Highlights:
Green and mature acorns of Quercus rugosa, Q. sideroxyla and Q. deserticola were analyzed.
Morphology varied according to species; Q. deserticola had larger acorns.
Acorn size varied among trees of the same species.
Acorn weight was most correlated with crown diameter and diameter at breast height.
Acorn chemical composition varied among species and among maturity stages.
Introduction
Oaks (Quercus sp.) are widely distributed in forest ecosystems. Oaks live in mixed stands with species of the genus Pinus and are harvested for commercial purposes to produce parquet and tool handles; branches with a diameter of less than 5 cm are used for charcoal production. Quercus species are carbon dioxide capture systems, leaf litter allows the incorporation of organic matter into the soil and helps as protection and food for wildlife (de la Paz-Pérez & Dávalos-Sotelo, 2008; Uribe-Salas, Rocha-Ramírez, Gregorio-Cipriano, Fernández-Pavia, & Alvarado-Rosales, 2019). There are about 450 species of the genus Quercus in the world (Sánchez-Burgos et al., 2013), of which 161 inhabit the American continent and 109 are native to Mexico, representing 68 % of the oaks in the American continent (Arizaga, Martínez-Cruz, Salcedo-Cabrales, & Bello-González, 2009); of these, 30 are found in the state of Durango (González-Elizondo, González-Elizondo, Tena-Flores, Ruacho-González, & López-Enríquez, 2012).
Acorns can have diverse uses based on morphological and chemical characteristics; for example, in Spain they are used as feed for pigs and represent an important source of food for humans, although their use has decreased in recent years (Arizaga et al., 2009). Bread supplemented with acorn flour has been found to develop resistance to aging and to have better sensory acceptance, because of its enrichment with protein, minerals and dietary fiber (Korus, Witczak, Ziobro, & Juszczak, 2015). However, acorns have a certain degree of toxicity, due to the presence of tannins and metabolites, which are bound to proteins that interfere with the gastrointestinal microbial flora; these can be considered as anti-nutritional agents for the animals that consume them (Smith et al., 2015). In this regard, in the case of Acacia farnesiana (L.) Willd., Barrientos-Ramírez et al. (2012) reveal that the elimination of tannins from the seed improves the assimilation of matter in the rumen of sheep.
Variation in chemical composition of acorns is attributed to geographic location, stage of maturation, climate (Belghith, Abidi, Trabelsi-Ayadi, & Chérif, 2015) and soil type where trees grow. On the other hand, germplasm quantity, size and weight are influenced by factors such as temperature and precipitation; when both decrease, seed production increases, while weight decreases with high temperatures (Carbonero & Fernández-Rebollo, 2014). Acorn weight variation varies from 1.2 to 6.5 g, depending on the genetic characteristics of trees, climatic conditions and soil properties (Gea-Izquierdo, Cañellas, & Montero, 2006). It has been reported that larger seeds have a higher germination rate, due to an important content of energy reserves in cotyledons (Rubio-Licona, Romero-Rangel, Rojas-Zenteno, Durán-Díaz, & Gutiérrez-Guzmán, 2011), and have more resistance to drought and nutrient scarcity; on the other hand, small seeds have a greater capacity for dispersal and establishment (Gómez, 2004).
Because of the poor information available in Mexico and importance of the subject studied, the objective of this study was the morphological, physical and chemical characterization of acorns of Quercus rugosa Née, Q. sideroxyla Humb. & Bonpl. and Q. deserticola Trel. The hypothesis is that there are differences in the above-mentioned characteristics among the species studied.
Materials and Methods
Study area and collection of material
The study was carried out in the surroundings of the community of San José de Causas, San Dimas, Durango, in the Sierra Madre Occidental, 180 km west of the city of Durango, within a polygon with the following geographical coordinates 24° 02’ 11.7” N - 105° 47’ 59.1” O; 24° 02’ 44.8” N - 105° 45’ 40.4” W; 24° 02’ 38.6” N - 105° 45’ 17.2” W; 24° 02’ 23.4” N - 105° 45’ 49.2” W; and 24° 01’ 39.6” N - 105° 46’ 46.4” W. With temperate sub-humid climate with average annual precipitation of 875.9 mm. Vegetation consists of pine forest, pine-oak forest and oak forest (Instituto Nacional de Estadística y Geografía [INEGI], 2020). The seed was collected from trees located on sites with slopes ranging from 0 to 30 %, with an average of 12 %.
Acorns were collected from 10 trees per species of Q. rugosa, Q. sideroxyla and Q. deserticola, with the best phenotypic characteristics (dominant height and crown) and absence of pests. From each tree, 30 acorns were collected in green stage (last week of October) and 30 acorns in mature stage (second week of November), with a period of 15 days between both collections; acorns were taken from the middle and lower part of the tree crowns.
