Introduction
Jatropha curcas L. is a plant that belongs to the family Euphorbiaceae; Mexico is the center of origin and domestication (Dias, Missio, & Dias, 2012). J. curcas plantations are distributed in tropical regions of America, Africa and Asia. In recent years, the species has become more important because of its potential for producing biodiesel (Basili & Fontini, 2012); reforestation of less fertile tropical soils (Fresnedo-Ramírez & Orozco-Ramírez, 2013); establishment of extensive plantations in warm coastal, dry and marginal areas, to capture atmospheric CO2 and climate change mitigation (Becker, Wulfmeyer, Berger, Gebel, & Münch, 2013); and recovery of degraded soils (Van Rooijen, 2014; Wani et al., 2012).
Terren et al. (2012) evidenced that roots, stems, branches, leaves, flowers, fruits and seeds of J. curcas are affected by insects and fungi that cause phytosanitary problems. Several species of insects belonging to the order Hemiptera cause damages in J. curcas. In particular, L. zonatus (Dallas) is one of the most damaging insects because it causes fruit abortion; reduces the size, weight and oil content in seeds; and leads to the formation of empty grains (Grimm & Somarriba, 1999, Terren et al., 2012). L. zonatus have been found in plantations the state of Morelos as a damage potential agent for the cultivation of J. curcas, because besides of causing damaging effects to the fruits, these insects also generate wounds that facilitate the entry of pathogenic fungi (Tepole-García, Pineda-Guillermo, Martínez-Herrera, & Castrejón-Gómez, 2012). These fungi alter the viability and quality of J. curcas seeds, specifically, F. verticillioides (Saccardo) Nirenberg affects J. curcas seed decreasing the amount of lipids, free fatty acid content and seeds viability (Dharmaputra, Worang, Syarief, & Tahudin, 2009), so it is necessary to address phytosanitary affectations taking into account the importance of the crop. An ecology alternative is the use of antagonistic bacteria from the rhizosphere with potential for biological control of pests and diseases.
Rhizospheric bacteria produce protein crystals that affect different insects and metabolites, lytic enzymes that inhibit the development of pathogenic fungi. However, the rhizosphere of J. curcas has been little studied, because there are few studies that address the use of antagonists bacteria obtained for controlling phytopathogenic fungi (Latha et al., 2011) and there are no previous reports on the use against crop insect pests. In this context, the aim of this work was to evaluate the antagonistic and entomopathogenic activity of rhizospheric bacteria of J. curcas against F. verticilloides and L. zonatus.
Materials and methods
Biological material
Microbial strains: The strains used in this study belong to the Laboratory of Phytopathology at Centro de Desarrollo de Productos Bióticos del Instituto Politécnico Nacional (CEPROBI-IPN). B. subtilis (Ehrenberg) Cohn, B. mojavensis Roberts, Nakamura & Cohan, B. thuringiensis Berliner and L. sphaericus comb. nov. were isolated from rhizospheric soil of J. curcas, and F. verticillioides, from crop seeds. The bacteria were cultured on nutrient agar and stored at 4 °C and the fungus on potato dextrose agar at 28 °C.
Insects: The nymphs of L. zonatus were obtained from a colony maintained at 25 °C with 60 % relative humidity and photoperiod of 12:12 at the Laboratory of Chemical Ecology at CEPROBI-IPN. Parents were collected in 2012 in a sorghum crop located in Diego Ruíz, Yautepec, Morelos (18° 49’ 22.85” N and 99° 06’ 13.68” W) and an experimental plantation of J. curcas located at CEPROBI-IPN. The insects were identified based on McPherson, Packauskas, Taylor, and O´Brien (1990) keys.
Establishment the breeding of L. zonatus
Nymphs and adults of L. zonatus were placed in acrylic cages (27 x 23 cm) and took to the Laboratory of Chemical Ecology at CEPROBI-IPN. Nymphs and adults were fed with young pods of green beans and maize, and received water with honey at 10% using Eppendorf perforated tubes (1.5 mL) at the base; food was changed every third day. Post mating, insects were isolated to obtain the eggs, which were counted and separated until the emergence of nymphs. These were selected immature in the fourth and fifth nymphal stage for subsequent bioassays.
