Tropical dry deciduous forests are characterized by seasonal drought and high photon flux densities (PFD), which are periodical hazards for many plant species. Moreover, environments in these dry forests are also characterized by high temperatures and elevated vapor pressure deficits (VPD) during the dry season, which in northern Yucatan can last up to three months (Orellana et al. 1999, Mendoza et al. 2007). When the dry season extends, photosystem reaction centers can be damaged, and alterations in the photophosphorylation and function of several enzymes involved in carbon fixation may also occur (Triantaphylidès & Havaux 2009, Fernández-Marín et al. 2020). In addition, Crassulacean acid metabolism (CAM) can be advantageous to cope with such desiccating conditions (Winter & Smith 1996, Ricalde et al. 2010, de la Rosa-Manzano et al. 2015), given that the diurnal fixation of CO2 by ribulose bisphosphate carboxylase/oxygenase (Rubisco) occurs under closed stomata. Such conditions generate high CO2 concentrations in the cytosol and chloroplasts, favoring Rubisco’s carboxylation activity over its oxygenase activity, providing additional photoprotection through maintenance of electron transport and preventing damage to photosystems (Niewiadomska & Borland 2008). Usually, excess photons are quickly dissipated as heat (Niyogi 2000, Yamori & Shikanai 2016), but photoinhibition occurs when the absorption of light energy exceeds the capacity for photosynthesis and the photoprotection mechanisms have been oversaturated (Takahashi & Murata 2008, Takahashi & Badger 2011, Nishiyama & Murata 2014, Adams et al. 2018).
Photoinhibition has been defined as a decrease in the photosynthetic efficiency that depends on the excess of light and leads to a partial loss of capacity to convert light into sugars and biomass allocation and, consequently, into growth (Long et al. 1994, Baker 2008, Blankenship 2014, Vialet-Chabrand et al. 2017). Because photoinhibition lowers productivity and plant growth, its avoidance is decisive for plant growth under variable environmental conditions (Long et al. 1994, Adams et al. 2008, Lawson et al. 2012, Evans 2013). Mechanisms of acclimation to cope with high sunlight can be particularly important for survival in the dry tropical forests of Yucatan, Mexico, where plants can receive up to nine times more PFD in the dry season, compared to the rainy season, and where the rain events during the dry season can be separated by more than 30 d (Graham & Andrade2004, González-Salvatierra et al. 2010).
Epiphytic species in the Bromeliaceae family show a wide range of strategies of light use, from those adapted to exposed sites, showing high light saturation points and low chlorophyll concentrations, to those adapted to the shade, with low values of light saturation and exhibiting photodegradation and photoinhibition when subjected to high light (Griffiths & Maxwell 1999, Benzing 2000, Hou-Sung & Niyogi 2008). These physiological adaptations are related to the forest type and to vertical gradients within the canopy (Smith et al. 1986, Griffiths & Maxwell 1999, Benzing 2000, Cach-Pérez et al. 2013, Cervantes et al. 2005, Keller & Lüttge 2005, Petter et al. 2016, Silvera & Lasso 2016).
Epiphytes have been postulated as species particularly vulnerable to prolonged droughts, given their strong coupling to the frequency of rain events, and their lack of access to water stored in the ground (Benzing 1998, de la Rosa-Manzano et al. 2014, Reyes-García & Griffiths 2009, Reyes-García et al. 2012, Zotz & Bader 2009). Moreover, epiphytic bromeliads show a low root to shoot ratio and the absorption of water and nutrients is primarily made by foliar trichomes (Benzing 2000, Zotz 2016); and, occasionally, they may rely on alternative sources of water other than rain, such as fog and dew, especially during part of the dry season (Andrade 2003, Guevara-Escobar et al. 2011, Reyes-García et al. 2012, Chávez-Sahagún et al. 2019). The aim of this study was to investigate the capacity for photosynthetic acclimation, under seasonal light micro-environment, for the CAM epiphytic bromeliad Tillandsia brachycaulos Schltdl., by measuring seasonal changes in chlorophyll fluorescence, nocturnal accumulation of tissue acidity, and water potentials, to characterize its responses to high light in the field. We expected that leaf tissues of T. brachycaulos would exhibit changes in photosynthetic parameters accordingly to the wide environmental changes that occur in its natural habitat, and these changes would be greater in the exposed microhabitats. This species grows in many forests within the Yucatan Peninsula with high density populations (Cach-Pérez et al. 2013). Furthermore, although T. brachycaulos shows lower morphological variation than other epiphytic bromeliads in the Yucatan Peninsula (Cach-Pérez et al. 2016), some studies reveal a high physiological plasticity in this species (Graham & Andrade 2004, Cervantes et al. 2005, González-Salvatierra et al. 2010, Cach-Pérez et al. 2018, Hernández-Robinson et al. 2020).
