Although many pollinators are generalists visiting a wide variety of flowers, close relationships between specific pollinators and plant species have developed throughout evolutionary history (Nicolson & Wright 2017). These relationships have led to the development of floral or pollination syndromes (Mangena & Mokwala 2018), namely, sets of floral characteristics (i.e., morphology, color, smell, size, rewards, and/or phenology) that are attractive to one or more groups of pollinators. Pollination syndromes can thus be used to predict the main pollinators in many cases (Stebbins 1970, Johnson & Steiner 2000, Rosas-Guerrero et al. 2014). In addition, flowers can be classified according to the diversity of their pollinators, i.e., generalist flowers, which are pollinated by a broad spectrum of pollinators, and specialists, which are pollinated by one or a few functional agents.
To ensure reproductive success, zoophilous plants attract pollinators (Moyroud & Glover 2016) using visual and/or olfactory cues and various rewards, such as food (pollen, nectar, or oil), egg-laying or mating sites, and heat (Faegri & van der Pijl 1979, Proctor et al. 1996). In some cases, however, the lures are deceptive, with no rewards (Vogel & Martens 2000). Depending on the pollinating animal group, several types of zoophily have traditionally been distinguished: entomophily (by insects), ornithophily (by birds), chiropterophily (by bats) and pollination by non-flying mammals (Faegri & van der Pijl 1979, Proctor et al. 1996). Entomophily is sometimes divided into cantharophily (by Coleoptera), myophily (by Diptera), myrmecophily (by ants), mellittophily (by Apidae), lepidopterophily (by Lepidoptera), as well as sapromyophily, which is based on deception towards scavenging and dung-eater Diptera and Coleoptera in traps. Melitthophily is the most common and important type of zoophily in angiosperms, the opposite of myrmecophily (Domingos-Melo et al. 2017, Del-Claro et al. 2019) that is restricted to approximately 50 species of angiosperms (Peakall & Beattie 1991, Delnevo et al. 2020); but this form of pollination can be very effective when many ants are active and visits are frequent (Gómez & Zamora 1992, de Vega et al. 2009, Delnevo et al. 2020). Lepidopterophily is usually subdivided into psychophily (diurnal, by butterflies) and sphingophily (nocturnal or crepuscular, by moths) (Faegri & van der Pijl 1979, Proctor et al. 1996). In some plants, the flowers do not rely on external vectors but instead self-pollinate, leading to autogamy. These flowers normally tend to be small, with inconspicuous parts, low amounts of pollen/flower, no odor or nectar, and a low pollen/ovule ratio (Orduff 1969, Faegri & van der Pijl 1979, Proctor et al. 1996).
The epidermis, the outermost plant-covering tissue, is in direct contact with abiotic factors (e.g., light, humidity, heat, temperature, etc.) and biotic agents (e.g., pollinators, nectar thieves, florivores and pollinator predators). This tissue is therefore essential for both attracting and repelling floral visitors selectively (Whitney et al. 2009 a, b, 2011). As a consequence, structures in the epidermis, especially in leaves or perianths, have been evolutionarily selected to optimize plant relationships with biotic and abiotic agents. Epidermal surfaces with papillose cells are most commonly found in angiosperm petals or petal-like structures (Kay et al. 1981) and are important in attracting pollinators (Costa et al. 2017); such cell types are known from the adaxial surfaces of c. 80 % of analyzed plant species (Whitney et al. 2009a, b, 2011), mainly with a grasping function for a wide range of pollinating insects (but see also Kraaij & van der Kooi 2020). In the case of inflorescences relying on a deception mechanism (e.g., Arum) papillose cells aid pollination by appearing on the surface of the spathe but here are modified to decrease the grip of the pollinating insects (Whitney et al. 2009b). In addition, papillose cells sometimes act as lenses that enhance color saturation of the petals (Papiorek et al. 2014) by focusing light on floral pigments (review in Bailes & Glover 2018). These cells also have an effect on floral surface temperatures under some conditions, reduce floral surface wetting, or act as self-cleaning mechanisms against dust and other particles (see revision in Whitney et al. 2011); they may even delay the attachment and biofilm formation of some bacterial strains (Cao et al. 2019). In contrast to a papillose surface, a flat epidermal surface on a corolla/perianth might repel bees or ants acting as thieves and thus prevent the theft of rewards destined for other pollinators (Whitney et al. 2009a, b, Ojeda et al. 2012, Papiorek et al. 2014).
Papillose and tabular cells are classified according to the ornamentation of their walls (striations, papillae, roughness) and the projection on the surface of these walls (e.g., Ojeda et al. 2009, Bailes & Glover 2018). Conical and tabular cell patterns may be found on both the adaxial and abaxial faces of the corolla/perianth and may even appear on the same surface. Surfaces that contact the pollinator, which are generally the adaxial ones, are the most important (e.g., Ojeda & Cronk 2008, Ojeda et al. 2009, Whitney et al. 2011, Costa et al. 2017, Piwowarczyk & Kasínska 2017, Kraaij & van der Kooi 2020). In addition, conical cells are more common in bee-pollinated flowers, whereas tabular epidermal cells are more frequent in plants pollinated by birds. In contrast, floral parts vulnerable to nectar theft are often flat in both bee- and bird-pollinated flowers (Papiorek et al. 2014). Epidermal cell shape is considered a multifunctional adaptive trait that depends on both the pollinator and the participating floral structure (Papiorek et al. 2014).
Knowledge of the microstructure of the flower is important in studies of floral and pollination biology and is also useful in systematics (e.g., Christensen & Hansen 1998, Ojeda et al. 2009, Piwowarczyk & Kasínska 2017, Coiro & Lumaga 2018, Song et al. 2020, Macedo et al. 2023).
Given the above context, we analyzed flowers with different pollination systems (melittophily, myrmecophily, sapromyophily, lepidopterophily, ornithophily, chiropterophily, or widespread self-pollination) according to their main animal pollinator group, the latter mostly based on literature reports and our own observations. Our primary goal was to verify whether corolla/perianth micromorphology was generally consistent with that expected for each species. We did not compare closely related or congeneric species and thus did not analyze evolution of the floral surface. Instead, we selected species based on their pollination systems, among them a species with widespread self-pollination. We specifically aimed to answer the following questions: (1) should adaxial epidermal cells be papillose if there is in contact with pollinators? (2) if papillous cells exist in ornithophilous species, should their density be greater if there is in contact with the pollinator?; (3) corolla mesophyll of chiropterophilous species should be thickest given the greater robustness of their pollinators? We must note that most studies of floral micromorphology have not distinguished among different types of entomophily (e.g., Christensen & Hansen 1998) and, in most cases, have only referred to bees and bumblebees. The same is true of ornithophily, with no distinction made between specialized (e.g., by hummingbirds) and generalist (pollinated by opportunistic birds, including sunbirds) ornithophily. Finally, we also performed analyses of three studied species in which we discriminated between male and female floral/sexual phases-a distinction not considered in most previous studies.
Material and methods
Studied species. We provide information on the following aspects of each studied taxon: the type of corolla and its symmetry (actinomorphic vs. zygomorphic), sexuality, type of pollination (and supporting references), and geographical distribution. Seven pollination systems were specifically represented: melittophily (Arbutus unedo L. and Teucrium fruticans L.), ornithophily (Nicotiana glauca L. and Grevillea robusta A. Cunn. ex R.Br.), lepidopterophily (psicophily: Dianthus lusitanus Brot. and sphingophily: Cestrum nocturnum L.); chiropterophily (Agave americana L. and Musa x paradisiaca L.), myrmecophily (Cytinus ruber Fourr. ex Fritsch and C. hypocistis subsp. macranthus Wettst.), sapromyophily (Aristolochia paucinervis Pomel), and widespread self-pollination (Stellaria media (L.) Vill.) (Table S1, Supplementary material).
