Introduction
Oregano (Lippia graveolens Kunth) is a species native to the arid areas of Mexico, where it is collected and marketed to generate income for the families of those areas; it is an aromatic species with great economic potential, since it has international demand due to its uses in the pharmaceutical and cosmetic industry (Pascual, Slowing, Carretero, Sánchez, & Villar, 2001; Silva, 2003). The oregano plant has been studied and tested as an anticancer, pesticide and antimicrobial food preservative Machado et al., 2010; Martínez-Rocha, Puga, Hernández-Sandoval, Loarca-Piña, & Mendoza, 2008; Zheng & Wang, 2001). The chemical composition of the stem has been studied due to its important content of flavonoids, which can contribute to the development of new compounds for agronomy and medicine applications (González-Güereca, Soto, Kite, & Martínez, 2007). Which gives even more importance to the plant.
Mexico is one of the countries with higher production of dry oregano in the world; in Huerta, 1997, reported that production was 4,000 t∙year-1 and that the country was one of the largest exporters, surpassed only by Turkey. Since the species is harvested in natural areas, there is no record of production statistics and there are no recent data available. However, Mexican oregano is considered the oregano with the highest quality, due to the chemical composition of its essential oils, which has promoted its commercialization in recent years (Comisión Nacional Forestal [CONAFOR], 2007). Most oregano production in Mexico comes from wild populations located in low precipitation and high temperature areas. This, combined with the harvesting during the flowering season, causes the plant’s natural regeneration rate to be slow. For this reason, reforestation programs are required to guarantee the permanence of oregano in its natural habitat.
Oregano may be an alternative crop in regions with low availability of water for irrigation. Because it is a native species of arid regions, water requirements are low (Villa-Castorena, Catalán-Valencia, Arreola-Ávila, Inzunza-Ibarra, & Román-Lopez, 2011); however, planting oregano in the field directly has a low probability of success, mainly because the seed is very small, has low percentage of germination and the seedling in its early stages is very fragile to withstand cultivation practices. This results in a low population density, which is reflected in a reduced harvest. The production of oregano seedlings in nursery massively is an option to obtain high quality plants with a well-developed root system and strong stems. So, when the plant is transplanted, it will have a good adaptation and establishment in the field.
The growing medium and the type of container are two very important factors in the production of nursery seedlings. They define the growth and development of both the root and the shoot. When selecting a growing medium, its physical and chemical properties should be considered, which determine the availability of water and oxygen, water mobility, ease of root penetration and nutrient uptake (Abad, Martínez, & Martínez, 1993; Cremades, 2005). A growing medium should promote good growth of seedlings within the confined space of the container. This is why the studies regarding the growth response of oregano seedlings for transplanting using different types of growing media and container are relevant. Knowing these factors helps to perform better planning of the nursery, since it is possible to determine the space required for the production of seedlings, as well as the costs required for this activity. Taking into account the above, the present study evaluated the effect of the type of growing medium and container on the growth and quality of oregano seedlings grown under nursery conditions.
Materials and methods
This study was developed in a greenhouse with polycarbonate cover on its front and lateral side and polyethylene roof, as well as extractors for ventilation. The greenhouse is located at 25° 30’ N - 103° 42’ W and at a height of 1,135 m, in the experimental field of CENID RASPA INIFAP in Gómez Palacio, Durango. The maximum and minimum temperatures inside the greenhouse ranged from 28.3 to 39.3 °C and 15.6 to 22.5 °C, respectively, while relative humidity ranged from 32 to 85 %.
A total of five growing media and five containers were evaluated for the production of oregano in nursery. The growing media were: S1 = commercial mixture of BM2 (peat moss + perlite + vermiculite, 80:10:10), S2 = mixture BM2 + river sand (1:1), S3 = mixture of BM2 + river sand (1.5:1), S4 = mixture of BM2 + vermiculite + perlite (1:1:1) and S5 = mixture of compost + sand (1.5:1); proportions were made on the basis of volume. Bulk density and moisture retention capacity of each growing media are shown in Table 1. The containers evaluated were: expanded polystyrene (EP) trays of 200 (CH200), 128 (CH128) and 72 (CH72) cavities, with volume of 16, 28 and 74 cm3, respectively; 250 cm3 polystyrene pot and black plastic bag (BP) caliber 150 μm of 10 x 15 cm and volume of 712 cm3.
