Introduction
Human activities emit significant amounts of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) into the atmosphere, contributing to the increase in the concentration of greenhouse gases (GHG) with consequences on global climate change (Intergovernmental Panel on Climate Change [IPCC], 2013). This situation worries scientists and society as a whole, since GHGs increased since the industrial era began in 1750: CO2 increased 30 %; CH4, 100 %; and N2O, 15 % (Olivo & Soto-Olivo, 2010). The use of natural resources has elevated the concentrations of CO2 in the atmosphere. In 2000, a total of 23.5 Gt were estimated worldwide; 60 % from stationary sources (World Meteorological Organization-United Nations Environment Program [WMO-UNEP], 2005). In 2004, emissions were attributed to the energy sector (25.9 %), industrial sector (19.4 %), forestry sector (17.4 %) and agricultural sector (13.5 %) (IPCC, 2007).
Wetlands store 40 % of the carbon generated on the planet (Moya, Hernández, & Borrell, 2005). The most representative are mangroves, submerged grasses, deltaic floodplains, estuaries, lakes and low flood forests (Yáñez-Arancibia & Day, 2010); together, they occupy between 4 and 6 % of the world's territory (Mistch & Gosselink, 2000). At a global scale there are 13.8 million ha of mangrove distributed in Asia (34 to 42 %), Indonesia (23 %), Africa (20 %), North and Central America (15 %), Oceania (12 %), South America (11 %) and Australia (7 %) (Kauffman, Heider, Norfolk, & Payton, 2014). According to Kauffman et al. (2013), 64 % of mangroves are concentrated in 10 countries: 42 % in Indonesia, Brazil, Australia and Mexico; the most diverse are located in Asia. Indonesia ranks first with 2 986 496 ha and Mexico ranks fifth with 964 438 ha (Hutchison, Manica, Swetnam, Balmford, & Spalding, 2014). In 2015, the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO) reported 775 555 ha.
In comparison with other ecosystems, mangroves have a potential impact on the global carbon flux (Dittmar, Hertkorn, Kattner, & Lara, 2006), because mangroves have the highest carbon sequestration rate (Brevik & Homburg, 2004): up to 1.023 Mg∙ha-1 in soil (Donato et al., 2011) and 7.4 Mg∙ha-1 in biomass (Lema & Polanía, 2006). In Mexico, the reserve of carbon in the wetlands has been estimated at 282 Mg∙ha-1, of which 223 Mg∙ha-1 correspond to biomass and 105 Mg∙ha-1 are found in soil, dead trees and fallen branches, while the rest is part of the decomposing matter, forest products and fossil fuels (Masera, Cerón, & Ordóñez, 2001). Recently, in Mexico, the total reserve of organic carbon in soil has been estimated at 2.21 Pg (Cartus et al., 2014), 30 % higher than the 1.69 Pg reported by the FAO (Food and Agriculture Organization) in 2010.
Mangrove coverage in Mexico decreased 10 % in the 25 years prior to 2014 (Valderrama et al., 2014) with an annual deforestation rate of 2.5 % (Instituto Nacional de Ecología [INE], 2005). The deterioration of mangroves in the last half century (30 to 50 %) allowed to estimate the loss of their functionality in only 100 years (Duke et al., 2007), because, when intervening, they become vulnerable to methanogenesis (production of CH4 by microorganisms) (Purvaja & Ramesh, 2001); because logging decreases biomass and releases CO2 and CH4, mangroves reverse their carbon sequestration capacity and become GHG emitters (Troxler et al., 2015).
In Mexico, little has been studied about the dynamics of CO2 and CH4 exchange, and carbon accumulation in wetland soils (Hernandez, 2009), therefore studies are required to determine global flows with precision (Betancourt-Portela, Parra, & Villamil, 2013). For the above, the objectives of this study were: i) to estimate the above-ground biomass and carbon content in live trees from El Sargento and Bahía del Tóbari in Sonora, using allometric equations, and ii) to compare carbon stocks of both mangroves. The hypothesis establishes that carbon stock in the above-ground biomass is greater in El Sargento, because it presents natural conditions that favor the development of biomass and carbon sequestration.
