Introduction
In soursop (Annona muricata L.), anthracnose is mainly attributed to the genus Colletotrichum and is considered the most economically important disease, because it causes necrosis and loss of inflorescences and fruits throughout the year. The highest incidence of this disease occurs during the periods of precipitation with high relative humidity (90 %), it also attacks leaves, buds and young branches (Rondón, 1999; Villanueva et al., 2006; Andrades et al., 2009; Hernández et al., 2014a). The affected inflorescence petals show small brown-black color, which are enlarged and sink, causing the premature fall of petals and even the total loss of the floral structure (Álvarez et al., 2002; Hernández, 2013; Hernández et al., 2014b). Colletotrichum theobromicola Delacr (sin. C. fragariae) belongs to the Glomerellaceae family of the Glomerellales order and the class Sordariomycetes, this species is widely distributed in tropical and subtropical regions, in a wide range of hosts such as strawberry (Fragaria × ananassa), cocoa (Theobroma cacao) and Annona (Annona diversifolia) (Rojas et al., 2010; Weir et al., 2012). Losses greater than 90 % in non-technified orchards have been recorded in Colombia (Álvarez et al., 2002). Hernández et al. (2014b) reported a 75.92 % anthracnose in soursop caused by Colletotrichum in Veracruz, Mexico. The state of Nayarit is the main soursop producer in Mexico, with a production of 21,810.86 t, corresponding to 75.6 % of the total production (SIAP, 2017). The anthracnose caused by C. gloeosporioides can diminish up to 90 % of the soursop yield (Pinto et al., 2005). Therefore, the damages caused by this phytopathogenic fungus reaffirm the need to develop and evaluate new, efficient and environmentally friendly fungicides (Juárez et al., 2010). In addition, the use of broadspectrum chemical fungicides is currently being regulated for inducing resistance in target organisms and due to the adverse effects on the environment and human health (Astúa et al., 1994). The use of antagonistic microorganisms as biological control agents is one of the alternatives that have demonstrated potential in the control of pathogenic diseases (Carrillo et al., 2005), mainly in strains of the genera Bacillus, Trichoderma and Streptomyces, showing antimicrobial potential against the phytopathogens such as Moniliophthora, Colletotrichum, Phytophthora, Rhizopus, Alternaria, Stemphylium, Fusarium, Lasiodiplodia, Botrytis, Penicillium, Aspergillus, Phytophthora, Rhizoctonia, Pseudomonas, Pectobacterium and Bacillus (Chavarría et al., 2005; Rodríguez & Veneros 2011; Rincón et al., 2014; Pérez-León et al., 2015; Rios-Velasco et al., 2016; Tenoriod & Mollinedo, 2016; Evangelista et al., 2017). The use of formulations based on synthetic molecules and vegetable extracts could represent another alternative for soursop anthracnose management. In fruit-trees and vegetables such as mango (Mangifera indica L.), papaya (Carica papaya L.), Andean raspberry (Rubus glaucus Benth.) and chili (Capsicum annuum L.) some of these formulations have been analyzed in the control of anthracnose, giving satisfactory results, especially with the application of difenoconazole, copper hydroxide, mancozeb, azoxystrobin, prochloraz and lavender extract (Lavandula stoechas Lam.) (Zavala et al., 2005; Arias & Carrizales, 2007; Santamaría et al., 2011; Gaviria et al., 2013; Pérez-León et al., 2015; Linu & Jisha, 2017; Villacís et al., 2017). Based on the above mentioned, the objective of the study was to evaluate the antifungal effect of six antagonistic microorganisms and seven commercial fungicides on the in vitro growth of C. theobromicola, the causal agent of anthracnose in soursop inflorescences in Nayarit, Mexico.