Table 1 Physical properties of growing media evaluated for the production of oregano (Lippia graveolens) grown under nursery conditions.
| Growing medium | Bulk density (g∙cm-3) | Moisture retention capacity (cm3∙cm-3) |
| Mixture BM2 | 0.08 | 0.75 |
| Mixture BM2 + sand (1:1) | 0.80 | 0.43 |
| Mixture BM2 + sand (1.5:1) | 0.60 | 0.49 |
| Mixture BM2 + perlite + vermiculite (1:1:1) | 0.16 | 0.46 |
| Compost + sand (1.5:1) | 1.03 | 0.40 |
BM2: Peat moss + perlite + vermiculite (80:10:10).
A randomized complete block design with 5 x 5 factorial arrangement, with four replications per treatment was used. Factor A comprised the types of growing media while factor B included the type of container. The combination of both factors resulted in a total of 25 treatments (Table 2). The experimental plot consisted of two trays of each type (200, 128 and 72 cavities), 10 pots and 10 bags.
Table 2 Treatments resulting from the combination of type of growing medium and type of container, for the production of oregano (Lippia graveolens) grown under nursery conditions.
| Treatment | growing medium | container |
| T1 | Mixture BM2 (S1) | CH200 (C1) |
| T2 | Mixture BM2 (S1) | CH128 (C2) |
| T3 | Mixture BM2 (S1) | CH72 (C3) |
| T4 | Mixture BM2 (S1) | POT (C4) |
| T5 | Mixture BM2 (S1) | BAG (C5) |
| T6 | Mixture BM2 + Sand 1:1 (S2) | CH200 (C1) |
| T7 | Mixture BM2 + Sand 1:1 (S2) | CH128 (C2) |
| T8 | Mixture BM2 + Sand 1:1 (S2) | CH72 (C3) |
| T9 | Mixture BM2 + Sand 1:1 (S2) | POT (C4) |
| T10 | Mixture BM2 + Sand 1:1 (S2) | BAG (C5) |
| T11 | Mixture BM2 + Sand 1.5:1 (S3) | CH200 (C1) |
| T12 | Mixture BM2 + Sand 1.5:1 (S3) | CH128 (C2) |
| T13 | Mixture BM2 + Sand 1.5:1 (S3) | CH72 (C3) |
| T14 | Mixture BM2 + Sand 1.5:1 (S3) | POT (C4) |
| T15 | Mixture BM2+ Sand 1.5:1 (S3) | BAG (C5) |
| T16 | Mixture BM2 + perlite + vermiculite 1:1:1 (S4) | CH200 (C1) |
| T17 | Mixture BM2 + perlite + vermiculite 1:1:1 (S4) | CH128 (C2) |
| T18 | Mixture BM2 + perlite + vermiculite 1:1:1 (S4) | CH72 (C3) |
| T19 | Mixture BM2 + perlite + vermiculite 1:1:1 (S4) | POT (C4) |
| T20 | Mixture BM2 + perlite + vermiculite 1:1:1 (S4) | BAG (C5) |
| T21 | Compost + Sand 1.5:1 (S5) | CH200 (C1) |
| T22 | Compost + Sand 1.5:1 (S5) | CH128 (C2) |
| T23 | Compost + Sand 1.5:1 (S5) | CH72 (C3) |
| T24 | Compost + Sand 1.5:1 (S5) | POT (C4) |
| T25 | Compost + Sand 1.5:1 (S5) | BAG (C5) |
BM2: Peat moss + perlite + vermiculite (80:10:10). Containers: Expanded polystyrene (EP) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, volume of 16, 28 and 74 cm3, respectively; EP pot of 250 cm3; and black plastic bags, caliber 150 μm of 10 x 15 cm and volume of 712 cm3.
The seeds were disinfected with a 5 % of chlorine solution for 60 s, rinsed with distilled water and then treated with a solution of gibberellic acid at a concentration of 250 mg∙L-1 of water and immersed for 12 h. Prior to sowing, the growing media were prepared in the proportions corresponding to each treatment, mixed with a shovel and moistened. Subsequently, the containers were filled and three seeds were sown in each cavity; after the emergency, the seedlings were discarded, leaving only one.