The following data were recorded for each selected tree: total height and slope of the site using a Suunto PM-5/360 clinometer, diameter at breast height using with a diametric tape and crown diameter with a tape measure (Table 1); measurements were taken according to the methodology described by Romahn and Ramírez (2010). Categories for variables diameter at breast height, crown diameter and slope were determined based on the methodology of Howard (2008).
Table 1 Tree-size measurements of oak species collected and slope of the land.
| Species | Diameter at breast height (cm) | Total height (m) | Crown diameter (m) | Slope (%) |
|---|---|---|---|---|
| Quercus sideroxyla | 54.60 ± 6.42 | 17.78 ± 1.72 | 12.04 ± 0.79 | 11 ± 2.52 |
| Quercus rugosa | 54.44 ± 5.40 | 16.01 ± 1.35 | 10.88 ± 0.79 | 15 ± 2.66 |
| Quercus deserticola | 32.75 ± 3.29 | 10.37 ± 0.89 | 7.03 ± 0.35 | 10 ± 1.77 |
± standard error of the mean (n = 10).
Morphological, physical and chemical characteristic of acorns
Equatorial diameter, polar diameter, shell weight and total weight were measured in mature acorns at the Forestry Engineering laboratory of the Faculty of Forestry Sciences of the Universidad Juárez del Estado de Durango. The first two variables were determined using the image analysis technique, which include capturing the images with a 32-megapixel digital camera and then performing the morphological analysis with the help of Image-Pro Plus version 4.5 software (Media Cybernetics®, 2002). Acorn weight was determined on a Velab model VE-210 analytical balance with an accuracy of ± 0.0001 g.
Physical and chemical characterization was performed on 30 mature and 30 green acorns collected per tree. Moisture, total dry matter, ash, crude fiber, crude protein, ethereal extract (Cunniff, 1995) and condensed tannins, estimated by the butanol/HC technique (Makkar, 2003), were determined in each acorn at the postgraduate laboratory of the Faculty of Veterinary Medicine and Animal Husbandry of the Universidad Juárez del Estado de Durango.
Statistical analysis
Data analysis was performed by multiple linear regression, where the independent variables (X) were species, tree and degree of maturity of the acorn, while the dependent variables (Y) were morphological, physical and chemical variables analyzed. The analysis of variance was performed using PROC GLM, regarding the data under a generalized linear model to detect whether independent variables had any effect on dependent variables. Once the existence of the effect was verified, the comparison of means was performed with Tukey's test (α = 0.05); all analyses were performed with the SAS statistical package version 9.0 (Statistical Analysis System, 2002). The relationship between tree-size measurements, slope and tree exposure with morphological characteristics of acorns was analyzed using the Pearson's correlation coefficient.
Results
Morphological comparison of acorns among species
According to Table 2, the three species showed statistical differences (P < 0.05) in polar diameter, equatorial diameter, average diameter and total weight. Quercus deserticola had the highest values, while Q. sideroxyla had the lowest values.
Acorn shell weight showed no significant differences (P > 0.05) between Q. sideroxyla and Q. rugosa, but significant differences were found with respect to Q. deserticola, which had the highest value (Table 2). For this species, total weight of acorns was also significantly higher than in Q. rugosa and Q. sideroxyla.
Table 2 Morphological comparison of acorns among Quercus species.
| Species | Polar diameter (mm) | Equatorial diameter (mm) | Average diameter (mm) | Shell weight (g) | Total weight (g) |
|---|---|---|---|---|---|
| Q. sideroxyla | 13.01 ± 0.078 c | 11.44 ± 0.067 c | 12.23 ± 0.058 c | 0.30 ± 0.009 b | 0.85 ± 0.015 c |
| Q. rugosa | 16.01 ± 0.139 b | 12.82 ± 0.111 b | 14.41 ± 0.112 b | 0.39 ± 0.009 b | 1.38 ± 0.035 b |
| Q. deserticola | 16.74 ± 0.110 a | 14.63 ± 0.093 a | 15.69 ± 0.083 a | 0.56 ± 0.049 a | 1.94 ± 0.040 a |
± standard error of the mean (n = 300). Means with different letters for the same variable are statistically different between species according to the Tukey's test (P < 0.05).
Morphological comparison of acorns between trees of the same species
Quercus sideroxyla
Table 3 shows that acorn morphology varied among individuals of the same species. Average diameter of acorns ranged from 10.54 to 13.05 mm; trees 1, 4, 5 and 7 recorded the highest values. Shell dry weight was higher for trees 7 and 10. Trees with larger mean diameter produced large acorns, while tree 3 provided the smallest acorns.