Materials and methods
Plant species and study site. Tillandsia brachycaulos Schltdl. is an atmospheric epiphyte found in tropical forests from southern Mexico through Central America to Venezuela (Ramírez et al. 2005). Within the Yucatan Peninsula, this species may be found in most types of forests, although it is most abundant in tropical dry deciduous forests (Olmsted & Gómez-Juárez 1996, Cach-Pérez et al. 2013). Tillandsia brachycaulos is an obligate CAM species (Graham & Andrade 2004).
The study was conducted at the Dzibilchaltún National Park (21° 05´ N, 89° 99´ W, 10 m asl), state of Yucatan, Mexico, whose vegetation is characterized as tropical dry deciduous forest with a maximum canopy height of 8 m (Thien et al. 1982, Mondragón et al. 2004, Valdez-Hernández et al. 2010). Mean annual precipitation is 700 mm and average annual temperature is 25.8 ºC (Orellana et al. 1999). The rainy season is between June and October and the dry season from March to May (Orellana et al. 1999), during which most of the trees are leafless (ca. 70 %; Mondragón et al. 2004, Valdez-Hernández et al. 2010).
Environment and micro-environment characterization. Environmental variables were measured using data collected with a meteorological station at the Dzibilchaltún National Park. Photon flux density (PFD) was measured with a quantum sensor (LI-190SB, Li-Cor, Inc., Lincoln, Nebraska), precipitation with a pluviometer (TR 525M, Texas Electronics, Inc., Dallas, Texas), and air temperature and relative humidity with a Vaisala shielded probe (HMP35C-L, Campbell Scientific, Logan, Utah). All variables were sampled at 15-s intervals and average values were recorded every 10 min with a datalogger (CR21X, Campbell Scientific).
The incident PFD was measured at 20 mm above the individual plants, with a quantum sensor (LI250-A, Li-Cor) in the rainy and dry seasons over three shaded and three exposed individuals; all individual epiphytes were between 1.8 - 2 m height, where the species is more abundant (Cach-Pérez et al. 2013). All measurements were taken every 3 h during the day on the first week of every month from August 2008 to May 2009. Both PFD above the canopy and on individual plants were integrated for each day during the season and an average was calculated.
Water potential and tissue acidity. Leaves of plants from both light microenvironments were collected during the dry and rainy seasons. To measure water potential (Ψ), leaf samples (n = 6 plants) were collected at predawn and at midday, stored at 4 ºC and transported to the laboratory (it took less than an hour from the field to the laboratory), where Ψ was measured using a chilled-mirror dewpoint meter (WP4, Decagon Devices, Inc. Washington). Samples were cut into square pieces, to cover the base of the sampling cups.
To characterize CAM, leaves from plants at both light micro-environments (n = 5 plants) were collected at dusk and before dawn the following day at each sampling season with a cork borer (1.54 cm2 leaf area). Plant material was cut and kept in a solution of 70% ethanol in 1.5-mL vials until laboratory analysis. The extraction was made by boiling, to remove the ethanol, macerating the plant material, and boiling in 10 mL of distilled water for 15 min; then 50 mL of distilled water was added, and the solution was titrated with 0.005 N NaOH to pH 7, using an electronic pH meter (Oakton® pH 510 series, Oakton Instruments Vernon Hills, Illinois, Zotz & Andrade 1998). Nocturnal acidification (ΔH+) was calculated from the hydrogen ion concentration (H+) at dawn minus the H+ at dusk.
Pigment concentration and fluorescence measurements. To determine chlorophyll and carotenoid concentrations, six leaf samples were collected from three exposed and three shaded individuals, during each sampling season, and transported at 4 °C to the laboratory. Pigments were quantified according to Hendry & Price (1993) for chlorophylls and to Wellburn (1994) for carotenoids. Extractions were performed from 50-mg samples (fresh weight) that were macerated with 2 mL of 80 % (v/v) cold acetone. The absorbance of the extracts obtained was measured with a spectrophotometer (DU650, Beckman Coulter, Indianapolis, Indiana) at 645 nm and 663 nm for chlorophyll and at 470 nm for total carotenoids.
A portable pulse-amplitude-modulated photosynthesis yield analyzer (Mini-PAM, H. Walz, Effeltrich, Germany) was used to evaluate chlorophyll a fluorescence, maximum quantum efficiency (Fv/Fm, variable fluorescence/maximum fluorescence), and related parameters (non-photochemical efficiency [NPQ], the quantum yield of photosystem II [ΦPSII], and the electron transport rate [ETR]). Measurements were carried out during the rainy and dry seasons on six individuals (three shaded and three exposed). Fv/Fm was assessed before dawn (05:00-06:00), while ETR, NPQ, ΦPSII and PFD were conducted every 3 h during a day.