Bacterial growth kinetics
The bacterial inoculum (B. subtilis, B. mojavensis, B. thuringiensis and L. sphaericus) was obtained by a loopful of the strain that was placed in 50 mL of nutrient broth and incubated under agitation (150 rpm) at 28 °C for 12 h.
Kinetics in nutrient broth medium: An aliquot of 0.5 mL of inoculum of each bacterial strain was added to flasks containing 50 mL of nutrient broth. Cultures were incubated under stirring (150 rpm) at 28 °C for 36 h. Every 4 h samples of 1 mL of the bacterial culture were taken and the absorbance was measured at an optical density of 590 nm using a spectrophotometer (Shimadzu UV-160, Japan). With the data obtained growth kinetics graphs were made using the SigmaPlot version 11.0 (Systat Software Inc., 2008).
Kinetics in nutrient broth medium supplemented with yeast extract and salts (SNB). B. thuringiensis and L. sphaericus were grown in SNB (nutrient broth plus 0.05 % yeast extract and salts solution) (Dussán, An-drade, Lush, & Vanegas, 2002). Samples were taken and processed as explained above to determine the growth phases.
Bioassays of in vitro antagonism
Antagonist activity of rhizospheric bacteria by dual culture: Antagonism tests were performed using the dual culture method in Petri dishes with nutrient agar. The rhizospheric bacteria B. subtilis, B. mojavensis, B. thuringiensis and L. sphaericus, and the phytopathogenic fungus F. verticillioides were used. A loopful of bacterial culture (24 h) was inoculated into four equidistant points (1 cm2) of the Petri dish and mycelial disc (5 mm) of F. verticillioides was placed in the center. The control consisted only of a disc of 5 mm of the fungus in nutrient agar medium. The treatments were incubated at 28 °C until the mycelium of F. verticillioides (control) completely filled the Petri dish. Then, the diameter of mycelial growth was measured with a digital vernier for calculating the percent inhibition using the equation of Guo et al. (2006):
Mycelial inhibition (%) = [1 - (Da /Db)] * 100 where:
Da = Diameter of mycelial growth zone of treatments (mm)
Db = Diameter of mycelial growth zone of the control (mm)
Antagonistic activity of rhizospheric bacteria grown in nutrient broth: From the kinetics of bacterial growth developed in nutrient broth, 5 µL from each bacterial strain grown for 12 and 24 h was taken and inoculated using the dual culture technique in four equidistant points of the Petri dishes with nutrient agar. Cultures were incubated at 28 °C until the mycelium of the control filled the Petri dish. Mycelial growth data and percent of inhibition were obtained as mentioned in the previous point.
Effect of rhizospheric bacteria in the hyphal morphology of F. verticillioides
A disc of 5 mm from the mycelial growth zone (F. verticillioides) inhibited by B. subtilis, B. mojavensis, B. thuringiensis and L. sphaericus was cut and placed on a microscope slide. Antagonism of rhizobacteria in dual culture in Petri dishes with nutrient agar was determined by blue staining cotton (0.5 g·100 mL-1 of lactophenol). The mycelium of the control treatment was processed in the same way. Samples were coverslipped and viewed under a light microscope (Nikon Eclipse 80i, Japan) with 20X and 40X magnifications. The morphology of the fungal mycelium was described based on that observed.