Table 3 Morphological comparison of acorns among Quercus sideroxyla trees.
| Tree | Polar diameter (mm) | Equatorial diameter (mm) | Average diameter (mm) | Shell weight (g) | Total weight (g) |
|---|---|---|---|---|---|
| 1 | 14.42 ± 0.184 a | 11.69 ± 0.139 b | 13.05 ± 0.139 a | 0.34 ± 0.017 c | 0.98 ± 0.036 b |
| 2 | 13.36 ± 0.115 b | 11.04 ± 0.106 d | 12.20 ± 0.090 b | 0.27 ± 0.012 c | 0.66 ± 0.020 d |
| 3 | 11.56 ± 0.107 d | 9.52 ± 0.099 e | 10.54 ± 0.091 c | 0.15 ± 0.016 d | 0.51 ± 0.015 d |
| 4 | 13.16 ± 0.202 b | 12.44 ± 0.110 a | 12.80 ± 0.136 a | 0.34 ± 0.008 b | 1.00 ± 0.036 b |
| 5 | 14.46 ± 0.221 a | 11.47 ± 0.107 c | 12.96 ± 0.149 a | 0.33 ± 0.019 c | 0.94 ± 0.040 c |
| 6 | 13.63 ± 0.167 b | 10.47 ± 0.141 d | 12.05 ± 0.140 b | 0.20 ± 0.013 d | 0.85 ± 0.023 c |
| 7 | 12.77 ± 0.214 c | 12.44 ± 0.169 a | 12.61 ± 0.178 a | 0.38 ± 0.023 a | 1.11 ± 0.048 a |
| 8 | 12.79 ± 0.227 c | 11.40 ± 0.139 c | 12.09 ± 0.140 b | 0.28 ± 0.014 c | 0.88 ± 0.044 c |
| 9 | 12.28 ± 0.148 c | 11.17 ± 0.099 c | 11.72 ± 0.093 b | 0.26 ± 0.008 c | 0.65 ± 0.023 d |
| 10 | 11.71 ± 0.172 d | 12.78 ± 0.159 a | 12.24 ± 0.151 b | 0.44 ± 0.030 a | 0.97 ± 0.044 b |
± standard error of the mean (n = 30). Means with different letters for the same variable are statistically different according to the Tukey's test (P < 0.05).
Quercus rugosa
Acorns showed morphological variation among trees; the largest acorns, defined by polar and equatorial diameter, were found in trees 10 and 7. These same trees had acorns with the highest shell weight, and tree 10 had the acorns with the highest total weight, which excelled in all variables. In contrast, trees 2 and 9 produced the acorns with the lowest diameter and total dry weight (Table 4).
Table 4 Morphological comparison of acorns among Quercus rugosa trees.
| Tree | Polar diameter (mm) | Equatorial diameter (mm) | Average diameter (mm) | Shell weight (g) | Total weight (g) |
|---|---|---|---|---|---|
| 1 | 14.45 ± 0.224 c | 12.32 ± 0.122 c | 13.38 ± 0.150 d | 0.37 ± 0.022 c | 1.07 ± 0.042 d |
| 2 | 13.86 ± 0.204 d | 10.97 ± 0.140 e | 12.41 ± 0.132 e | 0.35 ± 0.012 c | 0.66 ± 0.026 e |
| 3 | 16.62 ± 0.545 b | 14.54 ± 0.299 a | 15.58 ± 0.401 b | 0.41 ± 0.022 b | 1.70 ± 0.114 b |
| 4 | 15.88 ± 0.255 b | 13.15 ± 0.216 c | 14.51 ± 0.194 c | 0.39 ± 0.020 c | 1.37 ± 0.057 c |
| 5 | 16.41 ± 0.226 b | 14.21 ± 0.181 b | 15.31 ± 0.177 b | 0.39 ± 0.013 c | 1.82 ± 0.056 b |
| 6 | 12.96 ± 0.195 d | 12.18 ± 0.110 d | 12.57 ± 0.117 d | 0.31 ± 0.009 d | 0.97 ± 0.031 d |
| 7 | 18.66 ± 0.261 a | 14.22 ± 0.204 b | 16.44 ± 0.215 a | 0.50 ± 0.031 a | 1.98 ± 0.074 b |
| 8 | 16.84 ± 0.248 b | 10.74 ± 0.174 e | 13.79 ± 0.134 c | 0.39 ± 0.020 c | 1.05 ± 0.038 d |
| 9 | 15.21 ± 0.232 c | 10.51 ± 0.163 e | 12.86 ± 0.167 d | 0.24 ± 0.009 d | 0.87 ± 0.036 e |
| 10 | 19.23 ± 0.247 a | 15.33 ± 0.213 a | 17.28 ± 0.186 a | 0.52 ± 0.019 a | 2.26 ± 0.085 a |
± standard error of the mean (n = 30). Means with different letters for the same variable are statistically different according to the Tukey's test (P < 0.05).
Quercus deserticola
Table 5 indicates that the morphological variables had statistical differences (P < 0.05) except for shell weight. Trees 7 and 10 had acorns with higher diameter, being the largest and heaviest; on the other hand, tree 2 had the smallest average diameter and is part of the group of trees with acorns with lower total weight.
