diagram for microalgae taxa (▲) and physicoche-mical parameters (vectors) in the lagoon system of Mandinga, Veracruz, Mexico.
High salinity and temperature were related to the presence of dinoflagellates and Diatoms, such as: Prorocentrum micans, Protoperidinium oceanicum, Scrippsiella sp., Lithodesmium undulatum and Grammatophora oceanica (Figure 6).
Other microalgae such as Tripos furca, Navicula sp., Amphora sp. and Skeletonema costatum prefer environments having medium to high concentrations of dissolved oxygen, phosphates and nitrates. The diatom taxa Coscinodiscus sp., Tabellaria sp., Nitzschia sp. and Melossira moniliformis was present in 66 % of samples. The diatoms Pseudo-nitzschia sp., Asterionellopsis gracialis, Entomoneis sp., Guirnardia striata, Leptocylindrus danicus and Licmophora ehrenbergi predominantly occurred at medium to high concentrations of silicates and dissolved oxygen, and at lower temperatures. Other taxa such as Diploneis sp., Cylindrotheca closterium, Rhizosolenia setigera, Gyrosigma sp., Chaetocero sp., Thalassionema nitzschioides, Bacteriastrum sp. and Surirella sp. preferred environments with less salinity, dissolved oxygen, and nutrients, and lower temperatures.
According to Ayala (2008) Prorocentrum micans, Protoperidinium sp., occur under conditions of salinity and high temperatures. Álvarez-Gongóra et al. (2012), found that the dinoflagellate Tripos furca is related to the presence of salinity, temperature and nutrients (nitrates and phosphates). García et al. (2015), mention that diatoms, such as Leptocylindrus danicus and Pseudonitzschia seriata complex are favored by having availability of nutrients and low temperatures.
Bucheli (2016) refers that Cylindrotheca closterium is a species that has a greater relation with nutrients. On the other hand (García et al., 2015) related its growth to low temperatures. Unlike Ayala (2008) in a study conducted in two lagoons, different phytoplankton species such as Prorocentrum micans, Protoperidinium sp., Scrippsiella sp., Cylindrotheca closterium, Asterionellopsis gracialis, Rhizosolenia setigera have a successful correlation with values closer to the N:P ratio and high concentrations of oxidized nutrients. While, Lithodesmium undulatum, Protoperidinium sp., Pseudo-nitzschia sp., tends to predominate under conditions of high temperatures and concentrations of silicic acid.
According to Okolodkov (2008), dinoflagellates in the genus Protoperidinium proliferate at high salinities and are represented by 46 species in the Parque Nacional Sistema Veracruzano (PNSV). According Muciño-Márquez et al. (2011a), Prorocentrum spp. is widely distributed along the Gulf Coast of Mexico. In the Sontecomapan Lagoon, Veracruz, five species were identified including P. micans. Muciño-Marquez et al. (2011a), reported that salinity is a determining factor for the abundance and distribution of the genus Prorocentrum, which also was tested in the present study (Figure 6). However, we found no relationship between the presence of dinoflagellates and nutrient concentrations except for Tripos furca. Dortch (1990) states that there is significant variation between species, both in the inhibition and the preference of nutrients such as nitrogen and ammonium without any apparent pattern, which is influenced by environmental conditions. Gárate-Lizárraga et al. (2008), reported a proliferation of Prorocentrum micans during tidal flows in Magdalena Bay. This suggests that Prorocentrum occurs in shallow sampling sites, approximately 5 to 10 m in depth, and within a narrow temperature range. This information coincides with the results in the present study.
Reductions in freshwater input lead to increased salinity, and changes in the structure and composition of phytoplankton assemblages (Ferreira et al., 2005). This could lead to proliferation of opportunistic marine species, which can produce HABs, such as Tripos furca in the lagoons system of Mandinga (Guerra-Martínez & Lara-Villa, 1996). The presence of Tripos furca in the lagoons system of Mandinga was recorded during the windy season (January-February, 2012), and reported as a common species in the Gulf of Mexico and Pacific Ocean (Okolodkov, 2010). This dinoflagellate produces hypoxia and anoxia in these regions, causing death by suffocation of aquatic species (e.g. Puerto Escondido and Baja California in 2002).