Material and Methods
In vitro antagonism
We evaluated the in vitro antagonism of T. asperellum (Ta), T. harzianum (Th), T. longibrachiatum (Tl), and the actinobacteria Streptomyces sp. (A7), S. tubercidicus (A19) and S. viridochromogenes (A27) against C. theobromicola isolated from soursop inflorescences of Compostela and San Blas, Nayarit, Mexico. The identification and pathogenicity of this fungus were previously confirmed. The strain was placed in the microbial strain collection of Laboratory of Agricultural Parasitology (Cemic 03) of the Universidad Autónoma de Nayarit. Antagonists were provided by the Laboratory of Plant Pathology and Biological Control of the Research Center for Food and Development, A.C., ChihuahuaCampus Cuauhtémoc. Confrontations in Petri dishes (90 × 15 mm) containing the potato-dextrose-agar culture medium (PDA, DIBICO®) were carried out. The antifungal effect of the antagonist vs C. theobromicola was determined by direct confrontation method. Streptomyces spp. strains were grown in PDA culture medium for 10 d by placing 7 mm diameter culture discs in the four cardinal points of PDA Petri dishes. Thereafter, 7 mm culture discs of 7-day-old cultures of C. theobromicola (mycelium and conidia without quantifying) were placed in the center of Petri dishes. Trichoderma strains were confronted by dual cultures, that consisted in placing the antagonist in one side of the dish and the pathogen in the opposite side of the Petri dish, both were planted on the same day. Experiments were performed in triplicate, each replicate consisted of three Petri dishes, and nine PDA Petri dishes only inoculated with C. theobromicola were used as a positive control (Ruiz-Cisneros et al., 2017). Petri dishes were incubated at 28 °C in a Novatech® incubator (model EI60-AIA) in the absence of light and the radial growth of the confronted microorganisms was systematically measured every 24 h during 3 and 4 d for Trichoderma spp. strains and 12 d for Streptomyces spp. strains. The in vitro antagonism was evaluated estimating the percentage of radial growth inhibition of the pathogen (PICR) and calculated by the following formula PICR = (R1 - R2) / R1 × 100, where R1 was the radial growth of the control colony (pathogen) and R2 was the radial growth of the pathogen colony in the in vitro confrontation. The antagonism level of Trichoderma was classified using the Bell et al. (1982), which consists of five types: (1) The antagonist has completely overgrown the pathogens and fills the culture surface, entirely covering the pathogen; (2) the antagonist exceeds two-thirds of the culture surface; (3) the antagonist and the pathogen colonize approximately half of the culture surface and none dominates the other; (4) the pathogen overgrew the antagonist, colonizing threequarters of the culture surface; (5) the phytopathogenic completely overgrew the antagonist and filled the culture surface. The antagonism level of Streptomyces strains was determined according to the six-level scale proposed by Pérez-Corral et al. (2015). (1) No growth of the fungus (0 %); (2) radial growth (1 % to 25 %); (3) radial growth (26 % to 50 %); (4) radial growth greater than 50 %; without overgrowing the actinomycete; (5) presence of halo; and 6) the actinomycete has completely overgrown (100 %) by the fungus (without antagonism).
In vitro evaluation of fungicides
We evaluated the fungicide sensitivity of C. theobromicola to seven commercial fungicides: Maxtrobyn® (azoxystrobin SC), ExCitrus® (Citric extract + quercetin), Canelys® (Cinnamomum zeylanicum extract), Hidrocu® PH (Copper hydroxide), Pull75WG® (cuprous oxide), SCORE® 250 EC (Difenoconazole) and SWITCH® (cyprodinil + fludioxonil) 62.5 WG (Table 1). The doses were provided according to the manufacturer’s recommendations, half of the recommended dose [low dose (D1)], recommended dose [medium dose (D2)] and recommended dose plus half of this [high dose (D3)]. Five replicates (Petri dishes) for each dose with five controls for each treatment were carried out in this experiment. Each dose of the fungicide was mixed with PDA at 48 °C, stirred to homogenize the mixture and then poured into the Petri dishes (90 × 15 mm). Subsequently, disks (0.7 mm explants) of 7-day-old cultures of C. theobromicola were placed in the center of the Petri dishes. The control treatment was considered the PDA culture inoculated with C. theobromicola without the fungicides. Radial growth of the fungus was measured every 24 h for 12 d, time when the controls completely filled the Petri dishes (Gaviria et al., 2013). Based on these measurements, the PICR of C. theobromicola was calculated with the formula previously mentioned.