The seedlings were watered with water until the first true leaves appeared. From that moment until 45 days after the emergence (dae), the seedlings were irrigated with a nutrient solution at a concentration of 20- 40-30 mg∙L-1 de N, P and K, respectively. After 45 and up to 90 dae, the concentration of the solution was changed to 60-80-90. This solution was prepared with well water and soluble fertilizers. The contribution of nutrients from irrigation water was considered in the formulation of the nutritional solution. The nutritional solution was prepared with the commercial fertilizers: potassium monophosphate, potassium nitrate and ammonium nitrate. Phosphoric acid was also used to regulate the pH of the solution to 6.5. Every two or three days plants were watered according to the age of the plant and the humidity conditions in the trays, which was influenced by the temperature and humidity in the greenhouse; a micro spray system (nebulizer) was used for irrigation.
At 110 days after sowing (das), five plants were selected from each treatment and replication to measure plant height, leaf area and stem diameter. Plant height was measured from the stem base to the point of growth of the plant using a graduated scale, and the diameter was recorded at the base of the stem using a digital vernier (Auto Tec, Charlottre, NC, USA). All leaves of each plant (without petiole) were cut and measured with a leaf area integrator (LI-COR 3500, Lincoln, Nebraska, USA). In addition to the above, the dry weight of the aerial part (shoot) and dry weight of the root were obtained by drying the leaves (including the petiole), stem and root in forced air oven at 68 °C for 48 h. The sum of the dry weight of the leaves and stem resulted in the dry weight of the shoot. The morphological indices ratio between dry shoot weight and dry root weight, and the Dickson quality index (ICD) were also calculated (Olivo & Buduba, 2006). The latter was estimated by the following relation:
where:
TW |
Total weight of the plant (g) |
PH |
Plant height (cm) |
SDW |
Shoot dry weight (g) |
RDW |
Root dry weight (g) |
D |
Diameter of root crown (mm) |
Data were analyzed using a variance analysis according to the experimental design used. When the factors studied and their interaction were significant (P ≤ 0.05), the means comparison was done using the Tukey test (P = 0.05). Analyzes were carried out using the statistical package SAS version 9.0 (Statistical Analysis System [SAS Institute], 2002).
Results and discussion
Height of Lippia graveolens plant
The analysis of plant height variance (PH) indicated that the effects of the type of container, growing medium and interaction of both factors were significant (P ≤ 0.01). Figure 1 shows the graphical comparison in plant height, as a result of the interaction growing medium-container. The highest plants, in each growing medium studied, were produced in the bag, except for the S5. The combinations bags with S1 and S2 had a statistically similar effect on PH; on average, plants measured 23.4 cm and were superior to the rest of the combinations. The container CH128 in combination with all growing media, except for S1, produced the smallest plants with only 25 % of PH of the best combinations.
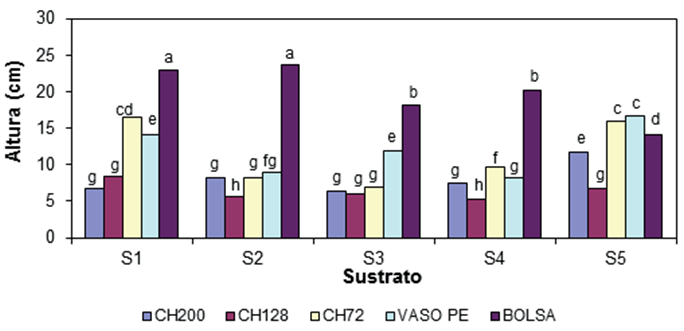
Figure 1 Interaction of the growing medium and type of container in the height of the oregano (Lippia graveolens) plant at 110 days after sowing. S1 = Mixture BM2 (Peat moss + perlite + vermiculite [80:10:10]), S2 = BM2 + Sand (1: 1), S3 = BM2 + Sand (1.5:1), S4 = BM2 + perlite + vermiculite (1:1:1) and S5 = Compound Mixture + Sand (1.5:1). Containers: Expanded polystyrene (PE) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, with volumes of 16, 28 and 74 cm3, respectively; 250 cm3 PE pot; and black plastic bag, caliber 150 μm of 10 x 15 cm and volume of 712 cm3. Bars with different letters indicate statistical difference between means according to the Tukey test (P = 0.05).
Leaf area of Lippia graveolens
The leaf area (LA) of oregano was significantly affected (P ≤ 0.01) by the growing medium, type of container and interaction of both factors. Figure 2 shows that the bag showed higher LA than the other containers in each growing medium, which could be due to the greater amount of water and nutrients available in that container. Studies on tomato, chili, and eggplant indicate that the volume of the container affected the number of leaves and leaf area of the plant (Romano, Paratore, & Rosi, 2003). On the other hand, the combination bag with S1, which had the highest retention capacity, showed the highest LA compared to the rest of the combinations (Figure 2). This confirms what has been reported in the literature regarding the area’s sensitivity to water availability (Kramer & Boyle, 1995; Steudle, 2000).