Table 5 Morphological comparison of acorns among Quercus deserticola trees.
| Tree | Polar diameter (mm) | Equatorial diameter (mm) | Average diameter (mm) | Shell weight (g) | Total weight (g) |
|---|---|---|---|---|---|
| 1 | 15.69 ± 0.277 d | 14.32 ± 0.216 c | 15.01 ± 0.195 d | 0.51 ± 0.035 a | 1.66 ± 0.077 e |
| 2 | 15.80 ± 0.289 d | 13.34 ± 0.266 e | 14.57 ± 0.196 e | 0.89 ± 0.048 a | 1.39 ± 0.060 e |
| 3 | 15.77 ± 0.270 d | 14.76 ± 0.170 c | 15.26 ± 0.175 c | 0.46 ± 0.037 a | 1.69 ± 0.073 e |
| 4 | 16.53 ± 0.307 c | 13.94 ± 0.220 d | 15.24 ± 0.197 c | 0.42 ± 0.022 a | 1.45 ± 0.066 e |
| 5 | 17.66 ± 0.422 b | 14.58 ± 0.274 c | 16.12 ± 0.318 b | 0.56 ± 0.018 a | 1.90 ± 0.070 c |
| 6 | 16.24 ± 0.318 d | 13.80 ± 0.193 d | 15.02 ± 0.221 d | 0.40 ± 0.012 a | 1.45 ± 0.077 e |
| 7 | 16.34 ± 0.324 c | 17.57 ± 0.244 a | 16.96 ± 0.254 a | 0.75 ± 0.057 a | 2.87 ± 0.107 a |
| 8 | 16.99 ± 0.299 c | 14.07 ± 0.178 d | 15.53 ± 0.221 c | 0.39 ± 0.031 a | 1.87 ± 0.080 d |
