Insect visitors shape floral microbiota by acquiring and depositing microorganisms onto flower surfaces during nectar and pollen collection (Keller et al. 2021). The flower microbiota can have important effects on plant (de Vega & Herrera 2012) and pollinator fitness (Adler et al. 2018). Therefore, it is crucial to better understand how insects influence floral microbial communities. Honey bees are known to introduce bacteria into the nectar while foraging (Aizenberg-Gershtein et al. 2013). In turn, the bacteria vectored to the flower change the chemistry of nectar and affect pollinator behavior and plant reproduction (Vannette et al. 2013, Junker et al. 2014, Rering et al. 2018, Vannette & Fukami 2018). However, less is known about the effects of honey bee foraging on the microbiota of pollen. As a reproductive structure, pollen is of great importance for the biological success of plants because it is directly linked to reproductive output (i.e., fitness). The few studies that have focused on the microbiota of pollen have found complex bacterial communities with a high level of species-specificity (Heydenreich et al. 2012, Manirajan et al. 2016). The highly sculptured exine surface of pollen grains present cavities filled with a heterogeneous coating of lipid and proteinaceous components that provide a unique habitat for bacteria (Heslop-Harrison & Heslop-Harrison 1985, Aleklett et al. 2014). This pollen coat is thicker in insect pollinated plants which could be a particular attractive habitat for microbes (Edlund et al. 2004, Aleklett et al. 2014). Bacteria on the pollen surface can be found alone or in small colonies forming small biofilms (Zasloff 2017). An interesting aspect of these pollen microbial communities is that they can potentially modulate pollen grain germination on the stigmatic surface (Eisikowitch et al. 1990, Christensen et al. 2021) and hence influence plant fitness.
The dominant bacterial phyla reported in pollen are Acidobacteria, Actinobacteria, Firmicutes and Proteobacteria (Heydenreich et al. 2012, Manirajan et al. 2016), at this taxonomic rank no pollen signature can be observed compared to other habitats of the plant. However, at a finer taxonomic rank, differences in microbiota composition can be found between insect- and wind-pollinated tree species, with more similar communities for insect-pollinated species (Manirajan et al. 2016). These findings suggest insect vectors affect the microbial composition of pollen by homogenizing the microbial communities across plant species.
Honey bees (Apis mellifera L.), one of the most economically important pollinators, harbour a distinct and taxonomically restricted microbiota (Cox-Foster et al. 2007, Martinson et al. 2011) that includes members of the Orbaceae family, which are all exclusively honey bee and bumble bee symbionts (Engel et al. 2013, Kwong & Moran 2013, Li et al. 2015). The sociality of Apis has been proposed as the key driver in symbiont transmission and the maintenance of a consistent gut microbiota (Martinson et al. 2011). Despite the restricted number of species identified in the honey bee gut, symbionts possess distinct functional capabilities linked to host interaction, biofilm formation, and carbohydrate breakdown (Engel et al. 2012). The genera previously detected in worker honey bees are Bartonella, Bifidobacterium, Bombella, Frischella, Gilliamella, Gluconacetobacter, Lactobacillus, Serratia, Simonsiella and Snodgrassella (Cox-Foster et al. 2007, Martinson et al. 2011, Engel et al. 2012, 2013, Li et al. 2015). The distinctive microbiota of honey bees provides an interesting framework to test the effect of pollinators on the pollen microbiota. As an initial approach towards the understanding of how pollinators influence the pollen microbiota, in this study we estimated the composition of pollen-associated bacterial communities of Brassica napus before and after it has been collected by the domestic honey bee.