Collection and preservation of samples and light microscope (LM) and scanning electron microscopy (SEM) study. Numerous flowers were collected at anthesis from three populations per species during 2017-2019 from locations in southern Spain (Badajoz and Almería provinces). Flowers were submerged for at least 24 h in fixative [70 % ethanol and acetic acid (3:1)] and then preserved in 70 % ethanol at 4 ºC until analysis.
For LM and SEM study, corollas (or petal-like structures) (hereinafter corolla or petals) were divided into two zones: (a) lobes, consisting of lobes from gamopetalous corollas or petal limbs from choripetalous corollas; (b) tube, which were tubes from gamopetalous corollas or petal claws from choripetalous corollas. Several samples from the middle portion of each zone were selected and analyzed. We discriminated between the abaxial or external face (ABA) and the adaxial or internal face (ADA) of each sample. More specifically, we analyzed the cellular characteristics of: (a) the adaxial face of lobes/limbs (hereinafter ADAL), (b) the abaxial face of lobes/limbs (ABAL), (c) the adaxial face of tubes/claws (ADAT), and (d) the abaxial portion of tubes/claws (ABAT). In the case of Agave americana, we studied the ADAL and ABAL of inner and outer tepals, respectively, as pollinators might more frequently come into contact with the faces of both series of tepals. In M. x paradisiaca, we studied the upper lobe (welding of five tepals) and the basal zone where this lobe merges with the lower one (tube). In the case of S. media and G. robusta, we only examined the corolla lobes, and we considered for the claw the lobes data in statistical analyses. When analyzing A. paucinervis, the perianth tongue was considered as the limb, and the utricle, which acts as a trap for pollinators, as the tube.
Samples used for the LM study were dehydrated in a graded ethanol series and then included in resin (Technovic 7100). Next, 3-µm thick cross sections were prepared with a rotary microtome (Leica RM 2145, Wetzlar, Germany), stained with ruthenium red contrasted with toluidine blue O, and mounted in Eukitt. The preparations were observed, photographed, and analyzed using a Nikon Eclipse Ci-S light microscope equipped with a digital camera (Nikon Ds-fi2, Tokyo, Japan). For the SEM study, samples were dehydrated, subjected to a CO2 atmosphere, placed on carbon fiber, and impregnated with gold at the University of Extremadura Research Support Service facility (SAIUEx, acronym in Spanish). After preparation of samples from both adaxial and abaxial faces, four high-resolution images were obtained per sample using a Quanta 3D FEG scanning electron microscope (FEI, Eindhoven, The Netherlands) equipped with a high-vacuum secondary electron detector (FEI, Eindhoven, The Netherlands).
Studied micromorphological variables. Variables in each zone (lobes and tube) were observed and measured from six flowers per population from three different populations. Three mean values per flower were calculated from 3 or 4 LM data and 10 or 20 SEM data, for a total of 18 values per species, with the exception of 24 values (12 per sexual phase) in A. paucinervis and 12 (female flowers) and 6 (male flowers) in Cytinus.
The following variables were chosen specifically for LM according to Costa et al. (2017): mesophyll thickness, adaxial and abaxial epidermal cell thickness, and height of the papillae, if present (Figure 1A). For SEM observations, we selected two variables: cell density (number of cells in a 100 µm2 grid; Figure 1B) and cell size (lengths of the longitudinal and transverse axes), and then this latter considered as longitudinal axis length minus transverse axis length (Figure 1C). Shape and cell surface (perimeter shape, projection, and surface) were also studied following to Ojeda et al. (2009) and Bailes & Glover (2018). The morphology and occupation of papillose cells were described according to Metcalfe & Chalk (1957), and the morphology of trichomes and other structures present in the epidermis were studied as well. All of these parameters were analyzed from LM and SEM images using Nikon NIS Elements imaging software (Tokyo, Japan).
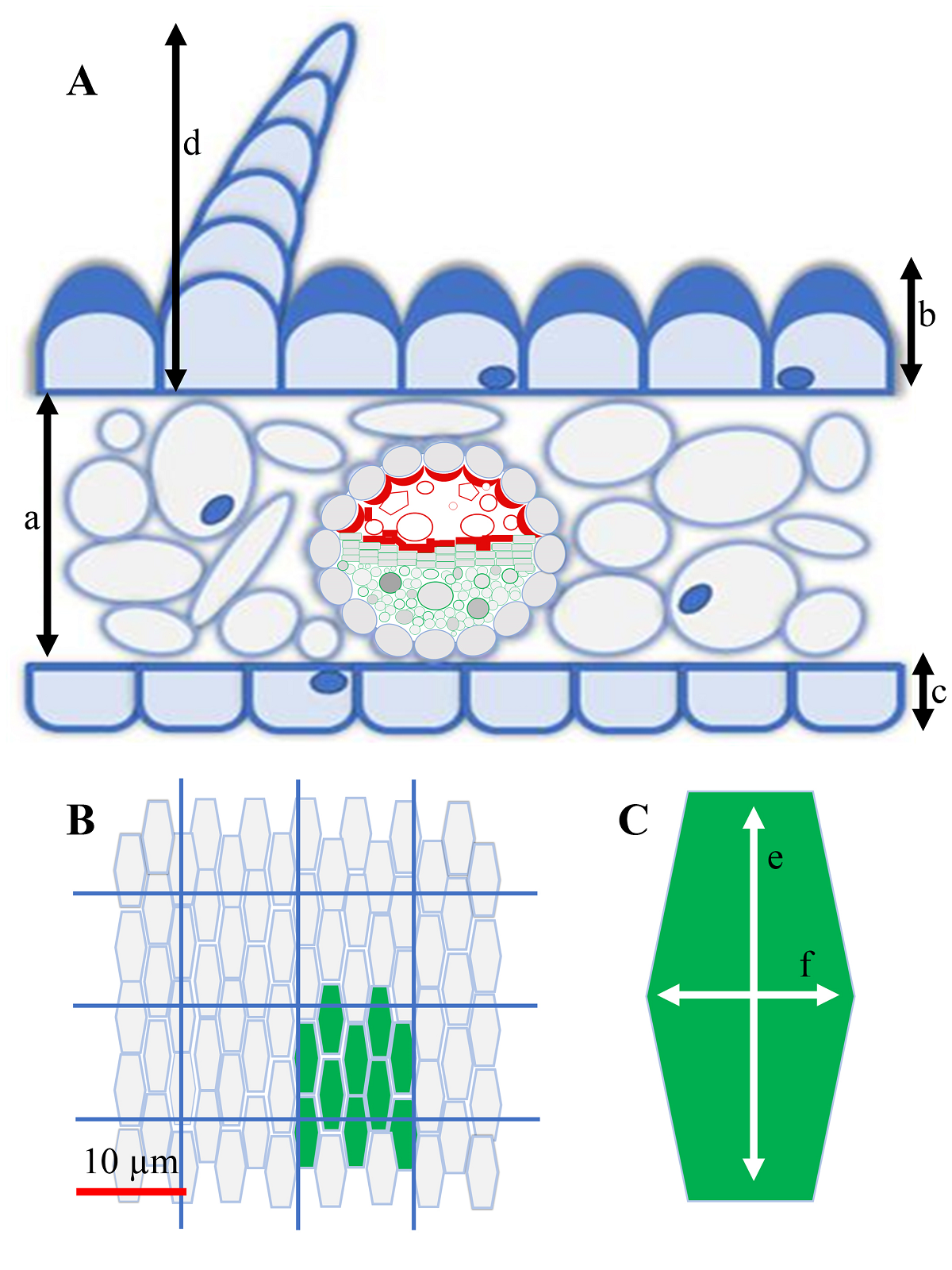
Figure 1 Measured parameters of corollas and petal-like structures. A. Parameters observed by light microscopy: (a) mesophyll thickness; (b) thickness of the adaxial epidermis; (c) thickness of the abaxial epidermis and (d) height of its papillae, if present. B. Cell density (example in green shows 10 cells contained in 100 µm²). C. Longitudinal (e) and transverse (f) axes of adaxial and abaxial epidermal cells. B and C were determined by scanning electron microscopy.