Table 1 Fungicides and doses evaluated in vitro against C. theobromicola.
| Treatment | Fungicide | Doses (mL o g/L-1) | |||
|---|---|---|---|---|---|
| Trade name | Active ingredient | D1 | D2 | D3 | |
| T1 | Maxtrobyn® | Azoxystrobin SC | 0.44 mL | 0.88 mL | 1.32 mL |
| T2 | ExCitrus® | Citrus extract + quercetin | 2.5 mL | 5 mL | 7.5 mL |
| T3 | Canelys® | Cinnamomum zeylanicum extract | 2.5 mL | 5 mL | 7.5 mL |
| T4 | Hidrocu® PH | Copper hydroxide | 2.5 g | 5 g | 7.5 g |
| T5 | Pull75WG® | Cuprous oxide | 2.5 g | 5 g | 7.5 g |
| T6 | SCORE® 250 EC | Difenoconazole | 1.5 g | 3 g | 4.5 g |
| T7 | SWITCH® | Cyprodinil + fludioxonil | 3 g | 6 g | 9 g |
Experimental design and statistical analysis
A completely randomized design was used to examine the variables. Analysis of variance and comparison by of means of the Tukey test (α = 0.05) was carried out to PICR data using Statistical Analysis System program (Sas Institute Inc., 2009).
Results and Discussion
In vitro antagonism
The six antagonists of Streptomyces and Trichoderma showed high PICR on C. theobromicola, the PICR showed significant differences in the Tukey test (α = 0.05) (Figure 1), highlighting the Streptomyces strains, which inhibited the pathogen growth between 90 and up to 100 %: Strain A27 completely inhibited the pathogen (100 %), followed by strains A19 and A7 with 96.18 and 90.4 %, respectively. The three strains A27, A19 and A7 showed inhibition halos of 26.35, 26.03 and 24.2 mm, respectively.
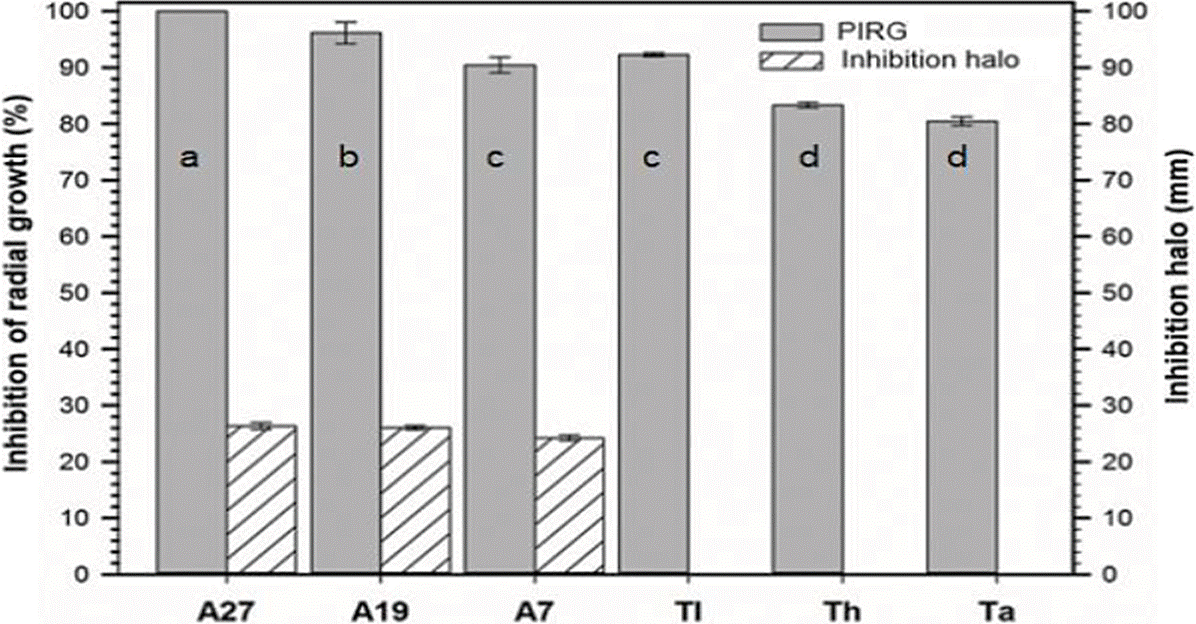
Figure 1 PIRG and inhibition halo of antagonists vs C. theobromicola. Streptomyces sp. (A7), streptomyces tubercidicus (A19), Streptomyces viridochromogenes (A27), Trichoderma asperellum (Ta), Trichoderma harzianum (Th) and Trichoderma longibrachiatum (Tl). Bars with the same letters are not statistically different (Tukey, 0.05).