Materials and methods
Study area
The present study was carried out in the estuary El Sargento and Bahía del Tóbari in Sonora, in the arid zone of northern Mexico. The areas have a different degree of anthropogenic pressure, but similar atmospheric conditions: very dry and warm climate, mean annual temperature greater than 22 °C, warm summer and intermediate rainfall regime between summer and winter (BW[h’]hw) (García & CONABIO, 1998).
El Sargento is located between the coordinates 29° 19´- 29° 34´´ NL and 112.3°-112° 22´ WL (Figure 1), covers 717 ha and 7 km long (Rodríguez-Zúñiga et al., 2013). The estuary represents a pristine zone of the Gulf of California (López-Medellín, Acosta-Velázquez, & Vázquez-Lule, 2009), where white mangrove species (Laguncularia racemosa [L.] Gaertn), black mangrove species (Avicennia germinans [L.] L.) and red mangrove species (Rhizophora mangle L.) are housed, which are considered under threat in the NOM-059-SEMARNAT-2010 (Secretaría del Medio Ambiente y Recursos Naturales [SEMARNAT], 2010).
The Bahía del Tóbari is located between the coordinates 27° 01´- 27° 07´ NL and 109° 54´-110° 03´ 18´´ WL (Figure 1), it occupies 16 700 ha and is part of the Protection Area of Flora and Fauna “Islas del Golfo de California” (Carmona et al., 2015). The body of water has deteriorated due to the construction of the road to Huivulai Island, pollution and sedimentation, which together modify the natural hydrodynamic circulation and interrupt the natural marine cleaning and vent streams (Domínguez, 2010).
Field research
The measurements were made from May 2014 to November 2015. In both study sites, an arrangement of 16 plots of 10 x 10 m was drawn. In each plot, two subplots of 4 x 4 m were established (Figure 2). In the plots of 100 m2, the mangrove species were identified; live trees were counted; trees with a diameter at breast height (DBH; 1.3 m) greater than 5 cm were measured with a measuring tape; height and width of canopy were obtained with the technique of Kauffman et al. (2013). In R. mangle, DBH was measured based on the highest root. In subplots of 16 m2, DBH trees < 5 cm were measured with the same technique.
Live above-ground biomass and carbon content
The AB was determined with allometric equations (Table 1) and was related to the estimation of carbon content by the factor 0.5 recommended by the IPCC (2013). The total above-ground biomass (TAB) was estimated by adding the average values of AB in correspondence with the species percentage.
Table 1 Allometric equations to determine the total above-ground biomass in mangrove species of El Sargento estuary and Bahía del Tóbari in Sonora.
| Source | Mangrove species | Allometric equation |
|---|---|---|
| Day, Conner, Ley-Lou, Day, and Navarro (1987) | Lr | AB = Exp [2.192 * (NL(DBH)) - 1.591] |
| Ag | AB = Exp [2.302 * (NL(DBH)) - 1.585] | |
| Rm | AB = Exp [2.507 * (NL(DR)) - 1.560] | |
| Smith and Whelan (2006) | Lr | AB = 10^[1.930 * (LOG10(DBH)) - 0.441] |
| Ag | AB = 10^[1.934 * (LOG10(DBH)) - 0.395] | |
| Rm | AB = 10^[1.731 * (LOG10(DBH)) - 0.112] | |
| Fromard et al. (1998) | Lr | AB = 0.1023 * (DBH^2.50) |
| Ag | AB = 0.14 * (DBH^2.40) | |
| Rm | AB = 0.1282 * (DBH^2.6) | |
| Imbert and Roller (1989) | Lr | AB = 0.209 * (DBH^2.24) |
| Ag | AB = 0.0942 * (DBH^2.54) | |
| Rm | AB = 0.178 * (DBH^2.47) | |
| Chave et al. (2005) | Common equation | AB = 0.168 * ρ * (D^2.47) |
| Komiyama, Poungparn, and Kato (2005) | Common equation | AB = 0.251 * ρ * (D^2.46) |
Ag = Avicennia germinans; Lr = Laguncularia racemosa; Rm = Rhizophora mangle. AB: above-ground biomass, NL: natural logarithm, DBH: diameter at breast height (cm), ρ: density of wood (Ag = 0.759, Lr = 0.762 [Ministerio del Ambiente y los Recursos Naturales, 1994]; Rm = 0.80 [Richter & Dallwitz, 2000]).