| 9 | 17.68 ± 0.328 b | 14.86 ± 0.217 b | 16.27 ± 0.232 b | 0.53 ± 0.027 a | 2.24 ± 0.098 b |
| 10 | 18.69 ± 0.214 a | 15.07 ± 0.203 b | 16.88 ± 0.187 a | 0.67 ± 0.034 a | 2.85 ± 0.119 a |
± standard error of the mean (n = 30). Means with different letters for the same variable are statistically different according to the Tukey's test (P < 0.05).
Correlation between acorn weight and tree characteristics
Based on Figure 1, in the three oak species, the Pearson's correlation showed a higher relationship of acorn weight with crown diameter and diameter at breast height, while the relationship with slope and tree exposure was lower.
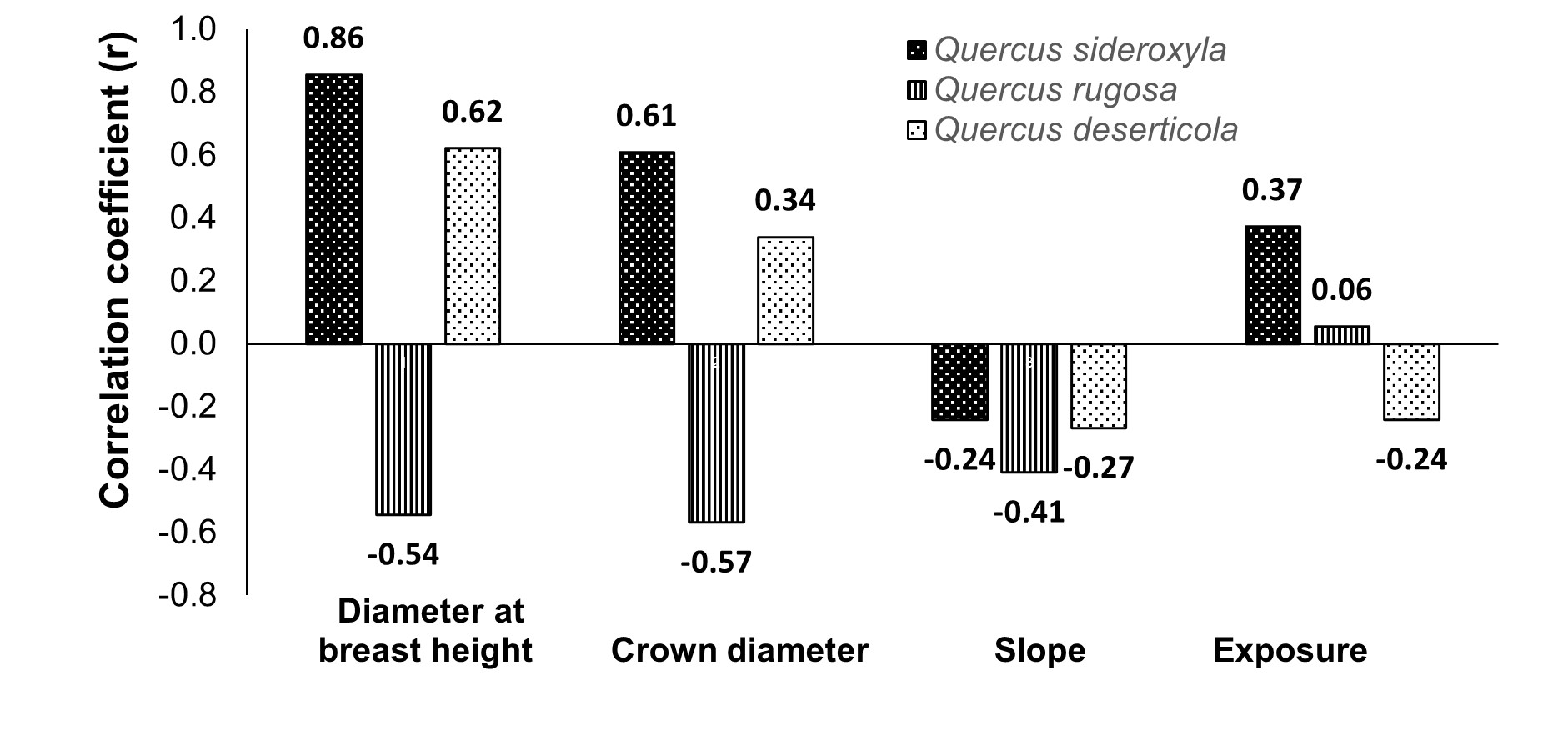
Figure 1 Pearson correlation coefficients (r) between acorn weight and tree-size measurements, slope and tree exposure.
Figures 2, 3 and 4 show intervals of the categories for diameter at breast height, crown diameter and slope on the horizontal axis, to graphically represent the development of acorn weight. The highest weights of Q. rugosa and