Introduction
Leucaena leucocephala (Lam.) de Wit is distributed in the tropics and subtropics (Yeung, Wong, & Wong, 2002) and has great biological diversity in Mexico (Grether, Martínez-Bernal, Luckow, & Zárate, 2006). The plant is fast-growing (Tewari, Katiyar, Ram, & Misra, 2004) and is used in the reforestation of eroded areas and for other purposes in various production systems (Wencomo & Lugo, 2013).
The species L. leucocephala easily adapts to different environments, probably due to the ability of the root system to establish symbiosis with diverse microorganisms, if one takes into account that the vegetation can be linked to the development of the underground community (Sanon et al., 2009). Among them are arbuscular mycorrhizal fungi (AMF), which influence the growth of plants through the transport of nutrients, especially phosphorus; in addition, they promote the absorption of minor elements (Smith, Anderson, & Smith, 2015), trigger defense mechanisms against pathogens (Jaizme-Vega & Rodríguez-Romero, 2008) and improve tolerance to adverse environmental conditions (Doubková, Vlasáková, & Sudová, 2013). AMF can be found in all terrestrial ecosystems; their universality implies vast taxonomic diversity (Jaizme-Vega, 2009). Native AMF populations have favored the sustainability of agricultural systems, while other introduced AMF may not adapt to the environment and present ecological specificity (Serralde & Ramírez 2004) or preference for the host (Daniell, Husband, Fitter, & Young, 2001). In the rhizosphere, in addition to microorganisms, there are abundant root exudates that have a selective effect on the soil microbiota (Offre et al., 2007).
The aim of the present study was to evaluate the effect of different collections of arbuscular mycorrhizal fungi from southeastern Mexico on the growth and phosphorus (P) content of L. leucocephala.
Materials and methods
The research was carried out under nursery conditions during the spring of 2015 in the Experimental Field of the Faculty of Agricultural Sciences C-IV in Huehuetán, Chiapas. The nursery is located at coordinates 15° 00´ NL and 94° 30´ WL at 44 masl. The area has an Am(w’’)ig climate, according to (1973). Mean annual rainfall is 2 326 mm and average annual temperature is 25.4 °C, according to García (1973).
The seed of L. leucocephala cv. Perú was harvested from a 10-year-old plantation in the Miguel Alemán neighborhood, municipality of Pijijiapan, Chiapas (15° 19’ NL - 92° 33’ WL). Four AMF isolates were collected in three tropical regions of Mexico. Two of them, “Caracoles” (Tenampulco, Puebla: 20° 08´ 30´´ NL and 97° 30´ 00´´ WL at 350 masl, 2 100 mm of precipitation and Regosol soils) and “San Rafael” (San Rafael, Veracruz: 20° 08´ 20´´ NL and 97° 02´ 57´´ WL at 20 masl, 1 600 mm of precipitation and Cambisol soils) were obtained from soil cultivated with Vanilla planifolia Andrews. The other two, “Rosario Izapa”, associated with Theobroma cacao L., and “Té limón”, associated with Coffea arabica L., were obtained in the Soconusco region of Chiapas (Tuxtla Chico, Chiapas: 14° 30´ NL and 92° 00´ WL at 425 masl, 4 252 mm of precipitation and Luvisol soils). These isolates were evaluated because their initial capacity to induce greater growth of the host plant during the multiplication stage has been observed. In addition to the above, Rhizophagus intraradices (Schenck & Sm.) Walker & Schüßler was evaluated, as a reference fungus, at a concentration of 40 spores·g-1 of soil and 95 % root colonization in the host plant Brachiaria brizantha (Hochst. ex. A. Rich.) (Data contained in the Micorriza INIFAP® product. This AMF has been used in the biofertilizer induction program for various annual and perennial crops in Mexico (Aguirre-Medina, 2006).
In the roots collected, the presence of AMF was verified by staining and clearing (Phillips & Hayman, 1970). In each soil sample, the spores were separated into groups, by wet sieving and decanting, in order to multiply the similar fungi (Gerdemann & Nicolson, 1963), according to the color, shape and size observed in a stereomicroscope. Spores with similar characteristics were propagated for 90 days in a sterilized river sand substrate (in 1-L containers), using sorghum as a host plant. At harvest, at least 100 spores·g-1 of soil with 93 % root colonization were achieved.