Light response curves (LRC) of ETR were determined for both exposed and shaded plants to determine the light saturation point in both seasons (Rascher et al. 2000). Plants were acclimated to the dark by covering them with a black clothing for 20 min. Light saturation pulses were applied; first, the maximal yield in the absence of actinic light (Fv⁄Fm) was measured, and then a series of eight consecutive yield-measurements at increasing light intensities were started, until a maximum of approximately 1,400 μmol m-2 s-1 was reached. ETR was calculated as ΦPSII × PFD × 0.5 × 0.84, where the standard factor 0.84 corresponds to the fraction of incident light absorbed by the photosynthetic tissue (Ritchie & Bunthawin 2010). Such a factor has been recently validated for two bromeliad species, with a 5 % variation (Stemke & Santiago 2011). The light saturation point was calculated with a nonlinear curve fit.
Statistical analysis. Differences in environmental PFD and VPD between seasons were tested using a Student’s t-test. Differences in water potential, tissue acidity and pigment concentration between seasons, and light levels were tested using two-way ANOVAs. Differences in maximum diurnal fluorescence parameters were also tested with a two-way ANOVA. When significant differences occurred, a Tukey test was conducted. All tests were made with the STATISTICA statistical package version 7.0 (Statsoft, Dell, Round Rock, Texas). The light saturation point was calculated in OriginPro 8 SRO (V 8.0724, OriginLab Corporation 1991-2007) with a nonlinear curve fit, where datasets fit converged with an allometric model (equation y = axb).
Results
Environment and light micro-environments. During the dry season (April-May 2009), the mean daily photon flux density (PFD) above the canopy for clear days (47.1 ± 1.23 mol m-2 d-1) was not different than for clear days of the rainy season (August-September 2008; 45.8 ± 0.96 mol m-2 d-1; P > 0.05). The mean of the maximum vapor pressure deficit was 3.48 ± 0.139 kPa and 2.65 ± 0.111 kPa for the dry and rainy season, respectively; and it was significantly different between seasons (Figure 1, P < 0.005).
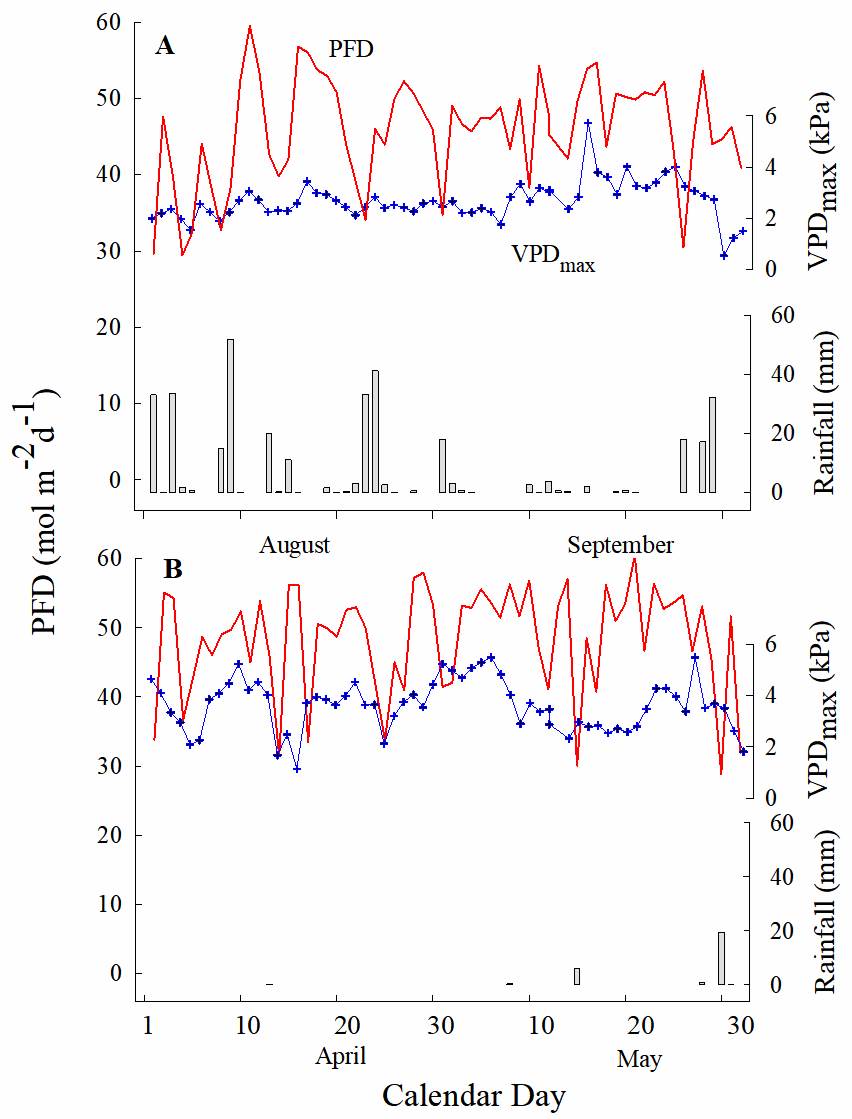
Figure 1 Monthly profile of daily photon flux density (PFD; line) above the canopy, maximum vapor pressure deficit (VPDmax; cross and line) and rainfall (bars) in Dzibilchaltún, Yucatan, Mexico, A. rainy (August-September 2008) and B. dry (April-May 2009) seasons.