Statistical analysis
The statistically significant differences between the means of carbon contents of both study sites, with respect to each mangrove species and global stocks, were detected with the T test for independent samples. The homoscedasticity of the data was determined by the F test with significance level of 0.05. Analyzes were carried out using the Excel program of Windows 10.
Results and discussion
Table 2 shows the characteristics of the species that make up the mangroves in El Sargento estuary and Bahía del Tóbari in Sonora. Based on the total number of trees sampled, a density of 4 406 trees∙ha-1 was estimated in El Sargento. The most abundant species was L. racemosa, followed by A. germinans and R. mangle. The R. mangle species had the lowest development: DBH of 1.3 cm, trunk height (TH) of 1 m, and canopy width of 0.4 m.
In the Bahía del Tóbari, density was 3 162 trees∙ha-1; A. germinans predominated over R. mangle (Table 2). The latter showed greater development and composition in Bahía del Tóbari (DBH = 8.24 cm, 16.6 %) than in El Sargento (DBH = 1.3 cm, 5.82 %), which suggests the adaptability of the species to the anthropic modifications of the site.
Table 2 Characteristics of the species under study in the mangroves of El Sargento and Bahía del Tóbari in Sonora
| Species | DBH (cm) | Trunk height (m) | Canopy width (m) | Canopy height (m) | Density (tree∙ha-1) | Composition (%) |
|---|---|---|---|---|---|---|
| El Sargento | ||||||
| Lr | 8.3 ± 1.3 | 2.3 ± 0.2 | 1.1 ± 0.1 | 1.5 ± 0.1 | 2 650 | 60.14 |
| Ag | 7.0 ± 1.4 | 2.1 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.2 | 1 500 | 34.04 |
| Rm | 1.3 ± 0.3 | 1.1 ± 0.3 | 0.6 ± 0.1 | 0.4 ± 0.1 | 256 | 5.82 |
| Total density (trees∙ha-1) | 4 406 | 100 | ||||
| Bahía del Tóbari | ||||||
| Ag | 4.8 ± 0.5 | 1.6 ± 0.2 | 0.9 ± 0.4 | 1.0 ± 0.4 | 2 637 | 83.4 |
| Rm | 8.24 ± 1.5 | 1.8 ± 0.8 | 0.8 ± 0.3 | 0.7 ± 0.3 | 525 | 16.6 |
| Total density (trees∙ha-1) | 3 162 | 100 | ||||
Ag = Avicennia germinans; Lr = Laguncularia racemosa; Rm = Rhizophora mangle. DBH: diameter at breast height. ± Standard error of the mean with 95 % confidence interval.
Determination of the mangrove above-ground biomass
Figure 3 illustrates, graphically, the estimate of AB of the species that make up the mangrove of El Sargento estuary, by means of different allometric equations. In El Sargento, the AB of L. racemosa varied between 81.5 (equation of Smith & Whelan, 2006) and 268.8 Mg∙ha-1 (equation of Komiyama et al., 2005); in A. germinans, between 26.6 (equation of Smith & Whelan, 2006) and 47.9 Mg∙ha-1 (equation of Komiyama et al., 2005); and in R. mangle, between 0.0064 (equation of Chave et al., 2005) and 0.038 Mg∙ha-1 (equation of Smith & Whelan, 2006). The rest of the equations produced intermediate values of those intervals.