The substrate used in the research was obtained in the grounds of the same experimental field, at a depth of 0 to 30 cm in soils of the eutric Fluvisol group (Instituto Nacional de Estadística, Geografía e Informática [INEGI], 2005). The substrate was mixed with river sand previously washed and sieved at a ratio of 1:1 (v/v). The final substrate had the following components and characteristics: sand (82.7 %), silt (14.3 %), clay (4.16 %), crumby sand texture, organic matter (2.8 %), pH 5.5, N (0.12 %), P (5.0 ppm), cation exchange capacity (CEC: 4.12 mg·100 g-1) and electrical conductivity (EC: 0.12 dS·m-1). Using this substrate, 5-kg bags were filled, perforated at the bottom to favor drainage and placed on iron terraces. Irrigation was carried out every other day.
The AMF were adhered to the L. leucocephala seeds with carboxymethylcellulose; the inoculum represented 6 % of the seed weight. The seeds were sown in pots, 3 cm deep, according to the following treatments: R. intraradices, “Caracoles”, “Rosario Izapa”, “Té limón”, “San Rafael”, fertilization with 15N-15P-15K and a control. The treatments were distributed in a completely randomized design with four repetitions.
The morphological variables (plant height, stem diameter, number of leaves and root colonization) and physiological variables (dry biomass of the root, stem and leaf) were recorded at 120 days after sowing (das). The height was obtained with a tape measure, from the root crown to the apical bud, and stem diameter was measured with a digital Vernier caliper (AutoTECTM, China), 5 cm away from the root crown to the apex. Mycorrhizal colonization (%) was determined in 100 root segments per treatment, with a length of 1.5 to 1.6 cm; they were prepared using the staining and clearing technique of Phillips and Hayman (1970) and were observed under a microscope with oil immersion objective (100x). On the other hand, the dry matter of the aerial and radical part was determined in a forced-air oven at temperatures of 75 to 80 °C for 72 h; subsequently, each component was weighed (Ohaus, Adventurer Pro, USA). With these variables, the quality index proposed by Dickson, Leaf, and Hosner (1960) was determined. Finally, the P content was quantified in a spectrophotometer (Thermo Fisher Scientific Model 400 ¼, USA).
The effects between treatments were determined through an analysis of variance for each variable with the PROC ANOVA procedure. Subsequently, Tukey’s range test (P ≤ 0.05) was performed using SAS computer software version 8.1 (Statistical Analysis System [SAS], 1999-2000).
Results and discussion
Table 1 shows the growth variables of L. leucocephala plants with the treatments applied. Plant height was statistically different among them (P ≤ 0.05); the maximum was 128 cm with the "Caracoles" collection and the increases were 15 % (19 cm) in relation to the control and 8.6 % (16 cm) compared to the fertilized treatment. The "Caracoles", "Rosario Izapa" and “Té limón” isolates were statistically similar with R. intraradices, with an average height of 124 ± 1.9 cm. In other perennial species such as T. cacao, Aguirre-Medina, Mendoza-López, Cadena-Iñiguez, and Avendaño-Arrazate (2007) report 9 cm more height in plants inoculated with R. intraradices compared to the control at 120 das.
Table 1 Morphological and physiological components of Leucaena leucocephala inoculated with different arbuscular mycorrhizal fungi in the nursery. Results at 120 days after sowing.
| Treatment | Height (cm) | Diameter (mm) | Number of leaves | Dry weight (g·plant-1) | DQI | ||
|---|---|---|---|---|---|---|---|
| Leaves | Stem | Root | |||||
| Rhizophagus intraradices | 123 ± 1.3 ab | 9.1 ± 0.0 a | 37 ± 0.8 a | 16.08 ± 0.5 a | 15.76 ± 0.5 a | 10.49 ± 0.5 c | 2.27 |
| Caracoles | 128 ± 2.7 a | 7.3 ± 0.4 b | 24 ± 0.9 b | 10.19 ± 0.3 b | 12.42 ± 0.6 b | 13.95 ± 0.7 ab | 1.80 |
| Rosario Izapa | 126 ± 3.2 ab | 7.3 ± 0.2 b | 21 ± 0.8 bc | 14.17 ± 0.4 a | 11.32 ± 0.6 bc | 14.64 ± 0.6 a | 1.87 |
| Té limón | 119 ± 3.3 abc | 7.0 ± 0.1 b | 18 ± 0.4 c | 9.05 ± 0.7 bc | 9.86 ± 0.1 bcd | 11.87 ± 0.2 bc | 1.68 |
| San Rafael | 86 ± 2.2 d | 7.0 ± 0.2 b | 24 ± 1.4 b | 8.00 ± 0.3 bc | 7.89 ± 0.9 d | 9.99 ± 0.5 c | 2.08 |
| 15N-15P-15K | 112 ± 4.3 bc | 6.7 ± 0.1 b | 19 ± 1.1 c | 8.56 ± 0.6 bc | 8.98 ± 0.3 cd | 12.60 ± 0.9 abc | 1.57 |
| Control | 109 ± 3.6 c | 6.8 ± 0.1 b | 19 ± 0.5 c | 7.80 ± 0.3 c | 8.48 ± 0.4 d | 12.31 ± 0.3 abc | 1.56 |
| CV (%) | 5.4 | 7.5 | 8.0 | 9.6 | 9.3 | 9.7 | |
CV: coefficient of variation. DQI: Dickson quality index. Values with the same letter within each column are equal according to the Tukey test (P ≤ 0.05).