Q. deserticola were found in crown diameters lower than 11 m, while Q. sideroxyla was distributed similarly in all categories unlike Q. deserticola (Figure 2).
As for diameter at breast height, the highest weights of Q. rugosa were found in diameters lower than 50 cm, while for Q. deserticola this occurred in higher diameters; Q. sideroxyla distribution in diameters smaller than 30 cm was null (Figure 3). Regarding slope, the highest weights of Q. rugosa were recorded in trees growing on slopes lower than 16 %, while in Q. deserticola on slopes lower than 10 % (Figure 4). Regarding exposure, the three oak species had the highest weights in trees growing in the southeast (Figure 5).
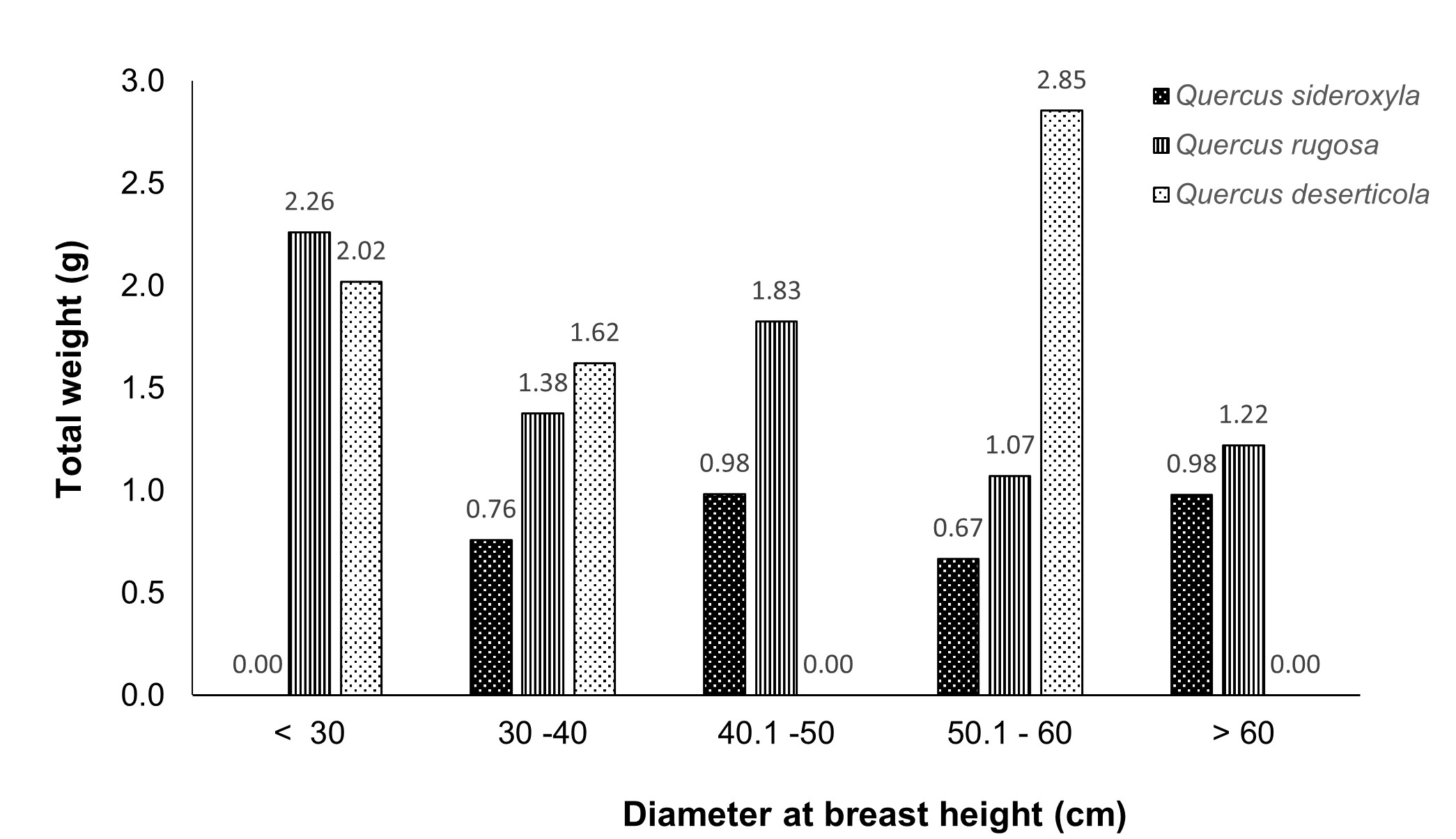
Figure 3 Distribution of weights among categories diameter at breast height of the three Quercus species studied.
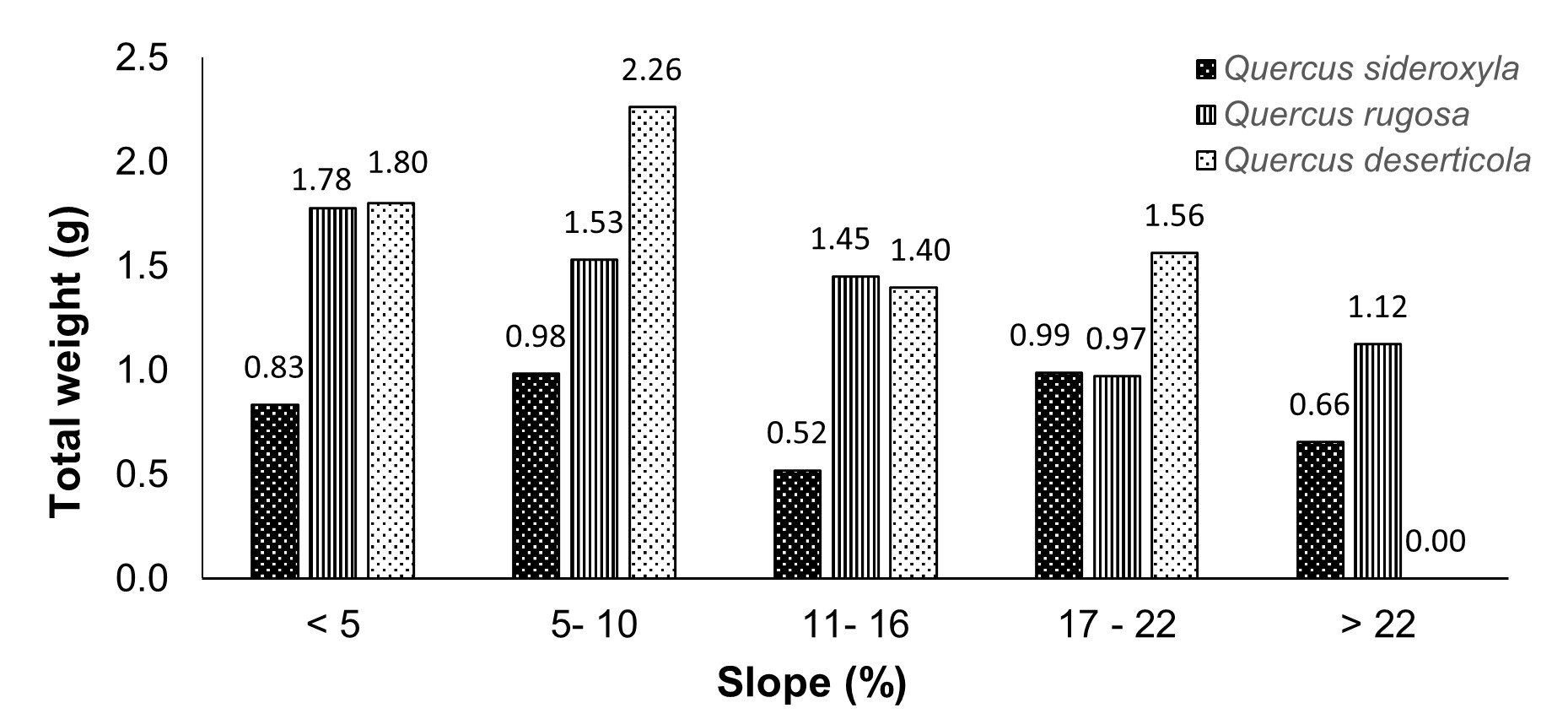
Figure 4 Distribution of seed weights of the species studied among slopes of collection sites in the state of Durango.