Materials and methods
Detailed methods can be found in Prado et al. (2020). Inside an insect proof tunnel Brassica napus L. were sown in the soil. Once the plants were in flower and before they had any contact with insects, clean pollen and nectar were collected. Pollen was collected by dissecting closed flower buds and separating the anthers. Anthers were left to dehisce for 4 h at room temperature in glass Petri dishes. To recover the pollen, the dried anthers were placed in a sterile steel tea ball and vibrated using a Vibri Vario pollinator. A total of three samples of anther collected pollen were recovered. Nectar was collected from the flowers with 2 μl capillary tubes between the base of the anthers and transferred to 2 ml Eppendorf tubes (N = 11). Afterward, a small five-frame honey bee (Apis mellifera L.) hive was introduced into the insect-proof tunnel, and honey bee foragers were allowed to forage freely on the B. napus flowers. Inside the tunnel B. napus flowers were the only resource available for bees to forage. Honey bee foragers were captured with a sweeping net and their pollen loads were removed and frozen for further analysis (N = 3 corbicular pollen). Honey bee foragers were sampled to obtain surface and gut microbial assemblages. Entire bees were sonicated in 1 ml of PBS buffer with 0.05 % Tween 20. After removing the liquid (bee surface samples, N = 3), insects were re-suspended in 1 ml of PBS and crushed to recover the microbes inside the bees’ gut (gut samples, N = 7). All the suspensions were centrifuged (12,000×g, 20 min, 4 °C) and pellets were stored at −20 °C until DNA extraction.
Total DNA extraction of pollen, corbicular pollen, nectar, bee washes and bee homogenates were performed with the PowerSoil DNA isolation kit (MoBio Laboratories) using the manufacturer's protocol. Amplification, purification, and pooling for amplicon library construction were conducted following the protocol described in Barret et al. (2015). PCRs were performed with a high-fidelity polymerase (AccuPrime Taq DNA polymerase; Invitrogen) using the manufacturer’s protocol and 10 μl of the DNA suspension targeting the V4 of the 16S rRNA gene with the PCR primers 515f/806r. After amplicon purification, a second round of amplification was performed with 5 μl of purified amplicons and primers containing the Illumina adaptors and indexes. All amplicons were purified, quantified, and pooled in equimolar concentrations. Finally, amplicons libraries were mixed with 10 % PhiX control according to Illumina’s protocols. Sequencing runs were performed in this study with MiSeq reagent kit V3 (600 cycles).
Data Analysis. Primers sequences of fastq files were first cut off using Cutadapt 1.8. Files were then merged and processed with DADA2 v. 1.8.0 according to the recommendations of the workflow “DADA2 Pipeline Tutorial”. The workflow was modified in the truncLen parameter to adjust it to the quality of the sequencing run. The 16S rRNA amplicon sequence variants (ASV) resulting from DADA2 were aligned with a naive Bayesian classifier against the Ribosomal Database Project training set 16 database. Data analyses and visualization were done with RStudio v. 3.3 using the R package phyloseq v. 1.24.2.
Results
Microbial assemblages of anther collected pollen consisted of Proteobacteria (42 %), Actinobacteria (42 %), Firmicutes (10 %), Acidobacteria (4 %) and Bacteroidetes (2 %). These microbial communities were mainly characterised by the Families Enterobacteriaceae, Pseudomonadaceae (Proteobacteria) and Microbacteriaceae (Actinobacteria) (Figure 1A). While collecting and packing pollen in their corbiculae, honey bees changed the relative proportion of Bacteria phyla and their overall diversity. Corbicular pollen samples contained Proteobacteria (40 %), Firmicutes (21 %), Actinobacteria (19.7 %), Bacteroidetes (9.8 %), Fusobacteria (2.8 %), Chloroflexi (2.8 %), Armatimonadetes (1.4 %) and Tenericutes (1.4 %). The change in the microbial assemblages of pollen was partly done by incorporating bee gut-associated symbionts in the Families Orbaceae and Neisseriaceae (Proteobacteria), bee pathogens in the Family Spiroplasmataceae (Tenericutes), nectar-associated taxa in the Families Sphingomondaceae (Proteobacteria) and Lactobacillaceae (Firmicutes) (Figure 1A). The abundance of Families Enterobacteriaceae (Proteobacteria) and Microbacteriaceae (Actinobacteria) was reduced in corbicular pollen as compared to anther collected pollen. Bee-gut symbionts such as Bombella, Frischella, Gilliamella and Snodgrassella were completely absent in anther collected pollen but present in corbicular pollen (Figure 1B). ASVs belonging to the genus Lactobacillus present in nectar were also incorporated into pollen samples by bees. Genera such as Escherichia/Shigella and Pseudomonas that were initially abundant in pollen were reduced in corbicular pollen (Figure 1C).