The percentage of daily PFD incident on shaded or exposed plants of T. brachycaulos during the rainy season was 5 and 70 %, respectively, increasing to 38 and 95 %, respectively, during the dry season (Table 1), when most trees shed their leaves in this forest. Incident PFD on individual plants was significantly different between seasons and between habitats (P < 0.05). The shaded individuals received an average of 13 mol m-2 d-1 more PFD in the dry season than in the rainy season.
Table 1 Daily incident photon flux density (PFD), total chlorophyll and carotenoids concentrations, maximum photochemical efficiency (Fv/Fm) for exposed and shaded plants of Tillandsia brachycaulos during the rainy and dry seasons in the tropical dry deciduous forest of Dzibilchaltún, Yucatan. Data are means ± S.E. (n = 21 for PFD, n = 3-5 for total chlorophyll and carotenoids concentrations and for Fv/Fm; fw = fresh weight). Different letters indicate significant differences between season and exposure (P < 0.05, Tukey`s test).
| Rainy season | Dry season | |||
|---|---|---|---|---|
| Exposed | Shaded | Exposed | Shaded | |
|
PFD (mol m-2 d-1) |
32.1b ± 4.82 | 2.3d ± 0.14 | 37.9a ± 2.04 | 15.3c ± 1.28 |
|
Total chlorophyll (μg g-1 fw) |
217.1bc ± 53.6 | 471.5a ± 77.7 | 116.7c ± 27.1 | 321.8ab ± 28.8 |
|
Carotenoids (μg g-1 fw) |
27.0c ± 3.6 | 31.3bc ± 2.8 | 55.3b ± 5 | 116.9a ± 13.3 |
| Fv/Fm | 0.70ab ± 0.06 | 0.80a ± 0.02 | 0.57c ± 0.02 | 0.67ab ± 0.05 |
Water potential and titratable acidity. Lower values (i.e., more negative) of predawn leaf water potential (Ψ) were observed for both, exposed and shaded plants of T. brachycaulos, during the dry season than during the rainy season (Figure 2, P < 0.013). During the rainy season, the values of predawn Ψ were significantly higher than at midday for both exposed and shaded plants (P < 0.05). Also, nocturnal acidification (ΔH+) in leaves was lower during the dry season than during the rainy season (P < 0.05), but no differences were found between exposed and shaded plants (Figure 3).
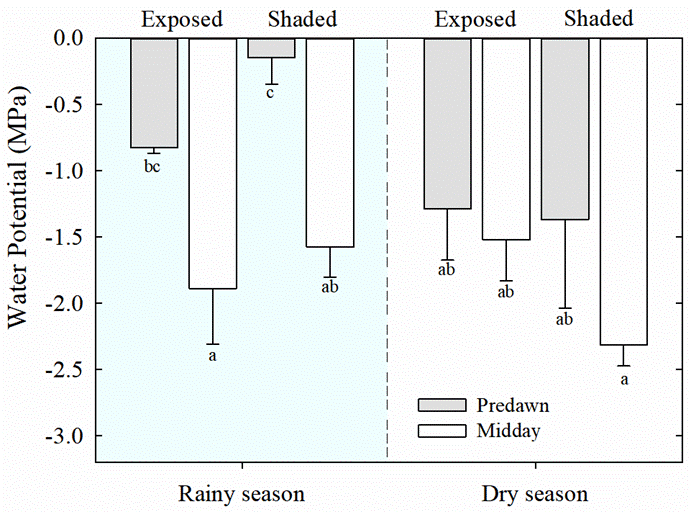
Figure 2 Predawn (gray bars) and midday (white bars) leaf water potentials (Ψ) of exposed and shaded plants during rainy and dry seasons, in the tropical dry deciduous forest of Dzibilchaltún, Yucatan, Mexico. Data are mean ± S.E. (n = 3-6). Different letters indicate significant differences between predawn and midday, and between seasons (P < 0.05, Tukey`s test).