Dinoflagellate in the genera Prorocentrum and Ceratium, two dominant HAB species found along the Pacific coast of Guerrero (Gárate-Lizárraga et al., 2008), Prorocentrum produces toxins that, when consumed, cause diarrheal shellfish poisoning (Hernández-Becerril et al., 2007). According to Calvo-Vargas et al. (2016), T. furca is associated with HABs such as P. micans at maximum concentrations of 20x103 cel L-1 during warm months (April), at temperatures near 27 °C, dissolved oxygen levels of 5.8 mg L-1, and salinities of 34 psu. The presence of S. costatum, also associated with HABs has been observed during the rainy season, at maximum abundances of 6.5x106 cel L-1, temperatures near 27 °C, 30 psu salinity and dissolved oxygen concentrations near 7 mg L-1. Such conditions also were recorded in Costa Rica during El Niño events from 2008 to 2010.
According to Wells et al. (2015), temperature is a parameter that affects various metabolic processes of organisms that produce algal blooms. This parameter has a strong influence on the presence and trajectory of the phytoplankton community. However, there is no significant evidence linking temperature changes to toxin production. In this investigation, the highest temperatures were recorded during March and April, identifying the presence of Coscinodiscu sp., Thalassiosira sp., Tripos furca and Prorocentrum micans, phytoplankton reported by other authors as algal blooms. However, no changes in water color or other characteristics indicating algal blooms were observed.
In this study, Pseudo-nitzschia had maximum abundance in November (6.7x104 cel L-1) and December 2011 (5x104 cel L-1) (Table 4). This genus was associated with low temperatures and high concentrations of dissolved oxygen and silicates, even though it has optimum growth at salinities of 32.5 to 34.4 psu and temperatures of 19 to 23 °C (Méndez and Ferrario, 2009). According to Aké-Castillo et al. (2014), the availability of high concentrations of orthophosphates and nitrates, and increases in water temperature are some factors that have triggered HABs in the coastal zone of Veracruz, Mexico. Thus, and in accordance with Rodríguez-Gómez et al. (2015), continuous monitoring of phytoplankton in the coastal zones of Veracruz is necessary for recognition of the diversity, abundance and seasonality of phytoplankton, especially with regard for those species involved in toxic algal blooms. This resistance to environmental variability may be influenced by the presence of non-endemic species that have been introduced into coastal areas by the ballast water of ships that land in the state of Veracruz (Okolodkov et al., 2007; Aké-Castillo et al., 2014). Water and sediments may contain dinoflagellate cysts capable of resisting for a long time (Meave Del Castillo, 2014). The strategic plan for biological diversity carried out by different foreign government agencies puts pressure on the Mexican legal framework, which requires decision makers to act more effectively in aspects related to invasive species already introduced and potential bio invasions through maritime transport (Okolodkov & García-Escobar, 2014).
According to Okolodkov et al. (2007), it is essential to develop legislation for emptying ballast water in Veracruz marine waters from ships and other vessels. In addition to strict regulatory oversight of the maximum permissible limits on discharges of wastewater into natural bodies of water. The construction of a drainage system in the areas surrounding coastal systems, could reduce the availability of nutrients and the formation of algal blooms.
Conclusions
Coastal lagoons, in the state of Veracruz, are a fundamental part of aquatic activities, since they are carried out in open or closed systems, as well as in cages or ponds to produce marine and freshwater organisms. The importance of phytoplankton species, in the Mandinga lagoon system, lies in its resistance to changes in environmental conditions and that tolerates high salinities and temperatures. These parameters influence the abundance and diversity of diatoms and dinoflagellates present in the study area.
Although low phytoplankton densities were obtained in the present study, species associated with algal blooms were identified, posing a risk to the ecosystem and public health. Phytoplankton in the lagoon system of Mandinga consists mainly of benthic and planktonic diatoms and dinoflagellates, and its abundance is associated with the presence of nutrients, mainly nitrates and silicates. The Mandinga lagoon system is cataloged by the Federal Commission for the Detection of Sanitary Risks (COFEPRIS), as a priority area for the certification of areas of oyster extraction destined for human consumption, in compliance with the sanitary specifications of the NOM-242 -SSA1-2009. It is because of this, that collaboration with public and private associations and institutions is required in order to identify the causes of HABs. In addition, it is necessary to plan and implement new strategies for the prevention and control of these phenomena, in order to reduce damage to aquatic systems and public health by the consumption of contaminated bivalve mollusk.











 texto en
texto en