Statistical analysis. All analyses were performed using SPSS v. 27 software (IBM SPSS, New York, USA). As some studied quantitative variables were not normally distributed or homoscedastic (all cell measurements and cell density), all variables were compared among plant species using a generalized linear model (GLM) fitted to a normal distribution with a logarithmic link function. To simplify the analyses and their interpretation, the population factor was not considered. In addition, the analyses were carried out independently for each studied area and face, with the only aim being to determine whether or not significant interspecific differences existed within the same area and face.
We performed a categorical principal components analysis (CATPCA) considering all quantitative and qualitative corolla micromorphological features obtained from LM and SEM and also conducted a hierarchical cluster analysis (Cluster) of the merged data to verify similarities of analyzed features. Both analyses were carried out on several different subsets: (a) cellular characteristics of each face (adaxial and abaxial) of the two zones (lobes and tubes) (i.e., ADAL, ADAT, ABAL, and ABAT); (b) cellular characteristics of all floral zones and faces; (c) characteristics of all zones and faces as well as the mesophyll thickness of both zones; and finally (d) all above analyses but considering sexual phases or sexuality of flowers of Aristolochia and Cytinus. For this latter subject, we performed a single analysis using both sexes separately and then two different analyses, each with one of the sexes. Cluster tree diagrams were constructed using the SPSS Baverage method based on Euclidean distance.
Results
Because the ADAL is the main face used for pollinator landing, as further corroborated by the results of CATPCA and cluster analysis, we mainly focused on the characteristics of ADAL cells. We additionally analyzed corolla lobe and tube thickness, as this feature is another important factor during pollinator visits.
Quantitative variables. Mean values (± SD) of the following quantitative ADAL variables are shown in Figure 2: cell size (length ( width) and cell density, both determined by SEM, and LM-measured lobe epidermal-cell thickness. Mesophyll thickness values of corolla lobes and tubes are likewise presented in Figure 3.
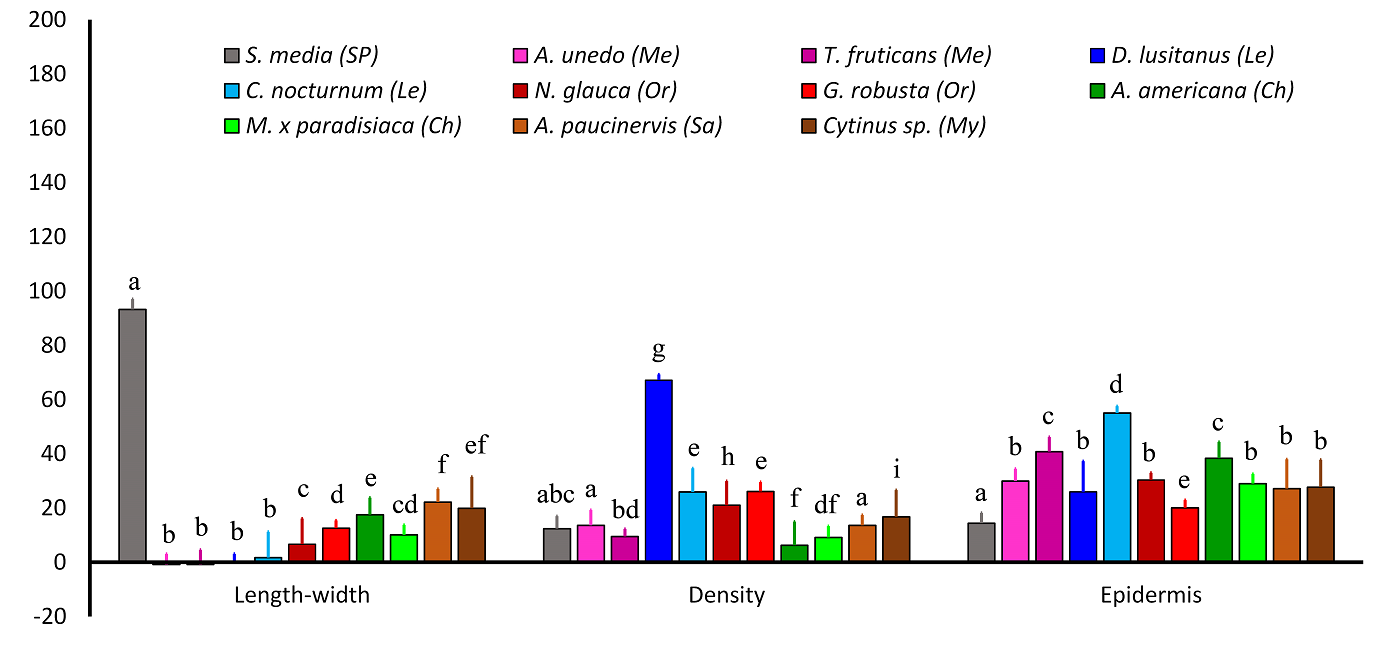
Figure 2 Analyzed quantitative variables (mean ± SD) on the adaxial side of the lobes/limb of the corolla (ADAL). Different superscript letters for a given variable indicate significant differences among species (P < 0.05). The following variables were analyzed: length-width (length - width, in µm), density (cells/100 µm2), and epidermis (epidermal cell thickness, in µm). Pollination systems are as follows: Ch, chiropterophily; Le, lepidopterophily; Me, melittophily; My, myrmecophily; Or, ornithophily; Sa, sapromyophily; and SP, widespread self-pollination.
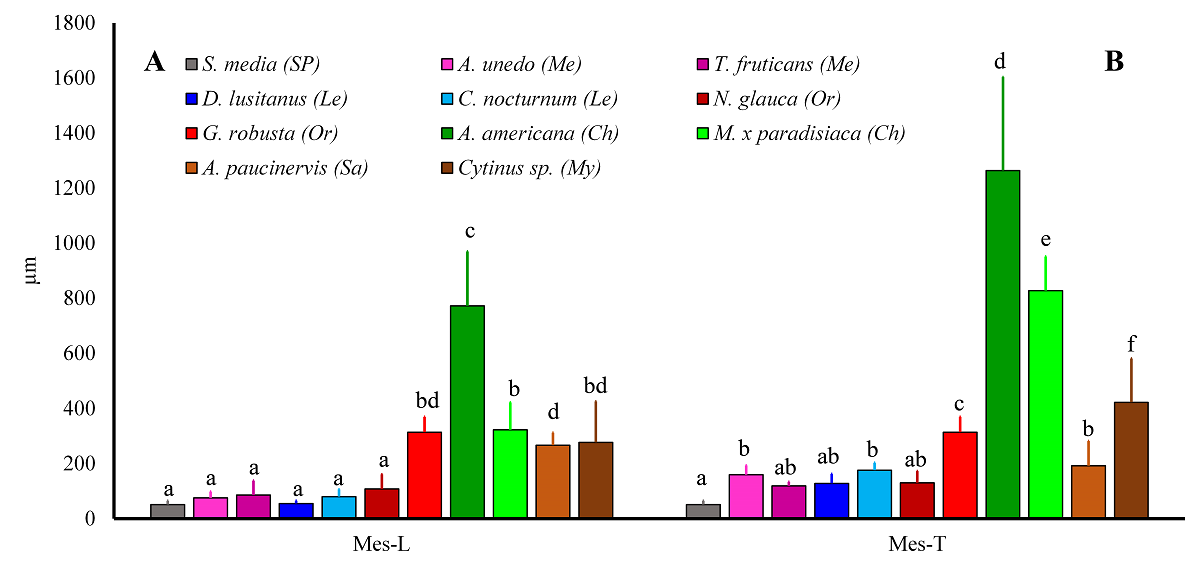
Figure 3 Mesophyll thickness in corolla lobes (Mes-L) and tubes (Mes-T). Different superscript letters for a given variable indicate significant differences among species (P < 0.05). Pollination systems are as follows: Ch, chiropterophily; Le, lepidopterophily; Me, melittophily; My, myrmecophily; Or, ornithophily; Sa, sapromyophily; and SP, widespread self-pollination.