In the case of stem diameter, the plants inoculated with R. intraradices were 25 % higher (P ≤ 0.05) than those of the control and fertilized treatment, and 21 % higher than those inoculated with the AMF collected. In other plants inoculated with R. intraradices such as Cedrela odorata L. and Coffea canephora (Pierre) ex Froehner, the increase was 8 and 20 %, respectively, with respect to the control (Aguirre-Medina, Mina-Briones, Cadena-Iñiguez, Dardón-Zunun, & Hernández-Sedas, 2014; Ibarra-Puón, Aguirre-Medina, Ley-De Coss, Cadena-Iñiguez, & Zavala-Mata, 2014). With other AMF such as Glomus fasciculatum (Thaxter) Gerdemann & Trappe inoculated in Tectona grandis L. F. and Astronium graveolens Jacq., the increase in stem diameter was 11.8 % compared to the control (Hernández & Salas, 2009).
Regarding the number of leaves, R. intraradices induced 18 more leaves than the control and fertilized treatment, and 14 more than the AMF collected (P ≤ 0.05). On average, the isolates induced two leaves more than the control and the treatment with chemical fertilization. In T. cacao inoculated with R. intraradices, the plants had four more leaves than the control (Aguirre-Medina et al., 2007), and the C. canephora plants had two more leaves with the same endomycorrhizal fungus in soil-sand as a substrate (Ibarra-Puón et al., 2014). The number of leaves increased in mycorrhized plants, with the exception of “Té limón”, possibly due to the greater nutrient absorption capacity (Leigh, Hodge, & Fitter, 2009), which induces increased photosynthetic activity after colonization, as in the case of bean (Aguirre-Medina, Kohashi-Shibata, Trejo-López, Acosta Gallegos, & Cadena-Iñiguez, 2005).
Leaf biomass production showed statistical difference (P ≤ 0.05) among treatments and the highest increase was recorded with the inoculation of the reference AMF R. intraradices. Among the fungi collected, "Rosario Izapa" decreased the dry matter allocation to the leaves by 11 % compared to R. intraradices, although statistically they were similar; however, the decrease was greater in the other isolates, on the order of 36 and 46 % for “Caracoles” and “Té limón”, respectively, and 46 % for “San Rafael” and the fertilized control. The results indicate that the accumulation of dry matter in the biomass of the leaf varies according to the isolate applied. Moora, Öpik, Sen, and Zobel (2004) point out variations in mycorrhization, nutrient uptake and plant productivity based on the interaction between plant species and AMF.
Stem dry weight also increased with R. intraradices and was statistically superior (P ≤ 0.05) to the rest of the treatments. Among the isolates collected, a differential response was presented. "Caracoles" and "Rosario Izapa" allocated 24 % less biomass to the stem compared to R. intraradices, while in “Té limón”, “San Rafael”, the control and the fertilized treatment, the decrease was 43 %. Doubková et al. (2013) showed that inoculation with AMF, in species of agricultural interest, increases the nutrition and growth of the plant, and allows it to overcome situations of biotic and abiotic stress. This background suggests the contrasting functionality of microorganisms in interaction with plants (Jäderlund, Arthurson, Granhall, & Jansson, 2008), as in the response found with L. leucocephala.
With regard to root biomass, there was a differential response among the isolates; the largest increase was recorded in “Caracoles” and “Rosario Izapa”. In relation to these two treatments, the biomass decreased 28 % in R. intraradices and “San Rafael”, and 15 % in the control and fertilized treatments. In C. arabica, R. intraradices induced lower root biomass compared to the control (Aguirre-Medina et al., 2011), but in C. odorata it increased (Aguirre-Medina et al., 2014). The decrease in the root biomass seems to be replaced by the hyphae of the fungus (Azcón & Barea, 1980); otherwise, it would be expected that treatments with a greater root system, such as those inoculated with the native and control collections, would have higher absorption of soil nutrients, but in this case the opposite happened with P, as observed in Figure 1.