The antagonism was of type 1 for A27 where the fungus growth was zero (0 %) and type 2 for A7 and A19 where the radial growth was from 1 to 25 %. These results differ with those obtained by Dávila et al. (2013), those authors evaluated in vitro isolates of Streptomyces sp. against phytopathogenic fungi such as Alternaria sp., Rhizoctonia sp., Fusarium sp. and Colletotrichum sp., reporting inhibitions percentages lower than 62 %, where the highest inhibition percentage was recorded against Colletotrichum sp. The antifungal capacity of the evaluated Streptomyces strains could be due to the production of some fungistatic compound (De Lima et al., 2012; Atta, 2015; Pathalam et al., 2017). In this regard, Srividya et al. (2012) mentioned that one of the biocontrol mechanisms of Streptomyces is through the production of mycolic enzymes, mainly chitinase and β-1, 3 glucanase as well as cellulase, lipase and protease. In a study performed by Savarana et al. (2014) found that the active principle of the strain SCA 7 of Streptomyces sp. was the compound 2,4-bis (1,1-dimethylethyl) phenol. In the same way, Shaik et al. (2017) found three Streptomyces isolates with an antifungal activity which produced the bioactive metabolites alpha-amylase, lipase, protease and cellulase. Atta (2015) mentioned that S. torulosus produces tunicamycin, a broad-spectrum antibiotic against Gram-positive and Gram-negative bacteria, and unicellular and filamentous fungi. The three strains of Trichoderma also showed in vitro antifungal potential by inhibiting C. theobromicola up to 92.27 %, T. longibrachiatum was the most effective strain, followed by T. harzianum and T. asperellum with inhibitions of 83.33 and 80.48 %, respectively. The three strains of Trichoderma showed antagonism type 1 for T. asperellum and T. harzianum (i.e the antagonist has overgrown completely the pathogens and fills the culture surface covering completely the pathogen) and type 2 for T. longibrachiatum (where the antagonist exceeded two-thirds of the culture surface). Trichoderma has been proposed as a potential agent of biological control against Colletotrichum spp., due to its rapid growth and the activity of its lytic enzymes such as chitinases, endoglucanases and exoglucanases (Hoyos et al., 2008; Rodríguez & Veneros 2011; Sanmartín et al., 2012; Vargas et al., 2012). Peláez et al. (2016) reported a 91 % in vitro growth inhibition of C. gloeosporioides by T. asperellum, and also observed an overgrowth in the phytopathogen. In addition, the same authors found a synergistic effect in the integrated use of T. asperellum and a low dose of the fungicide captan 50® (0.1 g L-1) which led to a 99 % in vitro growth inhibition of C. gloeosporioides. The antifungal potential (in vitro inhibition > 80 %) of our Trichoderma isolates, surpass up to four times the values reported by De la Cruz et al. (2018), who found in vitro inhibitions percentages of 9.5, 22.5 and 21.9 % by strains of T. harzianum, T. asperellum and T. longibrachiatum, respectively. Ghosh & Chakraborty, (2012) demonstrated the in vitro potential of T. viride strains as a biological control agent of C. gloeosporioides, the authors found that the spores of Trichoderma adhere to the cell wall of C. gloeosporioides and contract them. In this regard, Vargas et al. (2012) evaluated the in vitro antagonistic activity of T. longibrachiatum, T. asperellum and T. harzianum strains against Colletotrichum sp., showing an inhibition percentage higher than 70 % in the 33.3 % of their strains, highlighting the T. asperellum strains. On the other hand, Sanmartín et al. (2012) found in vitro inhibitions from 55.5 to 66.8 % against C. gloeosporioides using T. asperellum strains, this antagonistic activity was correlated with the production of chitinolytic and cellulolytic enzymes. In a study performed by Rodríguez and Veneros (2011), demonstrated that the hyphae of T. harzianum covered and degraded (mycoparasitism) the hyphae of the pathogens R. nigricans, C. gloeosporioides, A. alternata, S. lycopersici, F. oxysporum and L. thebromae.