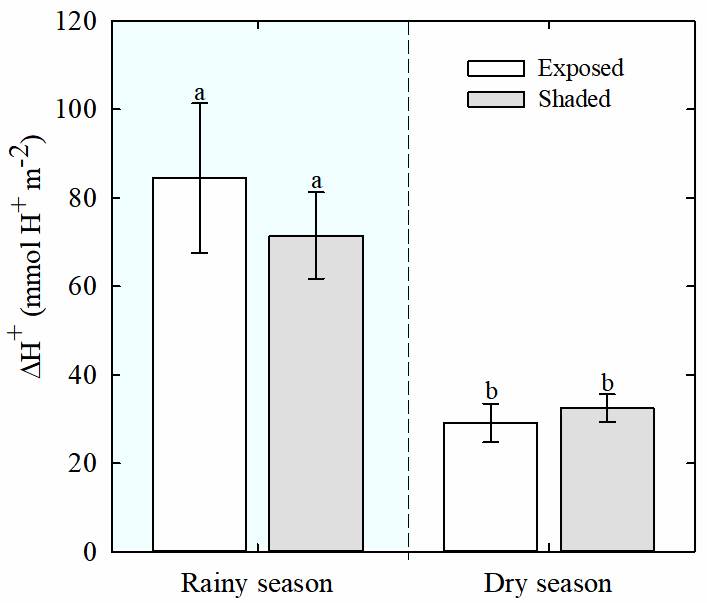
Figure 3 Tissue acidity (ΔH+; hydrogen ion concentration (H +) at dawn minus H+ at dusk) of Tillandsia brachycaulos for exposed (white bars) and shaded (gray bars) plants, during dry and rainy seasons, in the tropical dry deciduous forest of Dzibilchaltún, Yucatan, Mexico. Data are mean ± S.E. (n = 5). Different letters indicate significant differences between exposed and shaded plants (P < 0.05, Tukey`s test).
Pigment concentration and fluorescence parameters. A higher chlorophyll concentration was found for shaded plants compared with exposed plants, and the lowest values were observed for exposed plants during the dry season (Table 1, P < 0.05). Carotenoid concentration was different between seasons (P < 0.05) and between light microenvironments (P < 0.05). Shaded plants showed about 3.5 times greater carotenoids concentration during the dry season than during the rainy season, while exposed plants also showed a smaller, but significant increase during the dry season.
During the dry season, maximum quantum efficiency (Fv/Fm) values decreased in both shaded and exposed plants compared to the Fv/Fm values of the rainy season (Table 1, P < 0.05). The lowest mean Fv/Fm value (0.57) was recorded in leaves of exposed plants during the dry season, and the highest value (0.80) was found in leaves of shaded plants during the rainy season.
Quantum yield of photosystem II (ΦPSII) values showed significant differences between shaded and exposed plants (P < 0.001) and between seasons (Figure 4A and F; P < 0.003). During the dry season, ΦPSII values declined to very low levels upon exposure to high PFD and non-photochemical quenching (NPQ) was consistently low during the rainy and dry seasons in shaded plants. Particularly, during the rainy season, exposed plants showed that NPQ increased markedly early in the morning, declining to low levels for the rest of the day (Figure 4G and H). Electron transport rate (ETR) values were high during the dry season (Figure 4I and J), increasing at midday and ΦPSII values decreased (Figure 4E-F) with increasing PFD, with low NPQ values in both exposed and shaded plants (Figure 4G-H).