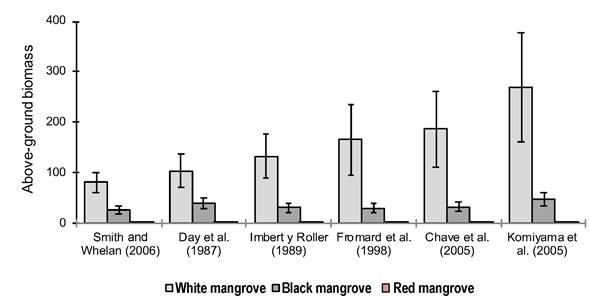
Figure 3 Above-ground biomass of El Sargento mangrove, Sonora, estimated with different allometric equations. The biomass of red mangrove (Rhizophora mangle) is lower in comparison with the white mangrove (Laguncularia racemosa) and the black mangrove (Avicennia germinans). The standard error of the mean with 95 % confidence interval is represented on the bars.
In Bahía del Tóbari, the AB of A. germinans varied between 60 (equation of Smith & Whelan, 2006) and 104.4 Mg∙ha-1 (equation of Komiyama et al., 2005), while in R. mangle the AB varied between 12.1 (equation of Smith & Whelan, 2006) and 32.3 Mg∙ha-1 (equation of Chave et al., 2005). The other equations provided intermediate values (Figure 4).
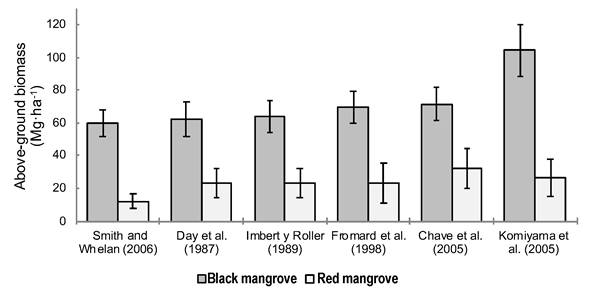
Figure 4 Above-ground biomass of the black mangrove (Avicennia germinans) and red mangrove (Rhizophora mangle) in Bahía del Tóbari, Sonora, estimated with different allometric equations. The standard error of the mean with 95 % confidence interval is represented on the bars.
In the Atasta peninsula in Campeche, Guerra-Santos et al. (2014) reported AB of 206.07 Mg∙ha−1 for L. racemosa, 161.93 Mg∙ha−1 for A. germinans and 181.70 Mg∙ha−1 in R. mangle. In the Yucatan Peninsula, Adame et al. (2013) reported 5.3 Mg∙ha−1 for R. mangle enano. On the other hand, in French Guiana, Fromard et al. (1998) estimated 31.5 Mg∙ha-1 for L. racemosa from two to three years of age, and 315 Mg∙ha-1 for A. germinans and R. mangle from 60 to 70 years; while in Africa, Fatoyinbo and Simard (2013) found 76 (in Benin) and 178 Mg·ha-1 (in Congo) as the average of the biomass of several species, among them A. germinans, L. racemosa and R. mangle. The differences of these values with respect to those obtained in the present study are justified by the typical environmental conditions of the ecoregions, the particular characteristics of the species and the different degree of deterioration by human activities.
Figure 5 shows the TAB estimates in the mangroves of El Sargento and Bahía del Tóbari. In El Sargento, the TAB varied between 108.1 and 316.78 Mg∙ha-1, while in Bahía del Tóbari, the TAB was estimated between 72.12 and 130 Mg∙ha-1. In both sites, the largest estimates derived from the equation of Komiyama et al. (2005), and the lower estimates by Smith and Whelan (2006). In El Sargento, the values estimated with the equation of Komiyama et al. (2005) equals 2.93 times those calculated with the equation of Smith and Whelan (2006); the same was observed in Bahía del Tóbari, whose relation was 1.81 times greater. The equation of Chave et al. (2005) generated lower values with respect to that of Komiyama et al. (2005), but higher to the rest; this was also reported by Komiyama, Ong, and Poungparn (2008), using the same value for density of wood in both equations.