Cellular size (length minus width), A. Lobes.- The largest ADAL cell-size values were observed in S. media, which is widespread self-pollinated, whereas the smallest (i.e., near 0) were those of melittophilous and lepidopterophilous species, which had more-or-less isodiametric cells. Values of the remaining species (exhibiting ornithophily, chiropterophily, myrmecophily, or sapromyophily) were intermediate between those of two previous groups (Figure 2), with no clear distinction observed among these four pollination types. Regarding the ABAL, the largest cell sizes were likewise those of S. media, and the two melittophilous species, psychophilous D. lusitanus, and ornithophilous N. glauca had the smallest. Values of the remaining species formed a group without exhibiting any obvious relation with pollination type (Figure S1A, Supplementary material).
Cellular size (length minus width), B. Tube.- The largest ADAT and ABAT cells by far were those of lepidopterophilous species, followed closely by those of S. media and, more distantly, N. glauca and T. fruticans. No relationship between pollination system and cell size was apparent with respect to the remaining species (Figure S1B-C, Supplementary material).
Cell density, A. Lobes.- The psychophilous species D. lusitanus had the highest ADAL cell density, which was considerably higher than that of the other species (Figure 2). Species exhibiting self-pollination, melittophily, chiropterophily, or sapromyophily typically had the lowest ADAL cell densities (Figure 2), whereas those of sphingophilous, ornithophilous, and myrmecophilous species were generally intermediate (Figure 2). The highest ABAL cell density was that of D. lusitanus, with no clear differentiation observed among the remaining species (Figure S1A, Supplementary material).
Cell density, B. Tube.- No obvious relation was found between ADAT or ABAT cell densities and the type of pollination system (Figure S1B-C, Supplementary material).
Epidermal cell thickness, A. Lobes.- No clear relationship was observed between the thickness of ADAL epidermal cells and pollination type. The thickest epidermal cells were generally found in C. nocturnum (sphingophilous), whereas the thinnest were in S. media and G. robusta (Figure 2). Similar to ADAL epidermal cells, no relation was found between the thickness of ABAL epidermal cells and pollination type. The thickest cells were those of A. americana and T. fruticans, and the thinnest were those of S. media and D. lusitanus (Figure S1A, Supplementary material).
Epidermal cell thickness, B. Tube.- No relationship was apparent between the thickness of ADAT or ABAT epidermal cells and pollination system. In both cases, the thinnest cells were observed in S. media, and the thickest were those in melittophilous species (A. unedo and T. fruticans; Figure S1B-C, Supplementary material).
Mesophyll thickness, A. Lobes.- Based on mesophyll thickness, the studied species could be divided into three groups: (a) thin mesophyll: S. media, melittophilous and lepidopterophilous species, and ornithophilous N. glauca; (b) thick mesophyll: the chiropterophilous species A. americana; and (c) intermediate mesophyll: myrmecophilous and sapromyophilous species, the chiropterophilous species M. ( paradisiaca, and ornithophilous G. robusta (Figure 3A).
Mesophyll thickness, B. Tube.- Corolla-tube mesophyll thickness had a species distribution pattern similar to that of the lobes. The thinnest mesophyll was in A. paucinervis plus the same taxa as in the lobes (Figures 3B and S2, Supplementary material). Mesophyll was thickest in chiropterophilous species, with G. robusta and Cytinus having intermediate values (Figures 3B and S2, Supplementary material).
Qualitative variables. Cell types (shape, projection, and surface) of studied areas and faces (ADAL, ABAL, ADAT, and ABAT) of each analyzed taxon are summarized in Table 1.
Table 1 Morphology of epidermal cells on adaxial and abaxial sides of corolla/perianth lobes (ADAL and ABAL, respectively) and tubes (ADAT and ABAT, respectively) of taxa in this study.
| ADAL | ABAL | ADAT | ABAT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shape | Projection | Surface | Shape | Projection | Surface | Shape | Projection | Surface | Shape | Projection | Surface | |
| Stellaria media | Tabular | Rugose | Smooth | Tabular | Rugose | Striate | ||||||
| Arbutus unedo | Papillose | Conical | Striate | Papillose | Lobular | Striate | Tabular | Rugose | Striate | Tabular | Flat | Striate |
| Teucrium fruticans | Papillose | Conical | Striate | Papillose | Conical | Striate | Tabular | Rugose | Smooth | Tabular | Rugose | Smooth |
| Dianthus lusitanus | Papillose | Conical | Striate | Tabular | Rugose | Striate | Tabular | Rugose | Striate | Tabular | Rugose | Striate |
| Cestrum nocturnum | Papillose | Lobular | Smooth | Tabular | Rugose | Striate | Tabular | Stepped | Smooth | Tabular | Rugose | Striate |
| Nicotiana glauca | Papillose | Knobby | Striate | Tabular | Rugose | Striate | Tabular | Flat | Striate | Tabular | Flat | Smooth |
| Grevillea robusta | Tabular | Rugose | Striate | Tabular | Flat | Smooth | ||||||
| Agave americana | Tabular | Rugose | Striate | Tabular | Rugose | Striate | Tabular | Rugose | Striate | Tabular | Rugose | Striate |
| Musa x paridisiaca | Tabular | Rugose | Striate | Papillose | Knobby | Smooth | Tabular | Rugose | Striate | Tabular | Rugose | Striate |
| Aristolochia paucinervis | Papillose | Conical | Smooth | Tabular | Rugose | Striate | Papillose | Conical | Smooth | Tabular | Rugose | Striate |
| Cytinus sp. | Papillose | Knobby | Smooth | Papillose | Knobby | Smooth | Tabular | Stepped | Striate | Papillose | Knobby | Smooth |
Cytinus sp.: Cytinus hypocistis subsp. macranthus y C. ruber.
Cell shape and surface.- Except in chiropterophilous species, the autogamous species S. media, and ornithophilous G. robusta, all ADAL epidermal cells were papillose (Figure 4). These papillose cells, which were more-or-less isodiametric and mostly conical, were smooth-surfaced in Cestrum, Aristolochia, and Cytinus (Table 1). Elongated tabular cells (i.e., with the longitudinal axis longer than the transverse axis) were almost 9.5 times longer than wide in species with heavier pollinators or in those species exhibiting autogamy (Figures 2 and 4).