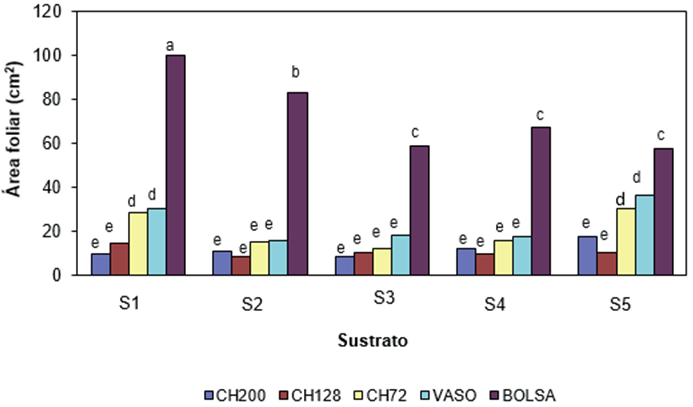
Figure 2 Interaction of the growing medium and type of container in the leaf area of the oregano (Lippia graveolens) plant at 110 days after sowing. S1 = Mixture BM2 (Peat moss + perlite + vermiculite [80:10:10]), S2 = BM2 + Sand (1: 1), S3 = BM2 + Sand (1.5:1), S4 = BM2 + perlite + vermiculite (1:1:1) and S5 = Compound Mixture + Sand (1.5:1). Containers: Expanded polystyrene (PE) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, with volumes of 16, 28 and 74 cm3, respectively; 250 cm3 PE pot; and black plastic bag, caliber 150 μm of 10 x 15 cm and volume of 712 cm3. Bars with different letters indicate statistical difference between means according to the Tukey test (P = 0.05).
Stem diameter of Lippia graveolens
The effects of type of container and growing medium on stem diameter were significant (P ≤ 0.01); on the other hand, the interaction of these two factors was not significant (P > 0.05). The comparison of means among containers indicated that the diameter of the stem was smaller as the volume of the container was smaller (Table 3). These results are consistent with other studies on types of containers in the production of pepper plants for traspalnting (Bar-Tal, Bar-Yosef, & Kafkafi, 1990), tomato (Kemble, Davis, Gardner, & Sanders, 1994) and watermelon (Liu & Latimer, 1995). Plants with high stem diameter values are better than those with less thick stems, since a larger diameter reduces wind damage (flattening the stem) and, consequently, have a better adaptation to the moment of transplantation.
Table 3 Stem diameter of oregano (Lippia graveolens) in each growing medium and container evaluated at 110 days after sowing.
| Growing medium | Stem diameter (mm) | Container | Stem diameter (mm) |
| Mixture BM2 (S1) | 1.55 a | CH200 | 0.96 d |
| Mixture BM2 + Sand 1:1(S2) | 1.27 bc | CH128 | 1.00 d |
| Mixture BM2 + Sand 1.5:1(S3) | 1.15 c | CH72 | 1.21 c |
| Mixture BM2 + vermiculita + perlita 1:1:1 (S4) | 1.25 bc | POT | 1.42 b |
| Composta + Sand 1.5:1(S5) | 1.45 ab | BAG | 2.08 a |
BM2: Peat moss + perlite + vermiculite (80:10:10). Containers: Expanded polystyrene (EP) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, volume of 16, 28 and 74 cm3, respectively; EP pot of 250 cm3; and black plastic bags, caliber 150 μm of 10 x 15 cm and volume of 712 cm3. Means followed by the same letter within columns are not statistically different according to the Tukey test (P = 0.05)
Growing media S1 and S5, which had the highest amount of organic matter, produced an average stem diameter of 1.5 mm, 19 % higher than the average observed in growing media S2 and S4, and 30 % higher compared to the growing medium S3 (Table 3).
Shoot dry weight of Lippia graveolens
The effects of the type of container, growing medium and interaction between both factors on shoot dry weight (SDW) were significant (P ≤ 0.01). In each growing medium, the highest SDW was recorded in the bag, except for S5 (Table 4). The combinations bag with growing medium S1 and S2 promoted the highest value of SDW with an average among them of 1.30 g. These combinations were higher in 51 % to the bags with S3 and S4, which were not statistically different (P > 0.05) and in 223 % to the bag S5. The treatments S1 with CH72 and with a pot, and S5 with CH72, pot and bag were not statistically different (P = 0.05), showing only 37 % of the SDW recorded in the best treatments. The remaining combinations had an average of 0.16 g SDW, which represents only 13 % of that observed in the best treatments (Table 4).