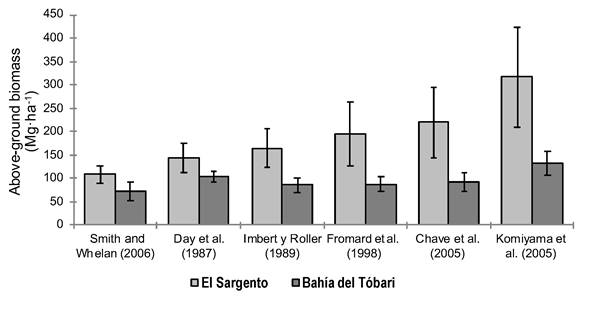
Figure 5 Estimates of total above-ground biomass in the mangroves of El Sargento and Bahía del Tóbari, Sonora, using different allometric equations. The standard error of the mean with 95 % confidence interval is represented on the bars.
Estimates of total above-ground biomass in the mangroves of El Sargento and Bahía del Tóbari, Sonora, using different allometric equations. The average error with 95 % confidence interval is represented on the bars.
The differences between AB calculated were attributed to the specificity or generality of the equations used, since the highest values correspond to those commonly used in the forest area (equations of Komiyama et al., 2005 and Chave et al., 2005); and the lower values, to those of particular use of each species. For some scientists, it is still controversial to state whether the allometry of a mangrove species is specific to a particular location (Komiyama et al., 2008); however, others determined similar equations for the same species at different sites, this is the case of Rhizophora apiculata Blume (Ong, Gong, & Wong, 2004).
Komiyama et al. (2008) compared the AB of Rhizophora species and found that the equation of Komiyama et al. (2005) had a relative error of -5.81 to -4.94 % in the estimate with respect to Imbert and Rollet (1989), and -8.44 to 6.79 % with respect to the equation of Fromard et al. (1998); in Avicennia, the relative error was -4.05 to -10.8 % for the equation of Imbert and Rollet (1989), and -11.7 to 3.99 for the equation of Fromard et al. (1998). This indicated that the estimate of AB with the commonly used equation of Komiyama et al. (2005) has a relative error of less than 10 %, which suggests that the allometric relationships of the same mangrove species do not differ between sites, and that the density of the wood can be the determining factor (Komiyama et al., 2008).
El Sargento had greater TAB (between 108.1 and 316 Mg∙ha-1) compared to Bahía del Tóbari (72.1 and 130 Mg∙ha-1) (Figure 5). In this interval, we found AB values reported by Hutchison et al. (2014) for North, Central America and the Caribbean (145.3 Mg∙ha-1), who also pointed out that the highest global averages come from the Pacific Islands (233.3 Mg∙ha-1) and Southeast Asia (230.9 Mg∙ha-1), while the lower averages are from East Asia (107.2 Mg∙ha-1).
Total above-ground biomass and carbon stock
There is a statistically significant difference in the average values of the carbon content of A. germinans (F = 0.12, P = 0.0004), R. mangle (F = 1.95-13; P = 0.0003) and the global (F = 0.01; P = 0.02) between both study sites. Laguncularia racemosa was excluded from the analysis because it was only found in El Sargento.
Table 3 indicates the carbon stock in the above-ground biomass of the mangroves of El Sargento and Bahía del Tóbari. In El Sargento, the carbon estimates in the AB of L. racemosa ranged between 40.7 and 134.44 Mg C∙ha-1; in A. germinans, from 13.3 to 24 Mg C∙ha-1; and in R. mangle, from 0.003 to 0.019 Mg C∙ha-1. In Bahía del Tóbari, the carbon stock in the AB of A. germinans varied between 30 and 52.2 Mg C∙ha-1; and in R. mangle, from 6 to 16.2 Mg C∙ha-1. The total carbon stock in the AB of El Sargento was estimated between 54.1 and 158.4 Mg C∙ha-1, while in Bahía del Tóbari it ranged from 36.1 to 65.5 Mg C∙ha-1.