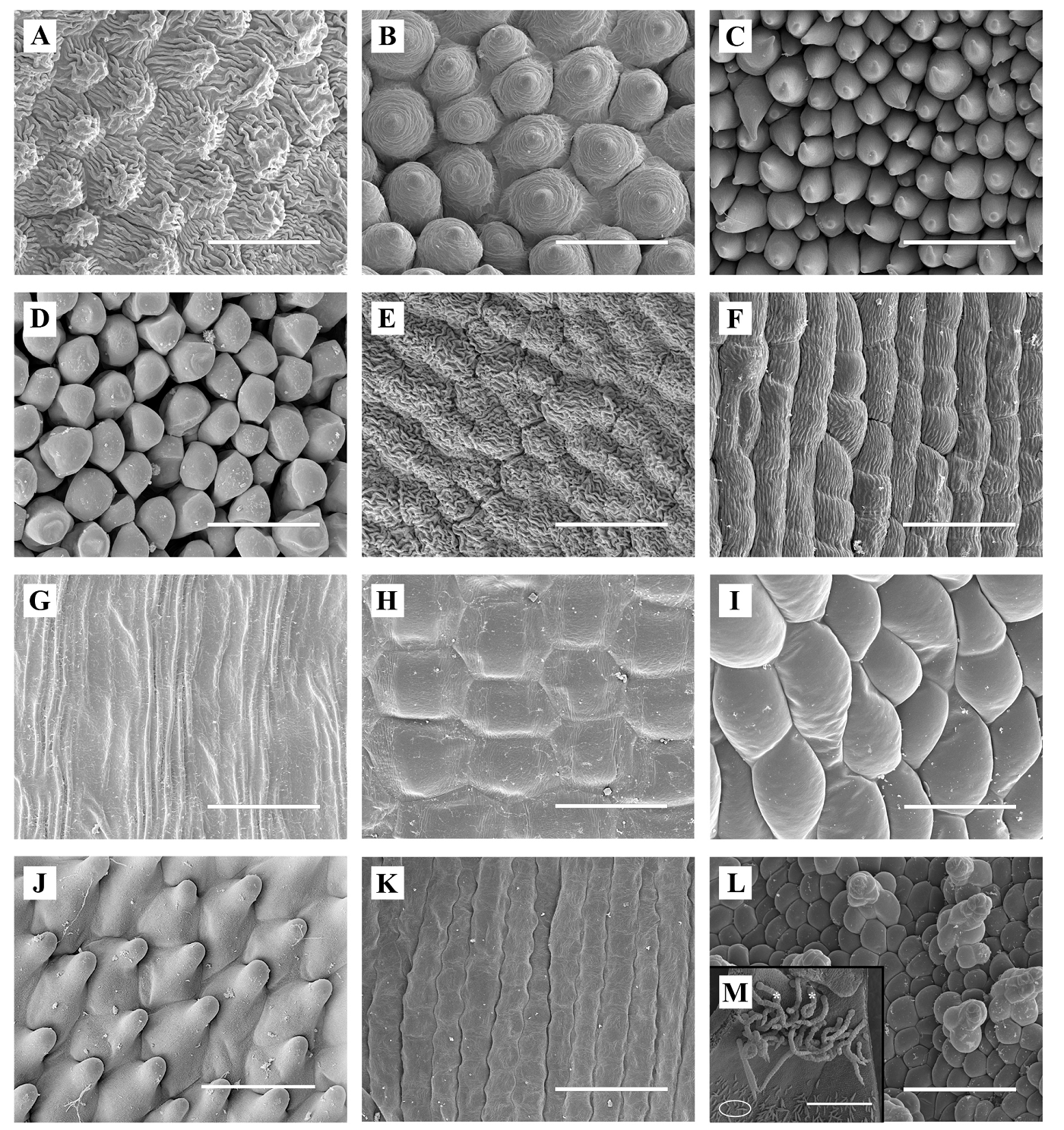
Figure 4 Micromorphology of epidermal cells on the adaxial side of corolla lobes (ADAL). A. Arbutus unedo (melittophilous). B. Teucrium fruticans (melittophilous). C. Dianthus lusitanus (psychophilous, lepidopterophilous). D. Cestrum nocturnum (sphingophilous, lepidopterophilous). E. Nicotiana glauca (ornithophilous). F. Grevillea robusta (ornithophilous). G. Agave americana (chiropterophilous). H. Musa ( paradisiaca (chiropterophilous). I. Cytinus hypocistis subsp. macranthus (myrmecophilous). J. Aristolochia paucinervis (sapromyophilous). K. Stellaria media (autogamous). L. Adaxial/abaxial Cytinus hypocistis subsp. macranthus trichomes from lobes and tube. M. Adaxial Aristolochia trichomes from utricles: trapping (*) and multicellular filiform trichomes (ellipse). Scale bar = 50 µm, except A. paucinervis (M) = 500 µm
In contrast to ADAL cells, ABAL, ADAT, and ABAT epidermal cells were tabular in all taxa except for Teucrium, Arbutus, Musa, Cytinus (ABAL and ABAT), and Aristolochia (ADAT). Tabular ADAL cells were elongated (Figure S1, Supplementary material), with the exception of Musa, where the transverse axis was slightly larger than the longitudinal one (ADAT; Figure S1B, Supplementary material). Cells on adaxial and abaxial sides of the petal limb were similar in Stellaria. Likewise, cells on adaxial and abaxial faces of the corolla tube were similar in both Dianthus and Cestrum and had longitudinal axes that were 9-12 times longer than the transverse ones (Figure S1C, Supplementary material).
CATPCA and Cluster analyses. When only ADAL (i.e., pollinator landing face) cellular characteristics were considered, CATPCA indicated that these features were responsible for 85.31 % of the total variance explained [component 1 (C1) = 52.13 %, component 2 (C2) = 19.88 %, and component 3 (C3) = 13.30 %; Figure 5A]. Although the analysis differentiated practically all pollination systems in this study, it did not separate ornithophilous N. glauca from melittophilous species. The two melittophilous species grouped together, and the same was true of chiropterophilous species. In contrast, the two lepidopterophilous species (psychophilous vs. sphingophilous) were separated from each other, as were the two ornithophilous species (hummingbird vs. passerine pollination). In the latter case, the passerine-pollinated species (G. robusta) was located closer to chiropterophilous species, whereas the hummingbird-pollinated species (N. glauca) was grouped with melittophilous ones (Figure 5A). The characters with the highest eigenvalues in the analysis were the presence/absence and height of papillae, cell shape, and cell projection (C1), cell surface area (C2), and cell density (C3) (Table S2, Supplementary material).
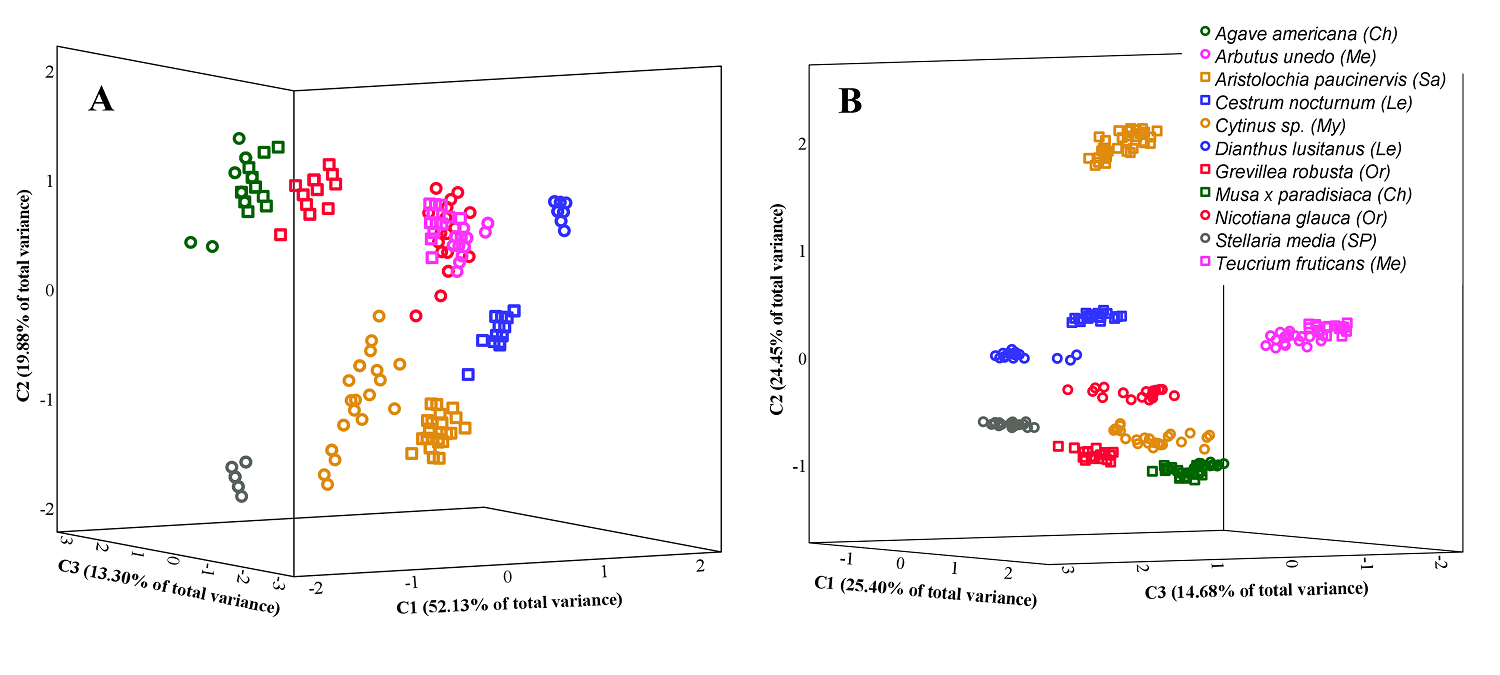
Figure 5 Tri-dimensional categorical principal components analysis (CATPCA) plots of floral variables. A. Plot based on eight qualitative and quantitative features of adaxial epidermal cells of lobes (total variance explained = 85.31 %). B. Plot based on 31 qualitative and quantitative features of adaxial and abaxial epidermal cells of the corolla/perianth and mesophyll thickness (total variance explained = 64.53 %). Pollination systems are as follows: Ch, chiropterophily; Le, lepidopterophily; Me, melittophily; My, myrmecophily; Or, ornithophily; Sa, sapromyophily; and SP, widespread self-pollination.