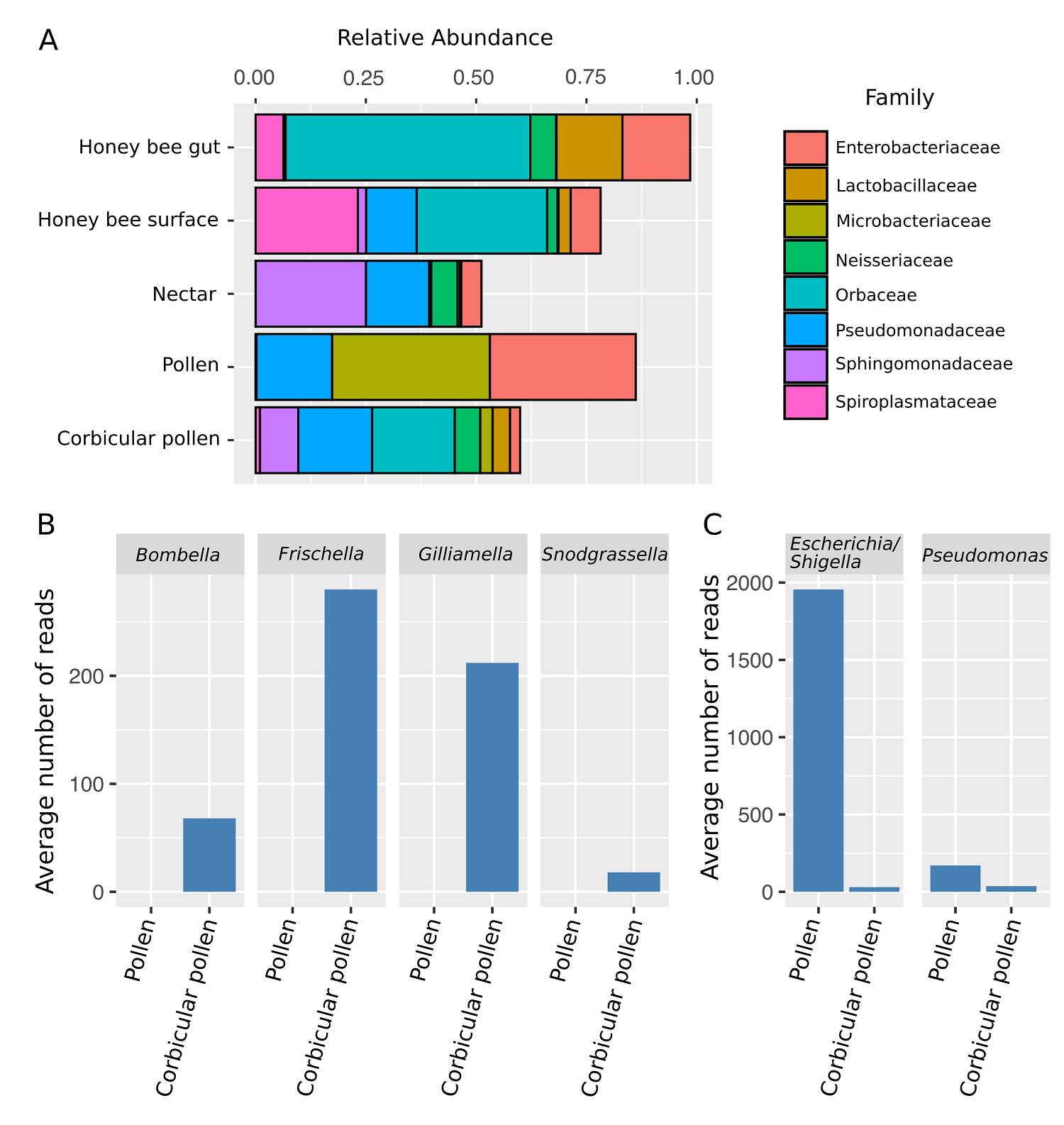
Figure 1 Changes in the microbial composition of pollen. A) Relative abundance of bacterial families. Only showing Families with more than 3 % relative abundance. B) Average number of reads in pollen samples for bee associated taxa. Honeybees introduce Bombella, Frischella, Gilliamella and Snodgrassella into the pollen. C) Average number of reads in pollen samples for genera that strongly decreased due to honey bee processing.