Table 4 Shoot dry weight (SDW) and root dry weight (RDW) of oregano (Lippia graveolens) at 110 days after sowing.
| Growing medium | Container | SDW | RDW |
| Mixture BM2 (S1) | CH200 | 0.125 d | 0.055 d |
| Mixture BM2 (S1) | CH128 | 0.224 d | 0.099 d |
| Mixture BM2 (S1) | CH72 | 0.404 c | 0.178 cd |
| Mixture BM2 (S1) | POT | 0.479 c | 0.227 c |
| Mixture BM2 (S1) | BAG | 1.428 a | 0.657 a |
| BM2 + Sand 1:1 (S2) | CH200 | 0.143 d | 0.075 d |
| BM2 + Sand 1:1 (S2) | CH128 | 0.116 d | 0.057 d |
| BM2 + Sand 1:1 (S2) | CH72 | 0.193 d | 0.087 d |
| BM2 + Sand 1:1 (S2) | POT | 0.258 d | 0.141 d |
| BM2 + Sand 1:1 (S2) | BAG | 1.184 a | 0.582 a |
| BM2 + Sand 1.5:1 (S3) | CH200 | 0.100 d | 0.053 d |
| BM2 + Sand 1.5:1 (S3) | CH128 | 0.108 d | 0.066 d |
| BM2 + Sand 1.5:1 (S3) | CH72 | 0.164 d | 0.071 d |
| BM2 + Sand 1.5:1 (S3) | POT / VASO | 0.215 d | 0.109 d |
| BM2 + Sand 1.5:1 (S3) | BAG / BOLSA | 0.795 b | 0.418 b |
| BM2 + perlite + vermiculite 1:1:1 (S4) | CH200 | 0.150 d | 0.070 d |
| BM2 + perlite + vermiculite 1:1:1 (S4) | CH128 | 0.103 d | 0.060 d |
| BM2 + perlite + vermiculite 1:1:1 (S4) | CH72 | 0.230 d | 0.100 d |
| BM2 + perlite + vermiculite 1:1:1 (S4) | POT / VASO | 0.230 d | 0.114 d |
| BM2 + perlite + vermiculite 1:1:1 (S4) | BAG | 0.931 b | 0.506 b |
| Compost + Sand 1.5:1 (S5) | CH200 | 0.247 d | 0.088 d |
| Compost + Sand 1.5:1 (S5) | CH128 | 0.184 d | 0.098 d |
| Compost + Sand 1.5:1 (S5) | CH72 | 0.439 c | 0.218 c |
| Compost + Sand 1.5:1 (S5) | POT | 0.529 c | 0.292 c |
| Compost + Sand 1.5:1 (S5) | BAG | 0.581 c | 0.289 c |
BM2: Peat moss + perlite + vermiculite (80:10:10). Containers: Expanded polystyrene (EP) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, volume of 16, 28 and 74 cm3, respectively; EP pot of 250 cm3; and black plastic bags, caliber 150 μm of 10 x 15 cm and volume of 712 cm3. Means followed by the same letter within columns are not statistically different according to the Tukey test (P = 0.05)
Root dry weight of Lippia graveolens
The root dry weight (RDW) was significantly affected (P ≤ 0.01) by the growing medium, type of container and the interaction of both factors. In each growing medium, the bag promoted a higher RDW with respect to the other containers, except for the growing medium S5, where the bag, pot and the CH72 showed a similar root biomass (Table 4). The combinations bag with growing media S1 and S2, which showed a similar RDW (P > 0.05), produced the highest value with an average of 0.62 g; the bags with S3 and S4 had, on average, 33 % less RDW. The combinations S1 with pot, and S5 with CH72, pot and bag had 59 % less RDW, while the rest of the combinations had 86 % less RDW.
The above results show that the highest volume of the growing medium in the bag positively affected the root growth, which may be due to the fact that that container had a greater reserve of water and nutrients than the others. In this regard, Van Iersel (1997) reported that the dry weight of the sage root increased linearly with the volume of the container. Also the results indicate that the mixtures of growing media interfered differently in root growth. This is because each of them provides different conditions of aeration and storage of moisture and, consequently, different storage of nutrients, because of the application of the nutritional solution, which influences the development of the root.