Physicochemical characteristics of mature and green acorns
Moisture and dry matter had no statistical differences (P > 0.05) among species, but the chemical variables ash, ethereal extract, crude fiber, crude protein and tannins had statistical differences among species for the two acorn maturity conditions (mature and green). Crude protein was similar among species (P > 0.05) for mature acorns.
Regarding the differences between mature and green acorns of the same species, it was found that there were no differences in moisture, dry matter, crude fiber and tannins for the three species under study. For the rest of the variables, Q. rugosa showed differences in ash and ethereal extract, Q. sideroxyla in crude protein and Q. deserticola in ash (Table 6).
Table 6 Physicochemical comparison of acorns in Quercus species.
| Physicochemical variables | Q. sideroxyla | Q. rugosa | Q. deserticola | |||
|---|---|---|---|---|---|---|
| Mature | Green | Mature | Green | Mature | Green | |
| Moisture (%) | 3.09 ± 0.24 A-a | 2.99 ± 0.47 A-a | 3.95 ± 0.38 A-a | 4.33 ± 0.51 A-a | 3.99 ± 0.26 A-a | 4.26 ± 1.09 A-a |
| Dry matter (%) | 96.92 ± 0.24 A-a | 97.01 ± 0.47 A-a | 96.05 ± 0.38 A-a | 95.67 ± 0.51 A-a | 96.01 ± 0.26 A-a | 95.74 ± 1.09 A-a |
| Ash (%) | 2.29 ± 0.12 A-a | 2.14 ± 0.14 C-a | 1.93 ± 0.10 B-b | 3.73 ± 0.12 A-a | 2.26 ± 0.05 A-b | 2.64 ± 0.11 B-a |
| Ether extract (%) | 8.88 ± 1.01 A-a | 9.68 ± 1.04 A-a | 4.65 ± 1.29 B-a | 2.01 ± 0.49 B-b | 2.72 ± 0.31 B-a | 2.46 ± 0.22 B-a |
| Crude fiber (%) | 26.38 ± 1.01 A-a | 25.82 ± 0.93 A-a | 25.49 ± 3.41 A-a | 25.41 ± 2.24 A-a | 15.50 ± 0.53 B-a | 14.33 ± 0.69 B-a |
| Crude protein (%) | 8.40 ± 0.37 A-a | 6.47 ± 0.04 B-b | 6.94 ± 0.48 A-a | 7.85 ± 0.70 A-a | 7.73 ± 0.40 A-a | 7.33 ± 0.26 A-a |
| Tannins (%) | 0.30 ± 0.04 B-a | 0.67 ± 0.33 B-a | 1.28 ± 0.23 A-a | 1.84 ± 0.54 A-a | 0.94 ± 0.04 A-a | 0.81 ± 0.05 A-a |
± standard error of the mean (n = 300). Means with different letters for the same variable are statistically different according to the Tukey's test (P < 0.05); capital letters indicate difference between species, small letters between degree of maturity of the same species.
Discussion
The size of oak acorns is strongly related to precipitation, altitude and temperature (Koenig & Knops, 2013; Pesendorfer et al., 2014). On the other hand, the genetic condition of the species has a strong influence on acorn morphology (Shi, Villar-Salvador, Li, & Jiang, 2019); the above agrees with this research, because significant differences in the morphology of acorns of each species were found, despite inhabiting sites with similar characteristics. Another determinant factor may be the subgenus of the species studied, in this case, Q. rugosa and Q. deserticola belong to the subgenus Leucobalanus, commonly known as white oaks, while Q. sideroxyla belongs to the subgenus Erythrobalanus of the red oak group (de la Paz-Pérez & Dávalos-Sotelo, 2008). In this regard, Rubio-Licona et al. (2011) found that white oaks tend to generate larger acorns compared to red oaks, which coincides with that found in this research, since Q. rugosa and Q. deserticola had larger acorns than Q. sideroxyla.
Q. sideroxyla showed significant differences in morphology of acorns of its individuals (Table 3). The size was negatively influenced by the slope of the land, since the trees located on land with slopes lower than 10 % produced larger acorns (Figure 4). This can be attributed to the greater amount of organic matter on the soil, as opposed to the slopes that have soils with less organic matter (Rodríguez-Estévez, García-Martínez, Mata-Moreno, Perea-Muñoz, & Gómez-Castro, 2008).