The microbial assemblages in corbicular pollen were richer than those present in the anther collected pollen with an average of 38 and 15.7 ASVs, respectively (Figure 2A). This enrichment represented 38 additional genera and 60 ASVs, and translated into a higher diversity as expressed by the Shannon index and Faith´s phylogenetic diversity (Figure 2B-C). Of the 71 ASVs present in the corbicular pollen 16.9 % are only shared with the bee gut while 7 % are only shared with the bee surface, suggesting the potential inoculum sources. The highest richness was observed in nectar and on the honey bee surface, with averages of 54.2 and 53.3 ASVs, respectively. The honey bee gut presented a simpler microbial community with an average of only 19.7 ASVs.
Discussion
The flower microbiota plays a crucial role on plant (de Vega & Herrera 2012) and pollinator fitness (Adler et al. 2018). Therefore, it is essential to understand the ecological processes involved in their assembly. This note focuses on the effect of honey bees on the pollen microbiota. Honey bees aggregate pollen grains by adding regurgitated nectar or diluted honey to transport it on the corbiculae of their hind legs. Unsurprisingly, we found that honey bees add bee and nectar-associated taxa to the pollen microbiota. Nonetheless, these effects could have important implications for plant reproduction and bee health. The pollen microbiota can have both deleterious and beneficial effects on pollen grain germination on the stigmatic surface. The ubiquitous nectar yeast Metschnikowia reukauffi inhibits pollen germination of Asclepias syriaca (Eisikowitch et al. 1990), while the nectar dwelling Acinetobacter can induce pollen germination of Eschscholzia californica (Christensen et al. 2021). By changing the pollen microbiota honey bees could potentially modulate pollen germination and hence plant fitness. However, pollen packed in the corbiculae is no longer available for pollination; only pollen grains that remain on other bee surfaces can be deposited onto the stigma. It is unknown if the pollen that remains on other bee surfaces and later deposited onto the stigma will suffer similar shifts in its microbial assembly as observed in this study. Nonetheless, honey bees have been shown to change the microbiota of seeds issued from bee pollination by increasing the variability of the microbial communities, and by introducing bee-associated taxa (Prado et al. 2020) suggesting a similar shift in the microbial assembly in the pollen deposited to the stigma. Here we show that while collecting pollen bees increase the diversity of pollen bacteria by incorporating nectar and gut-associated taxa. Hence, it is possible that the bee contribution to the seed microbiota is a consequence of how honey bees process pollen while foraging. Furthermore, pollinator behaviour (i.e., pollen vs nectar foraging) has been shown to significantly affect the acquisition and deposition of bacteria on to flower surfaces (Russell et al. 2019). If we want to understand better the effects of insect pollinators on the plant microbiota, it is crucial to understand the mechanisms involved in the acquisition and deposition of bacteria while insects forage on flowers. Particular attention should be paid to the bacteria vectored to pollen grains and the stigmatic surfaces.
Bee pollen provisions are not consumed immediately after collection; bee larvae usually consume pollen that has been aged. The main effects of pollen ageing are an increase in lactic acid due to the presence of lactic acid bacteria and hence a reduction in pH as well as the opening of the outer shells of the pollen grains (Lipinski 2018). During this process, the microbial diversity in pollen provisions of solitary and social bees changes (Lozo et al. 2015, Graystock et al. 2017). Microbes present in aged pollen provisions are central to bee health as a major dietary component (Dharampal et al. 2019) but also as nutritional mutualists allowing bees to digest some of the recalcitrant components of the pollen wall (Engel et al. 2012). Honey bees need to acquire their microbial symbionts twice during their lives, once as larvae and once as adults. Microorganisms acquired by honey bee larvae through food are eliminated during the single defecation prior to pupation. Hence worker bees emerging from the cells are usually devoid of microorganisms and re-acquire their microbiota through food and trophallaxis (Gilliam 1997). Alterations to the adult honey bee gut microbiota can lead to parasite and pathogen prevalence (Schwarz et al. 2016). Therefore, the presence of bacterial symbionts in the Orbaceae, such as Frischella and Gilliamella, and the Neisseriaceae, such as Snodgrassella, in the pollen provisions is of great importance for honey bees (Engel et al. 2012). Our analysis suggests the bee gut is an important source of inoculum of the corbicular pollen microbiota. In conclusion, the way honey bees forage for pollen affects the pollen microbiota with potential consequences on plant reproduction and honey bee health.











 nueva página del texto (beta)
nueva página del texto (beta)