Mortality and weight reduction of L. zonatus insect by the effect of B. thuringiensis and L. sphaericus rhizobacteria
Before bioassays, immature nymphs of fourth stage were individually placed in plastic cups (No. 4) without food for 4 h. Then, an Eppendorf tube perforated at the base was placed in each cup for feeding nymphs. A total of 1mL of the appropriate bacteria (B. thuringiensis or L. sphaericus), grown in SNB medium for 16 h, was placed in the tube; in addition, two tender corn kernels that were changed every other day were placed. A total of 1 mL of nutrient broth without bacteria was added with the control nymphs. Each treatment was integrated with 30 nymphs. It is important to mention that B. thuringiensis and L. sphaericus bacteria were evaluated based on the insecticidal activity demonstrated in previous studies (Baum et al., 2012; Berry et al., 2012). The mortality assessment was performed every 24 h for five days after the start of the experiment. Preliminary observations allowed determining that the duration of the fourth nymphal stage was five days. Dead insects were removed at each observation and mortality was calculated at end of the experiment according to the formula of Abbott (1925):
Mortality (%) = 100 [dead insects treated (%) - control dead insects (%)] / [100 - control dead insects (%)]
Moreover, weight data of fourth and fifth nymphal stage of L. zonatus were collected; in addition, it was observed whether any morphological change or delay occurred in the life cycle.
Data analysis
Experiments were performed in triplicate using a complete randomized design with simple arrangement. Data from in vitro antagonism and nymphal weight were processed by one-way analysis of variance and means were compared by the method of Holm-Sidak (P = 0.05). The effect of B. thuringiensis and L. sphaericus on mortality of L. zonatus was analyzed using the Chi-square test (Ӽ2) using the SigmaPlot version 11.0 statistical software (Systat Software Inc., 2008).
Results and discussion
Bacterial growth kinetics in two culture media
Growth kinetics of B. subtilis, B. mojavensis, B. thuringiensis and L. sphaericus rhizobacteria in nutritive broth and SNB are shown in Figure 1. All bacteria had the four phases of typical bacterial growth in a maximum of 36 h adaptation (0-6 h), exponential (6-12 h), stationary (12-24 h) and decline (from 24 h onwards). The phases were reflected equally in nutrient broth medium and SNB. The production of metabolites related and not related to bacterial growth plays a critical role in the antagonistic activity. Moreover, the use of a supplemented medium could facilitate the formation of toxins with entomopathogenic activity (Dussán et al., 2002). By means of staining using crystal violet we observed a large amount of protein crystals in B. thuringiensis and L. sphaericus strains. These results agree with those reported by Poopathi et al. (2014), who found entomopathogenic activity of Bacillus cereus Frankland & Frankland against mosquitoes with high protein production from 12 hours of culture in Luria Bertani medium.
In vitro antagonist activity of rhizobacteria on F. verticillioides
The antagonist activity of B. subtilis, B. mojavensis, B. thuringiensis and L. sphaericus to mycelial growth of F. verticillioides is reported in Table 1. All rhizobacteria inhibited the mycelial growth of F. verticillioides in nutrient agar medium regardless of the method of cultivation used for multiplication and antagonists metabolites released in liquid medium for 12 and 24 h.
Table 1 In vitro antifungal effect of Jatropha curcas rhizospheric bacteria on mycelial growth of Fusarium verticillioides at 28 °C.
| Treatments | Nutrient agar (7 days) | Nutrient broth (12 h) | Nutrient broth (24 h) | ||||
|---|---|---|---|---|---|---|---|
| MG (mm) | IMG (%) | MG (mm) | IMG (%) | MG (mm) | IMG (%) | ||
| Control | 85.31 ± 0.54 a | 0 | 85.43 ± 0.74 a | 0 | 85.43 ± 0.74 a | 0 | |
| Bacillus subtilis | 43.04 ± 3.46 c | 50.0 | 52.57 ± 2.46 c | 38.5 | 47.68 ± 3.57 c | 44.2 | |
| Bacillus mojavensis | 39.89 ± 1.74 c | 54.0 | 50.92 ± 2.48 c | 40.4 | 48.78 ± 4.58 c | 43.0 | |
| Bacillus thuringiensis | 63.98 ± 3.71 b | 26.0 | 62.03 ± 7.04 b | 27.4 | 61.18 ± 3.15 b | 28.4 | |
| Lysinibacillus sphaericus | 38.79 ± 4.68 c | 55.0 | 51.67 ± 4.11 c | 39.6 | 50.08 ± 2.90 c | 41.4 | |
MG: Mycelial growth. IMG: Inhibition of mycelial growth. One-way ANOVA. Same letters in the same column indicate no statistically significant differences according to the method Holm-Sidak (P = 0.05).