When ABAL, ADAT or ABAT cellular characteristics were considered, the explained variance also exceeded 80 % (ABAL = 84.18 %; ADAT = 91.13 %; ABAT = 82.44 %), but the pollination systems were not well separated (Figure S3, Supplementary material). ABAL cellular characteristics only separated melittophilous species from the remaining taxa (Figure S3A, Supplementary material). ADAT cellular characteristics were only able to distinguish A. paucinervis from the rest of the studied species (Figure S3B, Supplementary material), whereas those of ABAT basically only differentiated myrmecophilous and sapromyophilous species from the other taxa (Figure S3C, Supplementary material).
When we analyzed cellular characteristics of all zones and faces, the percentage of total explained variance decreased, an outcome that was true regardless of whether or not the thickness of lobes and/or tube mesophyll were taken into account (Figure 5B). When mesophyll thickness was considered, practically all species were differentiated according to their pollination system (Figure 5B). In lepidopterophily and ornithophily, subtypes of both pollination systems were also separated, with ornithophily by passerines being closer to chiropterophily, and ornithophily by hummingbirds nearer to lepidopterophily (Figure 5B). In this analysis, the percentage of the variance explained by the first three principal components was 64.53 % (C1 = 25.40 %, C2 = 24.45 %, and C3 = 14.68 %). Although this contribution to the variance was smaller than that from other combinations of variables, the pollination systems were more clearly differentiated. The most distinguishing characters were mainly the cell qualitative variables, especially those of the ADAL and ADAT, followed by the ABAL (Table S3).
Similar to the results of the CATPCA, cluster analysis of ADAL variables better separated the studied pollination systems than did those of the other faces (Figures 6A and S4, Supplementary material). Cluster analysis of ADAL variables differentiated the following groups of taxa: (a) self-pollinated (S. media); (b) psychophilous (D. lusitanus); (c) chiropterophilous, myrmecophilous, sapromyophilous, and G. robusta (ornithophilous); and (d) melittophilous, sphingophilous, and N. glauca (ornithophilous) (Figure 6). ABAL characters also clearly separated self-pollinated, psychophilous, and melittophilous species, but the other pollination systems were not clearly differentiated (Figure S4A, Supplementary material). When ADAT and ABAT variables were analyzed, only two groups were clearly delimited: lepidopterophilous and self-pollinated species (Figure S4B-C, Supplementary material). Cluster analysis of all cell characteristics (ADAL, ABAL, ADAT, and ABAT) plus mesophyll thickness (Figure 6B) best delimited the studied species according to pollination system. In the generated dendrogram, three main clusters were obvious. The first cluster, which comprised the two chiropterophilous species, was clearly differentiated from the remaining species. The remaining species were divided into two clusters: (1) a group made up of the myrmecophilous species and G. robusta, which was separated from the sapromyophilous species, and (2) a group divided into three well-differentiated subclusters: the self-pollinated species (S. media), lepidopterophilous species, and melittophilous species plus N. glauca (Figure 6B).
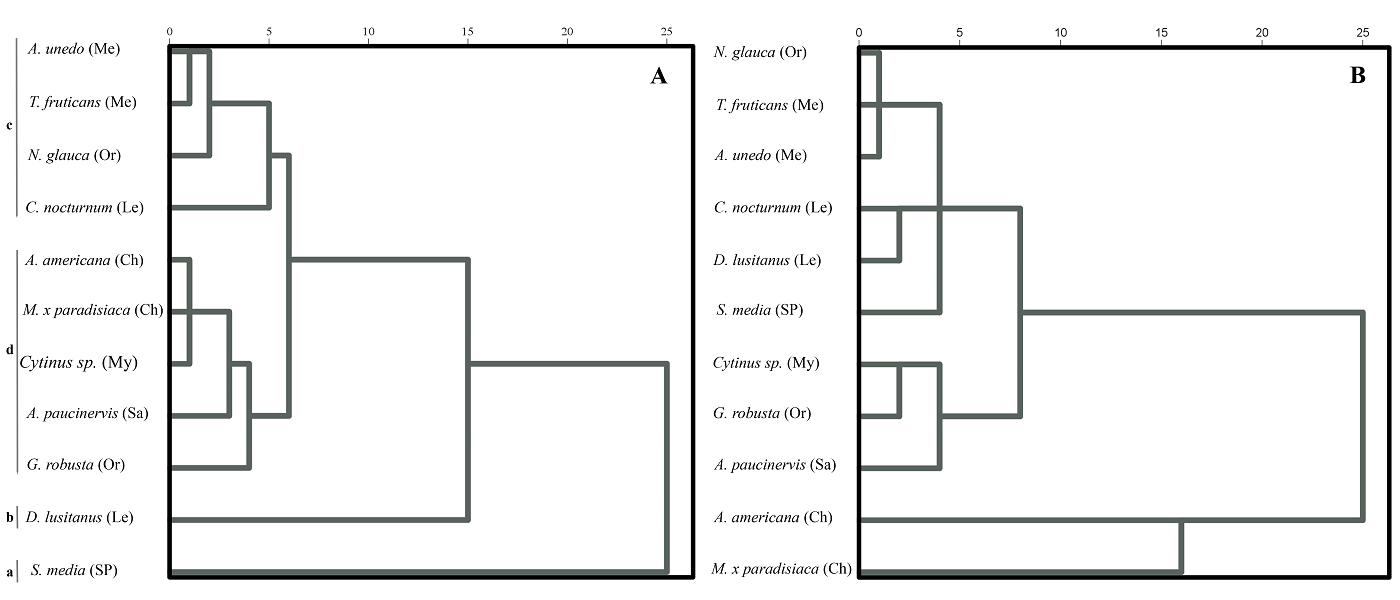
Figure 6 Dendrograms obtained from clustering analysis by Baverage’s method based on Euclidean distance. A. Based on eight qualitative and quantitative features of the adaxial epidermal cells of the corolla lobes. B. Based on 34 qualitative and quantitative features of the adaxial and abaxial epidermal cells of the corolla lobe and tube, and mesophyll thickness of both zones of the corolla. Pollination systems are as follows: Ch, chiropterophily; Le, lepidopterophily; Me, melittophily; My, myrmecophily; Or ornithophily; Sa, sapromiophily; SP, self-pollination.
In the preceding analyses, both floral types in Aristolochia (functionally female and male) and Cytinus (unisexual female and male) were used without differentiation based on sexuality. When ADAL characteristics were analyzed and considering only male flowers or both sexes separately the results were very similar to those obtained when both sexes were analyzed together (Figures 6 and S5, Supplementary material). When only female flowers were used, however, N. glauca clustered with A. paucinervis. If all zones and faces (ADAL, ABAL, ADAT, and ABAT) and mesophyll thickness were considered (Figure S6), the topology of the resulting dendrogram was identical to the one generated without considering flower sexuality (Figure 6B) with two exceptions: male flowers of A. paucinervis and Cytinus formed a sister group to melittophilous species plus N. glauca, and female flowers grouped with G. robusta (Figure S6, Supplementary material).