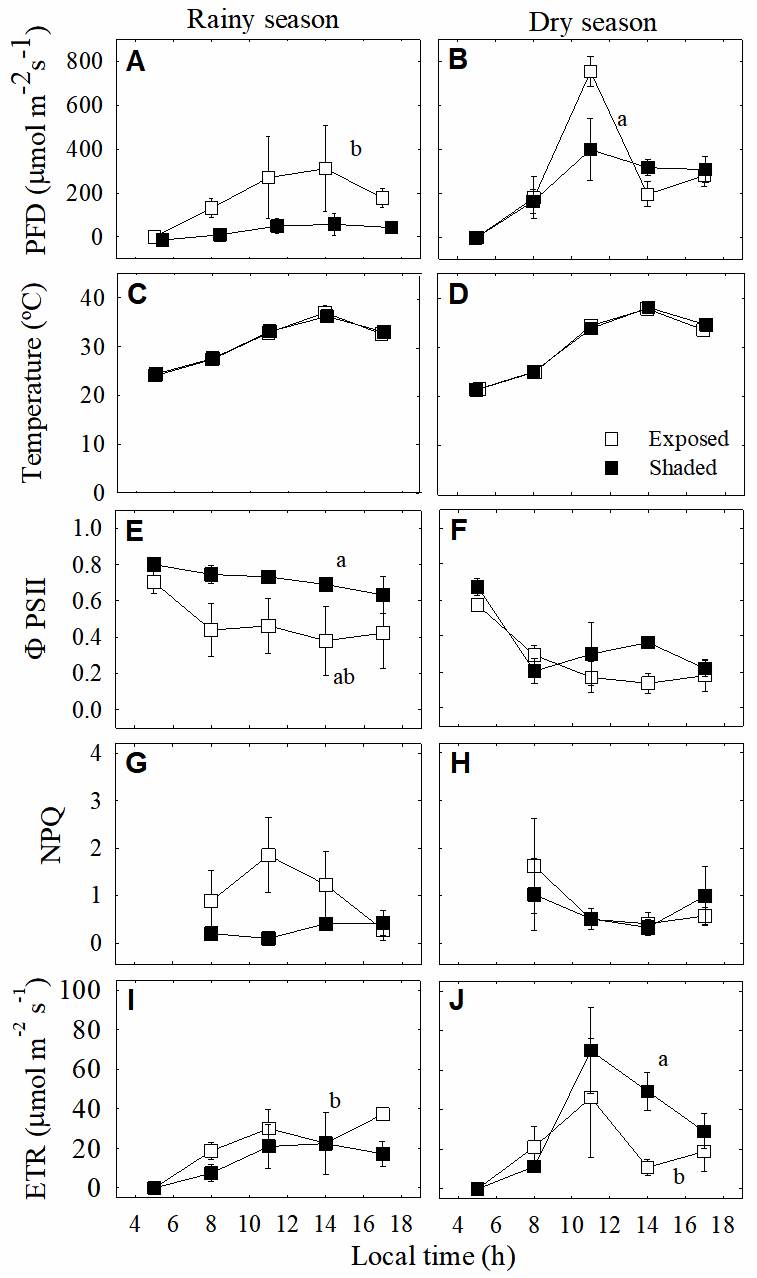
Figure. 4 A, B. Daily course of instantaneous photon flux density (PFD) and C, D. temperature in exposed (open squares) and shaded (closed squares) plants of the epiphytic bromeliad Tillandsia brachycaulos, and daily course measurements of chlorophyll fluorescence parameters: E, F. photochemical efficiency of PSII (ΦPSII), G, H. non-photochemical quenching (NPQ) and I, J. electron transport rate (ETR) during the rainy (left panels) and dry (right panels) seasons in the dry deciduous forest of Dzibilchaltún, Mexico. Values are mean ± S.E. (n = 3). Different letters indicate significant differences between seasons and microenvironments (P < 0.05, Tukey`s test).
The light saturation point was significantly different between seasons (P < 0.05), but no differences were observed between the leaves of exposed and shaded plants (Figure 5); during the rainy season, the light saturation point for leaves of both exposed and shaded plants was 800 μmol m-2 s-1 of PFD (Figure 5Aa); however, during the dry season, the maximum light saturation point decreased to less than 465 μmol m-2 s-1 (Figure 5Bb).