The highest percentages of inhibition were caused by B. subtilis (38.5 to 50 %), B. mojavensis (40.4 to 54 %) and L. sphaericus (39.6 to 55 %); statistically significant differences (P = 0.05) were observed between the mycelial growth of the control and that obtained with rhizospheric bacteria.
Colonial morphology of F. verticillioides is shown in Figure 2. The control had radially whitish mycelial growth, cottony texture and regular edges (Figure 2A). The presence of B. subtilis caused that F. verticillioides had square shaped delayed mycelial growth (Figures 2B, 2C and 2D). Latha et al. (2011) isolated B. subtilis from the rhizosphere of J. curcas and showed that the bacteria inhibited the phytopathogenic fungus Lasiodiplodia theobromae (Patouillard) Griffon & Maublanc under field conditions.
In this study, treatments with B. mojavensis caused major morphological damages in the mycelium of F. verticillioides; additionally, the bacteria induced fungal colonies with white and cottony central part (Figure 2E). Other morphological changes are highlighted in the development of irregular square fungal colonies (Figure 2F, 2G). It has been reported that B. mojavensis produced proteases, siderophores and volatile compounds inhibiting the mycelial growth of F. verticillioides (Bacon & Hinton, 2002; Bacon, Hinton, Mitchell, Snook, & Olubajo, 2012). The rhizobacteria B. mojavensis has a complex antagonist mechanism not yet fully known, where in addition to producing the above compounds also relates the production of surfactins with antifungal activity (Bacon et al., 2012). Bacillus thuringiensis treatments induced minor changes in color and appearance of F. verticillioides mycelium, but the mycelial growth of the fungus had also a square shape (Figures 2H, 2I and 2J). Recently, Rocha et al. (2014) demonstrated in in vitro tests that B. thuringiensis suppresses growth and inhibits the production of fumonisin of F. verticillioides, which concluded that the bacteria in addition to its ability to control insect pests, has potential as a biological control agent of phytopathogenic fungi. Moreover, L. sphaericus showed rapid growth and high inhibitory activity when grown dually with F. verticillioides (Figure 2K). A recent study showed a high activity of Lysinibacillus sp. against various bacteria and fungi (Ahmad, Iqbal, Haseeb, & Khan, 2014); antimicrobial activity was attributed to bacteriocins, suggesting that this bacteria is an alternative to control microbial deterioration of foodstuffs.
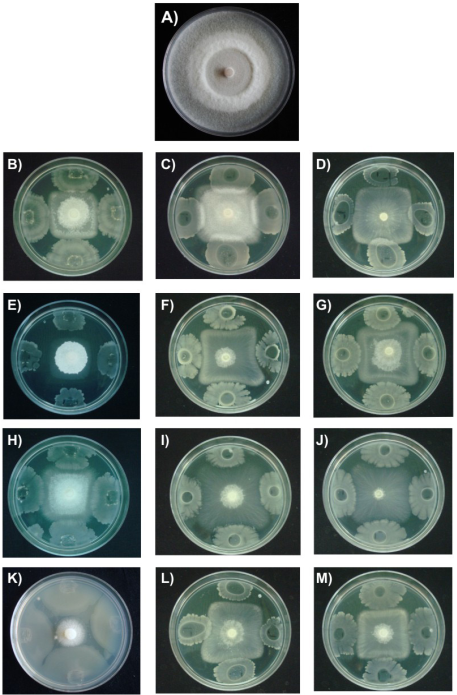
Figure 2 Effect of rhizospheric bacteria in the mycelial growth of Fusarium verticillioides on nutrient agar (NA) at 28 °C for seven days and nutrient broth (NB) for 12 and 24 h. A) Control, B) B. subtilis (NA); C) B. subtilis (NB 12 h); D) B. subtilis (NB, 24 h); E) B. mojavensis (NA); F) B. mojavensis (NB, 12 h); G) B. mojavensis (NB, 24 h); H) B. thuringiensis (NA); I) B. thuringiensis (NB, 12 h); J) B. thuringiensis (NB, 24 h); K) L. sphaericus (NA); L) L. sphaericus (NB 12 h); M) L. sphaericus (24 h).