In summary, ADAL variables better differentiated pollination systems compared with the performance of ABAL, ADAT, or ABAT characters. Analysis of all characteristics of corolla epidermal cells combined with mesophyll thickness grouped the pollination systems in a more logical way, although the correspondence was never perfect.
Discussion
Of the 12 selected species (Agave americana, Arbutus unedo, Aristolochia paucinervis, Cestrum nocturnum, Cytinus hypocistis subsp. macranthus, C. ruber, Dianthus lusitanus, Grevillea robusta, Musa x paradisiaca, Nicotiana glauca, Stellaria media and Teucrium fruticans), only C. hypocistis had been previously examined from a micromorphological point of view of its corolla (Christensen & Hansen 1998, but without specifying the subspecies studied), as well as Stellaria media (Kay et al. 1981) and Nicotiana glauca (Kraaij & van der Kooi 2020). Our work has thus increased the number of taxa studied at this level (nine species). At the generic level, we have analyzed two additional genera (Arbutus and Teucrium) that, to our knowledge, have not been investigated in regard to corolla micromorphology.
With respect to cell size, most epidermal cells ranged from 8.29-219.09 µm long and 8.49-48.71 µm wide. Christensen & Hansen (1998) have reported length and width ranges of 20-100 µm and 10-60 µm, respectively. The longest tabular cells in our study were those of two lepidopterophilous species, sphingophilous C. nocturnum (maximum lengths: ADAT = 219.09 µm, ABAT = 217.26 µm) and psychophilous D. lusitanus (ADAT = 190.62 µm, ABAT = 194.25 µm), and the autogamous species S. media (ADAL = 122.93 µm and ABAL = 144.43 µm). In a study by Kraaij & van der Kooi (2020), floral epidermal cells of selfing species were 35 % smaller in surface area than those of related allogamous species, a marginally significant difference, and a similar trend was found in moth-pollinated flowers.
In contrast to previous studies, our analyses have taken the sexuality of flowers into account, as some studied taxa have unisexual flowers (i.e., Cytinus) or exhibit dichogamy (i.e., protogyny in Aristolochia). According to our results, the main difference between sexual phases in the latter species may be related to tissue turgidity. Papillose cells of the tongue of flowers in the female state are turgid, whereas those in the male state appear desiccated (because their primary function has ended). In Cytinus, in contrast, sexual differences are mainly due to differences in mesophyll thickness. The mesophyll is much thicker in female flowers than in male ones, probably because female flowers must protect and nourish the developing fruit. We thus recommend that future investigations analyze sexes/sexual phases separately, rather than together, in species with such sexual characteristics.
Petal structure and function are of great importance in pollination biology (Kay et al. 1981). The relationship between the type of cells covering the corolla surface and pollination type has been indicated in several studies (e.g., Christensen & Hansen 1998, Whitney et al. 2009a, Papiorek 2014 for bees and birds, Costa et al. 2017 for chiropterophily, ornithophily, and melittophily), but not in others (e.g., Bräuer et al. 2017, Kraaij & van der Kooi 2020). Therefore, investigations of the corolla epidermal micromorphology of numerous other species whose pollination systems are known are still needed. In the present study, we tried to verify whether a relationship exists between epidermal cell type and pollination system, but we obtained mixed results. Given that the most important face of the corolla/perianth during pollination is the one that interacts with the pollinator, we now primarily turn our attention to the adaxial or internal surface of the corolla limb (Ojeda & Cronk 2008, Costa et al. 2017).
Our first question asked whether adaxial epidermal cells should be papillose when there is contact with pollinators, and the answer is not fully supported by our data, as papillose cells are found in the sphingophilous species C. nocturnum as well as the ornithophilous species N. glauca, where its pollinators presumably do not land on the corolla (but see below). In addition, species in which the pollinator does not land on the corolla have tabular cells (ornithophilous passerine-pollinated G. robusta, chiropterophilous A. americana and M. ( paradisiaca, and autogamous S. media). Tabular cells were present on the ABAL of all studied taxa except for Musa, Cytinus, and the two melittophilous species. Regarding the corolla tube (utricle in Aristolochia), tabular cells were present on both adaxial and abaxial faces in all taxa except for Aristolochia and Cytinus, which had papillose cells on adaxial and abaxial faces, respectively.
As reported by Kraaij & van der Kooi (2020), conical cells are rarely present on both adaxial and abaxial sides (see also Christensen & Hansen 1998). Conical cells were observed on both sides of the corolla limb in the two melittophilous species and the myrmecophilous ones (Cytinus). This symmetrical distribution of conical cells suggests that the nectar of these species can be stolen-even by their own pollinators (bees and bumblebees in the former case, and mostly ants in the latter). In Teucrium, the density of conical cells, many of which have no papillae, is lower on the ABAL than on the ADAL. Nevertheless, the presence of tabular cells on the corolla tube of the two melittophilous species would cushion against possible theft, an important function given that their pollinators have been frequently observed stealing nectar-even at the flower bud stage (A Ortega-Olivencia & T Rodríguez-Riaño unpubl. data).
In the case of Cytinus, the external surface of the corolla tube is papillose-knobby and smooth; this implies that ants, its main pollinators (de Vega et al. 2009), may thus also be able to steal nectar, which is the main mission that has been considered for them in the flowers of many species. In Cytinus, ants are primarily attracted by floral odor (de Vega et al. 2014). In addition, these insects probably use the scattered subconical trichomes that cover both surfaces of the corolla to move around the flower (Figure 4L). These trichomes are sometimes glandular, however, especially on the external side. In other species, glandular trichomes appear to repel ants (Whitney et al. 2009b, see Piwowarczyk & Kasínska 2017 and references therein), but this does not seem to be the case in Cytinus. According to de Vega et al. (2009), other pollinators, such as the fly Oplisa aterrima, forage for nectar and secretions from perianth glandular trichomes, which indicates that these structures have a nutritive function.
The existence of papillose contact surfaces on the ADAL of Cestrum and Nicotiana may not be meaningful, as such a surface would facilitate visits by nectar thieves such as bumblebees and ants that would be able to easily land on the long, narrow-tubed corollas. Cestrum nocturnum, native to Central America, only opens its strongly scented, small flowers [1.5-2.5 cm, with a very narrow tube (< 1 mm wide in the lower half); Gallego 2012a, T Rodríguez-Riaño unpubl. data] during twilight and at night. The plant flowers only briefly during the dry season, produces sucrose-rich nectar, and is pollinated by hawkmoths (Haber & Frankie 1989, Heath et al. 1992). Its corolla has patent lobes (4-6 mm, T Rodríguez-Riaño unpubl. data) that may permit these moths to occasionally land. Most likely, not all moths visit flowers by hovering (e.g., Faegri & van der Pijl 1979, Claessens et al. 2019, Funamoto & Sugiura 2021), and, given the nocturnal anthesis of this species and the narrow corolla tubes that prevent access to other nocturnal pollinators (e.g., bats), many moths pollinatings of C. nocturnum surely also perch. In any case, conical epidermal surfaces are also present in other sphingophilous species (Whitney et al. 2009a) and probably improve the grip of perching moths. Of course, papillose cells may have additional functions, such as those related to floral color, shape, odor, temperature, self-cleaning of the flower, etc. (Whitney et al. 2011).