Q. rugosa also had variation in acorn morphology among individuals, which was related to crown diameter, diameter at breast height and slope (Table 4; Figure 1); the largest acorns were found in trees with crown diameters lower than 11 m (Figure 2), diameter at breast height lower than 50 cm (Figure 3) and slopes lower than 16 % (Figure 4). For crown diameter, Martiník, Dobrovolný, and Palátová (2014) mention that trees with larger crown size have more resources available for acorn production; however, although quantity increases, size decreases. For tree slope and diameter, results coincide with those showed by Alejano, Vázquez-Piqué, Carevic, and Fernández (2011).
Q. deserticola also showed significant differences among acorns of trees studied (Table 5). There is a relationship between acorn weight and diameter at breast height, crown diameter, slope and exposure (Figure 1); the largest acorns were found in trees with crown diameter lower than 11 m (Figure 2), with diameter at breast height between 50 and 60 cm (Figure 3), slopes lower than 10 % (Figure 4) and grown in southeast exposure (Figure 5).
With the information mentioned above, it can be said that the weight of acorns in trees of the same species is influenced by the crown diameter of the tree (Pourhashemi, Dey, Mehdifar, Panahi, & Zandebasiri, 2018), since trees with small crowns produce large acorns, as they have the resources available; González and Parrado (2010) attributed these differences to the properties of soils where species grow. Another factor is the exposure of acorns; Alejano et al. (2011) indicate that acorns are larger in southern exposure because solar radiation is almost six times greater than in the northern exposure, which causes greater moisture loss and forces the tree to produce larger acorns to ensure regeneration (Mazzola, Kin, Morici, Bainec, & Tamborini, 2008).
The physical variables of moisture and dry matter showed no significant differences between degree of maturity and species. For chemical composition, species had differences in the amount of ash, ethereal extract, fiber, crude tannins and protein, which coincides with that reported by Rababah et al. (2008). On the other hand, degree of maturity influenced ash and ethereal extract content in Q. rugosa, ash content in Q. deserticola and crude protein in Q. sideroxyla. For the latter, variation is attributed to the fact that as acorn matures, protein levels decline and carbohydrate content increases (Belghith et al., 2015). In the case of green acorns, no research was found where values were reported that would make it possible to compare them with the results obtained here.
Mature acorns of Q. rugosa had the highest tannin content (1.28 %) including pulp and shell, with a value close to that of Q. ilex L. (1.4 %), which is the species most used in swine feed (Rodríguez-Estévez et al., 2008). In general, the tannin content of the three species is lower than in European oaks (Kamalak et al., 2015), so the Mexican species can be considered as livestock feed, because the low levels prevent the protein from degrading rapidly in the rumen.
Acorns of Q. rugosa and Q. deserticola had fat content of less than 5 %, which is in agreement with that reported by Akcan, Gökçe, Asensio, Estévez, and Morcuende (2017) for Q. ithaburensis Decne (1.27 %), Rababah et al. (2008) for Q. calliprinos Webb (2.71 %) and Kamalak et al. (2015) on Q. coccifera L. (4.50 %). Quercus sideroxyla had 8.88 % which was lower than that reported by Valero-Galván et al. (2011) for Q. ilex subsp. ballota Samp. (11.34 %); these differences may be attributed to genetic factors and environmental conditions of the site.
As for ash content, Q. rugosa (1.93 %), Q. deserticola (2.26 %) and Q. sideroxyla (2.29 %) had values similar to those reported by Rababah et al. (2008) for Q. calliprinos (1.91 %) and by Kamalak et al. (2015) for Q. coccifera L. (2.36 %); lower than those reported by Kilic, Boga, and Guven (2010) for Quercus robur L. (3.2 %); and higher than those obtained by Valero-Galván et al. (2011) for Q. ilex subsp. ballota (1.73 %). In these studies, differences between species are attributed to growth conditions, genotype, acorn maturity and environmental effects.
The percentage of acorn protein in the evaluated species exceed 4.62 % for Q. ilex subsp. ballota (Valero-Galván, et al., 2011), 2.35 % for Q. ithaburensis, 3.46 % for Q. calliprinos (Rababah et al., 2008) and 4.23 % for Q. coccifera (Kamalak et al., 2015). The difference can be attributed to the species and growing conditions.
Conclusions
Significant differences were found in the morphology of acorns of each species, even though they inhabit sites with similar characteristics; Q. deserticola produced acorns with the largest diameter and weight. Within species, acorns had morphological differences among trees, which were associated with diameter at breast height and crown diameter. Moisture content, dry matter, crude fiber and tannins were similar between mature and green acorns. In contrast, mature acorns had higher contents of ethereal extract and protein, and lower ash content. This study shows that Mexican oak acorns can be used as a feed supplement for livestock and, in the future, for pharmaceutical purposes, which would provide added value to this non-timber product that currently has no reported use. It is recommended to continue with research to determine micronutrients, macronutrients and antioxidants to contribute to the knowledge of the chemical composition of Mexican acorns.











 texto en
texto en 