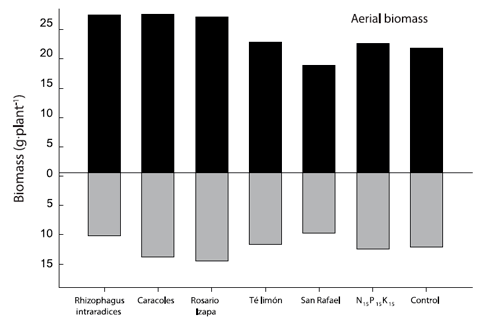
Figure 1 Aerial/root biomass ratio of Leucaena leucocephala inoculated with arbuscular mycorrhizal fungi. The values are averages of four repetitions per treatment.
The root-shoot ratio of L. leucocephala was modified with the biofertilization of some AMF under nursery conditions (Figure 1). The root system of L. leucocephala inoculated with R. intraradices represented 28.4 % of the total biomass, being the lowest percentage compared to the rest of the treatments (between 35 and 37 %). By contrast, in the case of aerial biomass, R. intraradices accounted for 71.6 %. In the rest of the treatments, the value fluctuated from 63 to 65 %. The above effect influences the determination of the plant quality index proposed by Dickson et al. (1960).
Kanno et al. (2006) evaluated native isolates of endomycorrhizal fungi in Brazil and found increases in aerial and root biomass in Brachiaria decumbens Stapf, B. brizantha (Hochst. ex A. Rich.) Stapf and Panicum maximun Jacq., but not in B. humidicola (Rendle) Schweick.
With respect to root colonization, the highest value was obtained with R. intraradices (78%), while the lowest (61%) was found in the control. This confirms the presence of other mycorrhizal fungi in the substrate used, but with less ability to stimulate the growth of Leucaena. These results confirm the colonization capacity of R. intraradices, as has also happened in other annual and perennial crops (Aguirre-Medina, Aguirre-Cadena, Cadena-Iñiguez, & Avendaño-Arrazate, 2012). As for the collections, the average colonization was: Caracoles (68 %), Rozario Izapa (72 %), Té limón (77 %) and San Rafael (63 %). These fungi, when introduced adhered to the seed, have a greater chance of colonization when the radicle emerges, unlike the fungi that are present in the substrate but may not be in the same vicinity. It is likely that colonization by native fungi occurs, but its effect is assumed in the ability to influence the growth of the host plant and the transport of P.
Figure 2 shows that the P content in L. leucocephala was statistically higher (P ≤ 0.05) in the treatment with R. intraradices. Plants in symbiosis with mycorrhizal fungi absorb P from the soil more efficiently than non-colonized plants (Aguirre-Medina et al., 2011; Kanno et al., 2006). Based on the results, AMF inoculated in L. leucocephala allow decreasing chemical fertilization in the nursery, without detriment to plant growth.
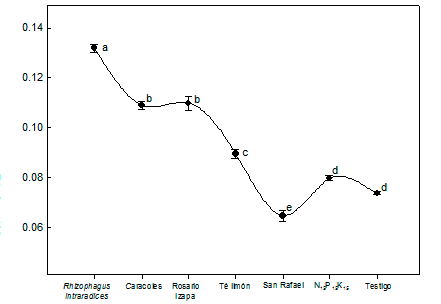
Figure 2 Variation in phosphorus content in the plant tissue of Leucaena leucocephala inoculated with arbuscular mycorrhizal fungi. Results at 120 days after sowing. Different letters indicate statistically significant differences according to the Tukey test (P ≤ 0.05). The vertical bars indicate the standard error of the mean (n = 4). Coefficient of variation = 3.7 %.
Conclusions
The morphological and physiological performance components of Leucaena leucocephala were modified in interaction with the isolates collected, but they maintained a similar proportion in the shoot-root ratio. Biofertilization with Rhizophagus intraradices promoted greater plant growth of L. leucocephala in the aerial part, lower in the root system and higher phosphorus content. With regard to endomycorrhizal fungi, "Caracoles", "Rosario Izapa" and “Té limón” increased the P content in the plant tissue compared to the control and fertilized treatment. The "Caracoles" and "Rosario Izapa" collections are good candidates as biofertilizers in perennial species. Based on the results obtained, AMF in L. leucocephala can help reduce chemical fertilization in the nursery, without detriment to plant growth.











 texto en
texto en 