Table 3 Carbon stock in the above-ground biomass of the mangroves of El Sargento and Bahía del Tóbari in Sonora.
| Carbon in above-ground biomass (Mg C∙ha-1) | Estimation equation | |||
|---|---|---|---|---|
| Laguncularia racemosa | Avicennia germinans | Rhizophora mangle | Total | |
| El Sargento | ||||
| 40.7 ± 9.8 | 13.3 ± 3.5 | 0.019 ± 0.014 | 54.1 ± 9.3 | Smith and Whelan (2006) |
| 52.1 ± 16.1 | 19.6 ± 5.1 | 0.011 ± 0.009 | 71.7 ± 16.0 | Day et al. (1987) |
| 66.7 ± 21.3 | 15.5 ± 4.2 | 0.006 ± 0.005 | 82.2 ± 21.0 | Imbert and Roller (1989) |
| 82.7 ± 34.8 | 15.1 ± 4.1 | 0.005 ± 0.004 | 97.8 ± 34.2 | Fromard et al. (1998) |
| 93.3 ± 37.9 | 16.5 ± 4.5 | 0.003 ± 0.002 | 109.8 ± 37.4 | Chave et al. (2005) |
| 134.4 ± 54.1 | 24.0 ± 6.6 | 0.005 ± 0.004 | 158.4 ± 53.3 | Komiyama et al. (2005) |
| Bahía del Tóbari | ||||
| 30.0 ± 3.9 | 6.0 ± 2.2 | 36.1 ± 5.6 | Smith y Whelan (2006) | |
| 31.3 ± 5.2 | 11.7 ± 4.4 | 42.9 ± 8.2 | Imbert and Roller (1989) | |
| 32.1 ± 4.9 | 11.8 ± 4.5 | 43.9 ± 8.1 | Fromard et al. (1998) | |
| 34.9 ± 4.9 | 11.6 ± 6.0 | 46.5 ± 10.0 | Chave et al. (2005) | |
| 35.9 ± 5.1 | 16.2 ± 6.1 | 52.0 ± 9.5 | Day et al. (1987) | |
| 52.2 ± 7.9 | 13.3 ± 5.6 | 65.5 ± 12.8 | Komiyama et al. (2005) | |
± Standard error of the mean with 95 % confidence interval.
Research in Mexico on AB of mangrove and its carbon content is scarce; however, estimates have been made for some regions, which are shown in Table 4. In the Gulf of Mexico and the central Pacific, 137.32 and 101.75 Mg C∙ha-1 are recorded, respectively (Herrera et al., 2016); in the Yucatan Peninsula, the carbon content varies between 2.5 and 84.6 Mg C∙ha-1 (Adame et al., 2013); the North Pacific, where the study sites are located, has an average of 42 Mg C∙ha-1, where the Agua Brava de Nayarit Lagoon (162.41 Mg C∙ha-1) and Bahía del Tóbari (3.4 Mg C∙ha-1) had the extreme values (Herrera et al., 2016). When comparing the previous data with those of this study, the minor stocks of El Sargento (54.1 Mg C∙ha-1) and of Bahía del Tóbari (36.1 Mg C∙ha-1) are close to the estimates of the North Pacific region (42 Mg Mg C∙ha-1), although there is contrast with respect to Bahía del Tóbari, where the comparison is complicated by the lack of information.
Despite the environmental differences between ecoregions, the Yucatan peninsula (Sian Ka'an) records AB (176.2 Mg∙ha-1) and intermediate carbon stocks (84.6 Mg∙ha-1) (Adame et al., 2013), with respect to the interval estimated in El Sargento (AB = 108.1-316 Mg∙ha-1 and C = 54.1-158.4 Mg∙ha-1) (Table 4). Different sites of the Atasta peninsula reported stocks from 36.8 to 235.77 Mg C∙ha−1 in R. mangle, and in Bahía de Cispatá (Colombia), of 64.85 Mg C∙ha−1 (Yepes et al., 2016). Approximations of the amounts previously reported with respect to El Sargento were attributed to L. racemosa being the dominant species in Sian Ka'an, although of moderate presence in Atasta.