In vitro evaluation of fungicides
The susceptibility of C. theobromicola to the seven fungicides was variable, since Canelys®, Hidrocu® PH and SCORE® 250 EC inhibited 100 % of the fungus growth with its three doses; Pull75WG® inhibited 100 % with D2 and D3; Switch® 62.5 WG from 93.1 to 98.67 %; Excitri® of 47, 77 and 98 % with doses D1, D2 and D3, respectively; Maxtrobyn® showed the lowest inhibition values (68.44, 71.60 and 65.05 %) with doses D1, D2 and D3, respectively. The three doses of Canelys®, Hidrocu® PH, SCORE® 250 EC and Pull75WG®, as well as the high doses of Switch® 62.5 WG and Excitri®, did not show significant differences according to the Tukey test (α = 0.05) (Figure 2). Based on the results exhibited here, we can assume that 5 fungicides showed a high potential of in vitro control against C. theobromicola (Canelys®, Hidrocu® PH, SCORE® 250 EC, Pull75WG® and Switch®). However, considering that soursop is an emergent crop where the management of diseases at low cost is sought, the fungicides Canelys® and Hidrocu® PH, could be a viable alternative for the control of anthracnose in field conditions, because they are considered low cost and environmentally friendly.
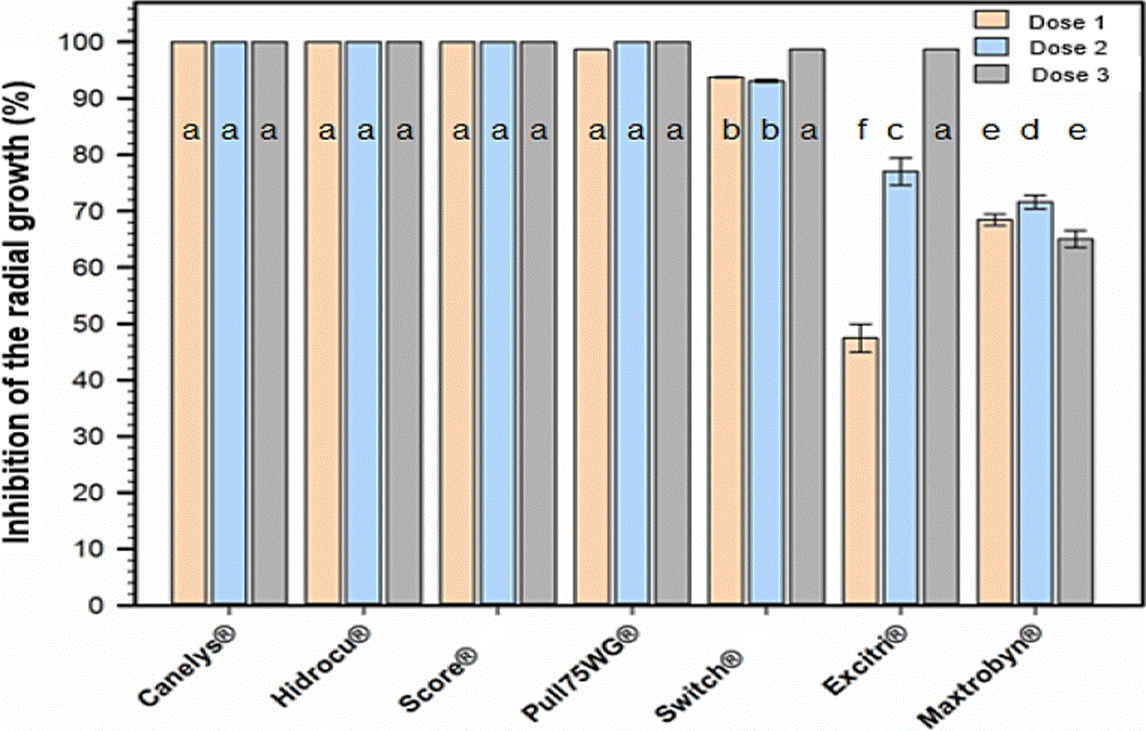
Figure 2 In vitro inhibition of the radial growth of C. theobromicola by commercial fungi cides. Bars with the same letters are not statistically different (Tukey, 0.05).
Gaviria et al. (2013) obtained similar results using Score 250® EC (difenoconazole 100 ppm) and Kocide® 101 (copper hydroxide 2460 ppm) with 100 % in vitro inhibition of C. gloeosporioides and C. acutatum. While the values obtained with Amistar® 50 WG (azoxystrobin 63 ppm) was not different since it ranges from 38.75 to 70 %. About that, Linu & Jisha, (2017) evaluated the in vitro effect of three fungicides (carbendazim 0.05 %, mancozeb 0.2 % and azoxystrobin 0.1 %) against C. capsici, obtaining inhibition percentages of 64.12, 73.47 and 62.21 %, respectively, where the inhibition of azoxystrobin was similar to the obtained in this investigation. Zavala et al. (2005), analyzed the in vitro effect of azoxystrobin against C. gloeosporioides, reporting a lower inhibition (59.8 %) than the found in our study, whereas when evaluated in vivo effect on the control of C. gloeosporioides the anthracnose severity in papaya was only reduced by 20.35 %. On the other hand, in vivo studies of some of these fungicides such as azoxystrobin mixed with cyproconazole at a dose of 50 g/100 L-1 have demonstrated that the incidence (below 25 %) of anthracnose caused by C. gloeosporioides in post-harvest mango fruits has been reduced (Arias & Carrizales, 2007). In another study conducted by Pérez-León et al. (2015) applied azoxystrobin in Sansevieria trifasciata var. Hahnii leaves, reporting a 100 % protection of anthracnose caused by C. sansevieria. In papaya maradol fruits, azoxystrobin showed an 87.5 % effectiveness in reducing the severity of C. gloeosporioides (Santamaría et al., 2011).
Conclusions
Both groups of antagonists showed in vitro biocontrol potential and therefore could be potential candidates to be evaluated in the field in order to control soursop inflorescence anthracnose. Strain A27 of Streptomyces completely inhibited the in vitro growth of C. theobromicola. The strains of Trichoderma inhibited C. theobromicola in values higher than 80.48 %.
Canelys®, Hidrocu® PH and SCORE® 250 EC fungicides were the most efficient for completely inhibiting (100 %) the in vitro growth of C. theobromicola. ExCitri® and Maxtrobyn® were the least effective, however, they showed percentages of inhibition higher than 47.43 %. Based on these results, the fungicides Canelys®, Hidrocu® PH, SCORE® 250 EC, Pull75WG® and Switch® 62.5 WG could be a potential alternative for the chemical control of anthracnose in the field as part of an integrated management scheme.











 texto en
texto en 