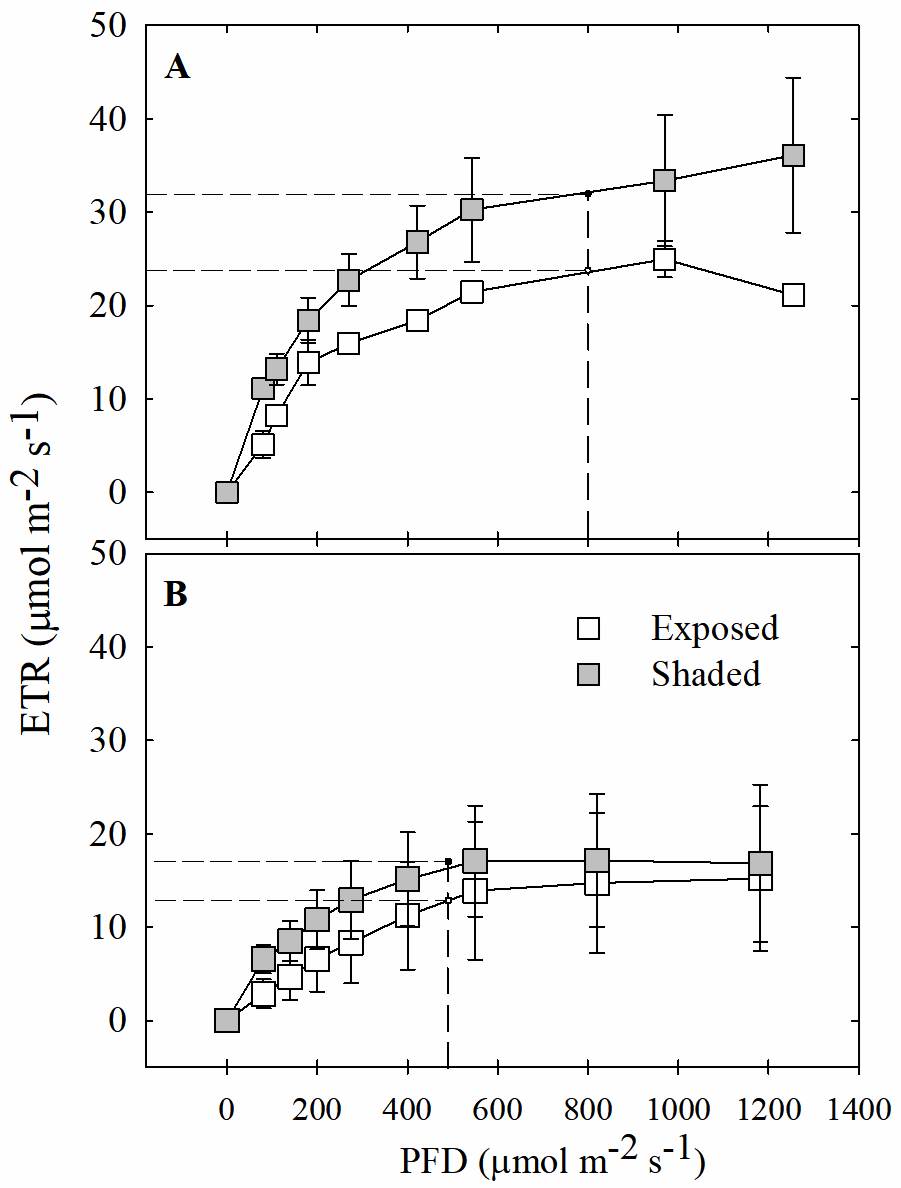
Figure 5 Light response curves (LRC) of photosynthetic electron transport rate (ETR) for exposed and shaded plants of Tillandsia brachycaulos. The curve shows the LRC of exposed (white circles) and shaded (black circles) plants during rainy A and dry B seasons. Values are mean ± S.E. (n = 3). Dashed line corresponds to the light saturation point calculated with a nonlinear curve fit.
Discussion
The leaf tissues of the epiphyte Tillandsia brachycaulos showed unusual water relations during the rainy season; a high daily oscillation in leaves water potential (Ψ) and low midday Ψ values were observed (Figure 2). It has been established that the nocturnal accumulation of malic acid in CAM plants decreases the leaf Ψ in the morning (as it decreases osmotic potential) and increases it during the day because malate decarboxylation occurs under closed stomata (Smith et al. 1986, Males & Griffiths 2017, Pereira & Cushman 2019). During the dry season, Ψ values were not significantly different between predawn and midday, but the predawn Ψ values were lower than during the rainy season. Although during the dry season acid accumulation is low, reduction of osmotic potentials would be by production of other organic solutes rather than malic acid. Actually, this decrease in osmotic potentials occurs during the early dry season in preparation for the extreme dry season where osmotic potentials are the lowest (Hernández-Robinson et al. 2020). Furthermore, during the rainy season leaf temperature for individuals of this species is lower than that of the air, indicating stomatal opening during the day (Andrade 2003, Hernández-Robinson et al. 2020). In fact, well-watered T. brachycaulos plants fix as much as 20 % of their total CO2 via rubisco mainly during phase IV of CAM (Graham & Andrade 2004).
Nocturnal changes in tissue acidity during the dry season were small for leaves of T. brachycaulos compared to the rainy season, regardless of light exposure. This also occurs for terrestrial CAM plants from shaded microhabitats in tropical deciduous forests since they show a greater nocturnal accumulation of tissue acidity when growing in exposed sites, owing to their massive leaves and stems (Nobel 2003, Ricalde et al. 2010). Moreover, the observed reduction of tissue acidity during the dry season is more likely to occur because of higher nocturnal temperatures than during the rainy season (Nobel et al. 1991, Cervera et al. 2007, Andrade et al. 2009). Additionally, increased light incidence without a proportional increase in the malate supply would predispose this epiphytic species to photoinhibition, which, along with drought stress, would increase the negative effects of the high radiation (Skillman & Winter 1997, van Tongerlo et al. 2021). In fact, studies show that stress caused by drought or extreme temperatures increases the risk and severity of photoinhibition in plants in arid regions and in tropical epiphytes (Athar & Ashraf 2005, Hasanuzzaman et al. 2013, Chaves et al. 2018, Arroyo-Pérez et al. 2017).