In general, rhizobacteria are very diverse and may have potential as biocontrol agents based on their antagonistic activity, highlighting the species of the genera Bacillus and Pseudomonas, which can inhibit the growth of phytopathogens such as Fusarium oxysporum Schlechtendal, Rhizoctonia solani Kuhn, Pythium spp. (Beneduzi, Ambrosini, & Passaglia, 2012). In particular, in the rhizosphere of J. curcas bacteria have been isolated from the genera Enterobacter, Bacillus and Pseudomonas with the aim of promoting plant growth, but without evaluating the antifungal activity of these rhizobacteria (Jha, Patel, & Saraf, 2012; Patel & Saraf, 2013); therefore, the results obtained in this study are of interest for the management of fungal diseases of a crop.
Rhizobacteria effect on the morphology of hyphae of F. verticillioides
Figure 3 shows the hyphae of F. verticillioides treated with different rhizobacteria. At 7 and 14 days, the control presented rectilinear septate hyphae with uniform thickness and homogeneous cell content (Figures 3A and 3F). The rhizobacteria caused damages on hyphal morphology expressed in several ways. B. subtilis caused curvature and thickening provoking cell vacuolization at 14 days (Figures 3B and 3G); B. mojavensis induced thickening (seven days) and vacuolization (14 days) (Figures 3C and 3H); B. thuringiensis caused slight swellings (seven days) and curvature (14 days) (Figures 3D and 3I), while L. sphaericus generated major changes in morphology by forming closer septa and cell vacuolization from the seven days of culture (Figures 3E and 3J).
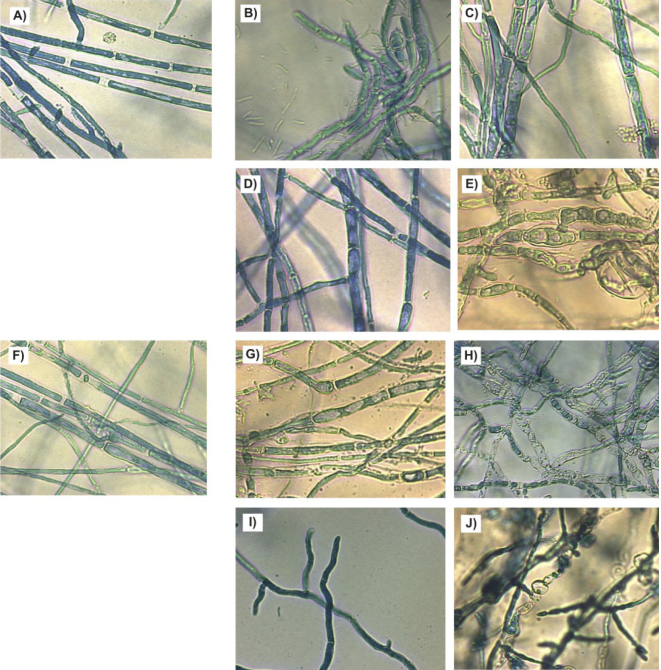
Figure 3 Hyphal morphology of F. verticillioides by effect of rhizospheric bacteria for seven and 14 days of growth on nutrient agar at 28 °C. A) Control 7 d, B) B. subtilis 7 d, C) B. mojavensis 7 d, D). B. thuringiensis 7 d, E)L. sphaericus 7 d, F) Control 14 d, G) B. subtilis 14 d, H) B. mojavensis 14 d, I) B. thuringiensis 14 d, J) L. sphaericus 14 d.