Relationship between shoot dry weight and root dry weight (SDW/RDW)
One of the indicators of seedling quality for nursery-grown transplants is the shoot dry weight (PSV) ratio between root dry weight (PSW). This relationship was significantly (P < 0.01) affected by the container, but not by the growing medium nor the interaction between containers and growing media. According to Table 5, the highest value was recorded in CH200, which indicates that the lower volume container affected root growth more than shoot growth. On the other hand, the smallest value of SDW/RDW was recorded in the containers pots, CH128 and bag.
Table 5 Ratio between shoot dry weight (SDW) and root dry weight (RDW) of oregano (Lippia graveolens) at 110 days after sowing.
| Container | SDW / RDW |
| CH200 | 2.66 a |
| CH128 | 1.53 c |
| CH72 | 2.16 b |
| POT | 1.20 c |
| BAG | 1.71 c |
Containers: Expanded polystyrene (EP) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, volume of 16, 28 and 74 cm3, respectively; EP pot of 250 cm3; and black plastic bags, caliber 150 μm of 10 x 15 cm and volume of 712 cm3. Means followed by the same letter within columns are not statistically different according to the Tukey test (P = 0.05)
In general, the lower the ratio SDW/RDW ratio, the better the quality in the plant. When RDW is high, field survival at the time of transplant is higher, as the greater number of roots guarantees better absorption of water and nutrients that favor the growth of the plant. Moreover, plants with less SDW⁄RDW can maintain a better water status with more moderate water consumption in water deficient situations (Leiva & Fernández-Alés, 1998; Stewart & Bernier, 1995). Plants with SDW/RDW values above 2.5, such as those developed in container CH200, should be established under favorable environmental conditions or where there is the possibility of applying irrigation during the establishment phase (Haase & Rose, 1993).
Dickson Quality Index of Lippia graveolens
With respect to the Dickson quality index (DQI), there was a significant statistical difference (P < 0.01) between growing media and containers, but there was no interaction of both factors (P > 0.05). Growing medium S1 produced plants with the highest value of DQI, while the bag did it inside the containers (Table 6). The higher value of DQI, better plant quality (Sáenz, Villaseñor, Muñoz, Rueda, & Prieto, 2010); this implies that the development is large and that, at the same time, the fractions of the shoot are balanced and, therefore, the plants will have greater probability of success in the field.
Table 6 Dickson quality index (DQI) of oregano (Lippia graveolens) at 110 days after sowing.
| Growing medium | DQI | Container | DQI |
| Mixture BM2 (S1) | 0.067 a | CH200 | 0.020 c |
| Mixture BM2 + Sand 1:1 (S2) | 0.047 c | CH128 | 0.026 c |
| Mixture BM2 + Sand 1.5:1 (S3) | 0.040 c | CH72 | 0.035 bc |
| Mixture BM2 + vermiculite + perlite 1:1:1 (S4) | 0.045 c | Pot | 0.048 b |
| Composta + Sand 1.5:1 (S5) | 0.053 b | Bag | 0.124 a |
BM2: Peat moss + perlite + vermiculite (80:10:10). Containers: Expanded polystyrene (EP) trays with 200 (CH200), 128 (CH128) and 72 (CH72) cavities, volume of 16, 28 and 74 cm3, respectively; EP pot of 250 cm3; and black plastic bags, caliber 150 μm of 10 x 15 cm and volume of 712 cm3. Means followed by the same letter within columns are not statistically different according to the Tukey test (P = 0.05)
Conclusions
The growing media and types of containers evaluated definitively affected growth and quality of the oregano plant. The combination bag and growing medium S1 (BM2 = Peat moss, perlite and vermiculite [80:10:10]) produced plants with higher height, leaf area, shoot dry weight and root dry weight. Growing media S1 and S5 (composite mixture with sand, 1.5: 1) promoted thicker stems, and so did the container of black plastic bag (150 μm of 10 x 15 cm). Also the growing medium S1 and the bag produced the highest Dickson quality index. On the other hand, the largest containers (CH128, pot and bag) showed the highest ratio of dry stem weight and root dry weight. With the information generated and the knowledge of the number of plants required, it is possible to define the dimensions of the nursery as well as the management practices for the mass production of plants, either to reforest natural areas or to establish as a crop.











 texto en
texto en 