The chlorophyll content confirmed that the leaves of T. brachycaulos possess several characteristics typical of exposed and shaded-adapted plants depending on the microhabitat where these were sampled (Givnish 1988, Martin et al. 1999, Lambers & Oliveira 2019.), with a higher total chlorophyll concentration in leaves of shaded plants compared to leaves of exposed individuals, indicating modifications to the light harvesting apparatus, e.g., reduction in the number of photosynthetic reaction centers in exposed plants. Total chlorophyll values observed here were similar and comparable to those found for other epiphytic and terrestrial bromeliads (Griffiths & Maxwell 1999, Martin et al. 1999, Benzing 2000, Graham & Andrade 2004, Matsubara et al. 2009). During the dry season, leaf tissues of both exposed and shaded plants had reduced chlorophyll and increased carotenoid contents, a pigment combination that prevents excessive light absorption. Similarly, the leaves of some bromeliads and orchids growing under high-light conditions show an increase in carotenoid concentration, particularly zeaxanthin, which reduces oxidative damage by light saturation (Königer et al. 1995, Skillman & Winter 1997, Matsubara et al. 2009, de la Rosa-Manzano et al. 2015).
A previous study shows that leaves of exposed individuals of T. brachycaulos have an anthocyanin concentration that is four-fold higher than that of the sympatric terrestrial Bromelia karatas (González-Salvatierra et al. 2010). Additional mechanisms of photoprotection were found in the present study, such as low chlorophyll and high carotenoid concentrations during the dry season, together with a high NPQ. The low values of Fv/Fm observed during the dry season can represent a photo-inactivation or even photo-damage of PSII (Maxwell et al. 1992, Zotz & Winter 1994, Chow et al. 2005, Ritchie & Bunthawin 2010). This photo-inactivation rapidly reverses as the rainy season progresses and promotes survival of the species in its natural habitat (Xiong et al. 2000). Consequently, the drought stress observed, during the dry season, for T. brachycaulos plants was significant to assume that more prolonged droughts and lower frequency of rain events could be potentially dangerous for the epiphytic bromeliad populations, although Tillandsia species are highly plastic (Cach-Pérez et al. 2018, Rosado-Calderón et al. 2020).
During the rainy season, leaves of exposed plants of T. brachycaulos dissipated excess energy as heat, decreasing the likelihood of photoinhibition, as suggested by the high ΦPSII values measured in the early morning, producing photochemical and non-photochemical dissipation, and avoiding permanent damage to the photosynthetic apparatus (Demmig‐Adams & Adams 2006). The down-regulation of ΦPSII should mean an increase of NPQ (Nogués & Baker 2000). However, NPQ values in leaves of shaded plants during both seasons remained consistently low and did not show diurnal differences. During the dry season, an increase of ETR for both shaded and exposed plants, associated with increased light incidence, resulted in a ΦPSII inactivation process and, therefore, NPQ and ΦPSII remained low during the day, decreasing oxidative damage (Adams et al. 2013). Thus, the NPQ value refers to the mechanism used by plants to dissipate excess heat energy when subjected to high levels of environmental stress and is, therefore, a mechanism to avoid photoinhibition (Maxwell & Johnson 2000, Chow et al. 2005).
In conclusion, the epiphytic bromeliad Tillandsia brachycaulos, showed fast photoprotection responses and specialized physiological and morphological strategies related to their light tolerance and their ability to endure the dry season in this forest. Yet, despite presenting adaptations common to other epiphytic bromeliads to cope with drought and the large seasonal changes in light (Maxwell et al. 1992, 1994), this species has a reduced growth and reproduction in certain microhabitats, mainly due to leaf temperatures that are 2-5 ºC higher than the air during the afternoon and an apparently reduced water accessibility (Cervantes et al. 2005). Furthermore, in those hot microhabitats T. brachycaulos plants increase their leaf relative capacitance to cope with those extreme changes (Hernández-Robinson et al. 2020). More detailed common garden and laboratory experiments, as well as long-term field observations of populations of this and other epiphytic bromeliads, are necessary to understand and even predict potential changes in their populations in response to changes in the environment in tropical dry deciduous forests of Yucatan.











 nueva página del texto (beta)
nueva página del texto (beta)