Table 4 Above-ground biomass and organic carbon in different mangrove forests.
| Study site | Dominant species | Mangrove characteristics | Above-ground biomass (Mg∙ha-1) | Carbon (Mg∙ha-1) | Source | |
|---|---|---|---|---|---|---|
| Height (m) | DBH (cm) | |||||
| Mexico | ||||||
| El Sargento, Sonora | Ag y Lr | 108.1-316 | 54.1-158.4 | Present study | ||
| Bahía del Tóbari, Sonora | Ag y Rm | 72.12-130 | 36.1-65.5 | |||
| Sian Ka'an, Yucatán | Lr | 176.2 | 84.6 | Adame et al. (2013) | ||
| Rm | > 5 | 144.9 | 69.6 | |||
| Rm | 3-5 | 114.2 | 54.8 | |||
| Rm | <1.5 | 5.3 | 2.5 | |||
| Reserva de la Biósfera La Encrucijada, Chiapas | Ag, Lr y Rm | 20-40 | 8-11 | 421.1 | 215 | Adame et al. (2015) |
| North Pacific | 42.09 | Herrera et al. (2016) | ||||
| Central Pacific | 101.75 | |||||
| Gulf of México | 137.32 | |||||
| South Pacific | 139.65 | |||||
| French Guyana | Lr (Age = 2-3 years old) | 31.5 | Fromard et al. (1998) | |||
| Ag (age = 50 years old) | 180 | |||||
| Ag y Rm (age = 60-70 years old) | 315 | |||||
| Dominican Republic | Rm | <3 | 11.9 | 10 | Kauffman et al. (2014) | |
| Lr and Rm | 3-10 | 54.2 | 47 | |||
| Rm | >10 | 240.7 | 161 | |||
| Colombia | ||||||
| Bahía de Cispatá | Rm | 129.69 | 64.85 | Yepes et al. (2016) | ||
| Delta del Río Atrato | Rm | >15 | 165.9 | 82.9 | Blanco-Libreros, Ortiz-Acevedo, y Urrego (2015) | |
| Ensenada de Rionegro | Rm | <2 | 115.6 | 57.8 | ||
| Puerto César-Punta | Ag, Lr and Rm | 5-16.5 | 85.4 | 42.7 | ||
| Turbo | Ag, Lr and Rm | < 5 | 75.8 | 37.9 | ||
| USA (East Coast of Florida) | Ag, Lr and Rm | > 2 | 114 | 55 | Doughty et al. (2016) | |
Ag = Avicennia germinans, Lr = Laguncularia racemosa, Rm = Rhizophora mangle. DBH: Diameter at breast height (1.3 m).
The differences or similarities of the AB between the study sites are justified by the diversity and abundance of the species. Environmental components, management practices and disturbances in hydrological regimes also influence the AB development. Height decreases as latitude increases, a pattern that produces small amounts of AB and decreases carbon sequestration (Morrisey et al., 2010). In contrast, primary or mature mangroves have higher AB (lower in temperate zones) at lower latitudes, which is associated with climatic conditions (temperature, solar radiation, precipitation and frequency of storms) (Komiyama et al., 2008).
The structural characteristics of the vegetation provide a certain tolerance of the species to the effects of global climate change (Doughty et al., 2016). The properties and nutrient condition of the soil affect the biomass growth rate and carbon sequestration capacity (Komiyama et al., 2008). This correlates with the size of trees and the potential carbon sequestration in R. mangle: Adame et al., (2013) indicated that trees smaller than 1.5 m store 2.5 Mg C∙ha-1 and greater than 5 m store 69.6 Mg C∙ha-1. Kauffman et al. (2014) coincide with the above: trees smaller than 3 m capture 10 Mg C∙ha-1 and greater than 10 m capture up to 161 Mg C∙ha-1.
Conclusions
El Sargento is a pristine environment which recorded higher amounts of above-ground biomass and carbon reserves compared to Bahía del Tóbari, whose mangrove has anthropic impacts. The storage of carbon in natural habitats is essential to mitigate the effects of global climate change. Mangrove conservation and management policies are required at the national level, to favor their protection and maintain their ecological and environmental functions.











 texto en
texto en 