In general, rhizobacteria affected the morphology of hyphae of F. verticillioides, primarily with regard to thickness, roughness, vacuolization and probably at the output of intracellular material. Other research has shown that rizobacteria can affect the morphology of Curvularia lunata (Wakker) Boedijn (Basha & Ulaganathan, 2002). In particular, it is known that various species of the genus Bacillus affect the morphology of hyphae of different fungi and has been proposed that antagonist microorganisms may induce various defense mechanisms in fungi, among them the deposition of chitin in the cell wall. However, it has been reported that some Bacillus species are able to overcome these defenses and provoke severe damage in the fungal cells (Chérif et al., 2002).
Mortality and weight reduction of L. zonatus by the effect of B. thuringiensis and L. sphaericus
Under the study conditions, B. thuringiensis and L. sphaericus had no statistically significant effect (P = 0.05) on the mortality of the nymph 4 of L. zonatus regarding the control. Similarly, there was no significant effect of bacteria on the weight of the nymph 4 to the nymph 5 regarding the control (Table 2).
Table 2 Effect of Bacillus thuringiensis and Lysinibacillus sphaericus on mortality and weight of Leptoglossus zonatus.
| Treatments | Leptoglossus zonatus | ||
|---|---|---|---|
| Mortality (%)* | Nymph weight 4 to 5 (mg)** | ||
| Control | 33.33 a | 50.16 ± 18.80 a | |
| Bacillus thuringiensis | 46.66 a | 42.61 ± 12.04 a | |
| Lysinibacillus sphaericus | 36.66 a | 44.02 ± 17.21 a | |
*Chi-square (Ӽ2), **One-way ANOVA. Same letters in the same column indicate no statistically significant differences according to the method Holm-Sidak (P = 0.05).
In this study, B. thuringiensis and L. sphaericus had no entomopathogenic effect on L. zonatus. There are few studies where the application of B. thuringiensis and L. sphaericus has been successful on the control of Hemiptera. In this regard, Schünemann, Knaak, and Fiuza (2014) mentioned little is known about the toxicity of B. thuringiensis on the Hemiptera order, because there are few studies evaluating the effect. Most successful studies on entomopathogenic activity of bacteria have been tested in the larval stage of holometabolous insects that feed chewing leaves. After the bacteria are ingested, toxins are inserted into the peritrophic membrane of the midgut which act by forming pores, causing vacuolization of the cytoplasm and subsequently a cell disruption, which causes that the insect cannot eat and eventually die. Hemiptera evolved to feed liquid diet (Stockhoff & Conlan, 2003), developing a sucking-biting oral cavity, so the midgut morphology varies, functional activity, lacks peritrophic membrane and particular hydrolytic digestive enzymes such as trypsin, necessary for the action of toxins. For this reason, toxins of B. thuringiensis did not interact with the digestive tract of the insect; however, recently it has succeeded in expressing an insecticidal protein of 35 kDa of this bacteria affecting survival and development of Lygus hesperus (Knight) in transgenic cotton plants (Baum et al., 2012). This means that there may be toxic proteins against bedbugs, but we have to investigate how such proteins interact with the digestive tract of bedbugs. Therefore, in later studies it is important to seek the most effective way of providing bacteria to insects.
Taking into account that this is the first study that evaluates the entomopathogenic activity of B. thuringiensis and L. sphaericus on L. zonatus, because so far there are no reports of studies performed in these organisms, it is necessary to consider the study in other biological stages of the insect and concentrations of protein crystals. It is especially important to find a mechanism for toxic proteins of these bacteria can act in the digestive system of the Heteroptera, through an enzyme capable of initiating the process in the digestive tract of bedbugs.
Conclusions
Bacillussubtilis, B. mojavensis, B. thuringiensis and L. sphaericus rhizospheric bacteria inhibited mycelial growth and affected the hyphal morphology of F. verticillioides regardless of the medium and times of culture used. B. thuringiensis and L. sphaericus rhizobacteria did not affect mortality or the development of L. zonatus. In general, strains of rhizospheric bacteria showed antagonistic activity against F. verticillioides and showed no entomopathogenic activity against L. zonatus. This study can extend the potential use of antagonistic bacteria that may be used within the strategies of integrated management of pests and disease.











 texto en
texto en 