In N. glauca, the adaxial surface with its papillose cells corresponds to five very small lobes (2-4 mm, Gallego 2012b). These lobes are not arranged perpendicular to the tube but are instead more-or-less erect; consequently, the corolla landing surface for visitors is not very large. In its native habitat (South America, from Bolivia to Brazil) and other New World locations, flowers of this bush are pollinated by hummingbirds (Grases & Ramírez 1998, Ollerton et al. 2012). It is pollinated by sunbirds in some areas of the Old World (e.g., Israel and South Africa) and also very infrequently by bees and Dipteran insects in California, places where it is introduced. Nevertheless, many visitors, both insects and birds, act as nectar thieves (e.g., Xylocopa, Bombus, Apis, Syrphidae, Lepidoptera, sunbirds, common whitethroat, Canarian blue tit, etc.; see Table S1). Some insects surely land on the lobes to steal from the corolla tube, and passerine birds do the same while perched on pedicels and branches near the flower. Christensen & Hansen (1998) have argued that conical cells cause greater color saturation by refracting light more efficiently than do tabular cells; consequently, birds perceive papillose petals as being darker than tabular petals. Furthermore, it cannot be ruled out the possibility that ornithophilous flowers with papillose petals evolved from entomophilous flowers without changes in their surface pattern (Papiorek et al. 2014). This pattern of change from conical epidermal cells in entomophilous lineages to tabular cells in ornithophilous lineages has been confirmed, however, in Macaronesia (Ojeda et al. 2016), with petal micromorphology suggested to be a labile trait during pollinator shifts. In addition, papillose cells are usually absent in those species in which pollinators have limited mechanical interaction with flowers (Ojeda et al. 2016). It is assumed, therefore, that flat cells in ornithophilous flowers hinder the ability of nectar-stealing insects to land (Ojeda et al. 2016).
According to Whitney et al. (2009b) conical cells in the spathe of the deceptive inflorescence of Arum help in pollination but have modifications that decrease the grip of pollinating insects. In A. paucinervis, which has protogynous sapromyophilous flowers, smooth conical cells are also present on the inner surface of the tongue and the utricle, but there is a long junction zone between tongue and utricle where there are tabular cells. These tabular cells are accompanied by many large, retrorse, multicellular trichomes (trapping trichomes, according to Oelschlägel et al. 2009), which are turgid in the female phase and withered in the male phase. The retrorse orientation of these trichomes prevents pollinators from grabbing hold and facilitates their slide into the trap (utricle) when the flower is in the female state (see asterisks in Fig. 4M). In addition, multicellular filiform trichomes cover most of the utricle epidermal surface (see ellipse in Figure 4M). In flowers in the female state, these trichomes probably retain trapped insects by secreting nectar; although we have not verified this possibility, a similar phenomenon has been observed in A. bianorii (Alpuente et al. 2023). Similar to other species in the genus (Oelschlägel et al. 2009), the trapping trichomes in A. paucinervis are more abundant and longer at the utricle entrance (Figure 4M). Papillose cells might also be involved in odor emission and/or elevation of floral temperature to attract pollinating insects (Table S1, Supplementary material), as indicated in other cases (Whitney et al. 2011).
The presence of tabular cells on the internal surfaces of the corolla in chiropterophilous species A. americana and M. x paradisiaca is unsurprising, as Costa et al. (2017) has stated that minimum interaction with bats is expected in regard to some other chiropterophilous taxa (but see below). The same may be true of S. media, which undergoes regular self-pollination and does not usually come in contact with pollinating insects.
In this study, the highest cell densities (adaxial and abaxial) were observed in the psychophilous species D. lusitanus, followed to a much lesser degree by sphingophilous C. nocturnum and ornithophilous G. robusta, and the lowest density was recorded in chiropterophilous species. Significant highest density in Dianthus is due to the large number of striated, conical cells, whose rough surface should help butterflies, their main pollinators, more firmly grip the corolla; the same should be true of Cestrum and the perching ability of its pollinating moths indicated above. The lower cell density of chiropterophilous species would in principle support less physical interaction with bats. A common assumption is that these animals forage by hovering, but this is not always the case. In Musa, for example, various bat species approach the inflorescence in three different ways: by hovering (glossophagine species), by upside landing (Glossophaga soricina, Cynopterus sphinix) and by downside landing (Phyllostomus discolor) (Pedrozo et al. 2018, see also Figure 3 in Raghuram et al. 2011). Regarding Agave americana, little is known about the main pollinators of this species in its native habitat (southern USA and Mexico), but eight species of insectivorous birds act as nectarivores on Tenerife (Canary Islands), where it is naturalized (Rodríguez et al. 2015). In other Agave species, the primary pollinators are bats, some of which perch on the inflorescence (Trejo-Salazar et al. 2015). In contrast, the pollinators of G. robusta are perching birds (Kalinganire et al. 2001), which may come in direct contact with high cell density epidermal surface.
For our second question -that the density of papillate cells in ornithophilous species, if they exist, should be greater if there is contact with the pollinator we would expect Nicotiana glauca, whose main pollinators (hummingbirds) do not perch on the flowers, to have a lower cell density compared with G. robusta. Although this premise is true, conical cells do not appear in G. robusta; instead, rough and striated tabular cells are present on the internal surface, which contrasts with other ornithophilous species in the genus (e.g., G. plurijuga, Christensen & Hansen 1998, see also Kraaij & van der Kooi 2020). When their surfaces are rough and striated, however, tabular cells may be nearly equivalent to papillose cells. In addition, the higher cell density of G. robusta allows ants and bees to act as nectar thieves (Kalinganire et al. 2001). Because the pollinators are perching birds, however, significant physical contact with flowers is not assumed, as the birds typically perch near the flower, not actually on it. As mentioned above, conical cells only appear in N. glauca on the inner side of the tiny lobes, which do not act as a contact surface. The external surface of the N. glauca tube, which is flat-tabular and smooth, is more comparable to the rough, striated-tabular internal surface of G. robusta. No trichomes are present on the adaxial side of the perianth of G. robusta (Mast et al. 2015), whereas the external surface of the corolla tube of N. glauca is densely pubescent-glandular, with multicellular filiform trichomes. Although Marinho (2013, in Costa et al. 2017) has reported that glandular trichomes in some other species may act as secretory structures involved in pollinator attraction and even guidance (Piwowarczyk & Kasínska 2017), we do not know if this is true in N. glauca.
Our third question (chiropterophilous species have the thickest corolla mesophyll) is supported by our results of the lobes and tube of A. americana. In Musa, only the corolla tube accords with this answer, as the thickness of the lobes is similar to those of G. robusta and Cytinus sp. In general, the larger the pollinator, the heavier its target flowers (see Opler 1983). The corollas of chiropterophilous species, while morphologically variable (Faegri & van der Pijl 1979, Proctor et al. 1996), are typically robust -probably in line with the wingspan of bats- and Old World species are generally heavier than those from the New World (Proctor et al. 1996). Chiropterophilous flowers usually emit more nectar than do flowers pollinated by other agents and probably have larger nectaries. A perianth with a thick mesophyll would therefore help protect the flowers, which is an issue that deserves to be explored in more depth in future studies. Rather than being an adaptation to pollination, the relatively thick mesophyll of Cytinus flowers likely serves to protect the ground-level or semi-buried inflorescences (de Vega et al. 2009). The expectation that species pollinated by larger animals (bats or passerine birds) have thicker corollas than other species thus seems reasonable.
Tabular cells were found on the external surfaces of lobes and/or tubes of most of the species in this study. Aside from serving as slippery surfaces to discourage antagonistic insects (e.g., ants), these cells might enhance petal coloration to increase their attractiveness to pollinators (Whitney et al. 2009c).
The results of the CATPCA and cluster analysis confirm that cellular characteristics of the ADAL, the pollinator-contacting face, are the best features to use for differentiating pollination systems (Costa et al. 2017). These characters are not always reliable, however, and are sometimes insufficient for delineating the different pollination systems. Using epidermal cell data from all areas and faces of the corolla usually separates pollination systems even better, especially when mesophyll thickness is included. This last character seems to be very important, as highlight by the cluster analyses for differentiating pollination systems associated with large pollinators (e.g., chiropterophily) (Costa et al. 2017).
In conclusion, petal structure and function are continuing topics of interest in floral and pollination biology. Although papillose cells are indeed found on adaxial epidermal cells of corollas in contact with major pollinators, this cell type is also present in the sphingophilous species C. nocturnum and hummingbird-pollinated ornithophilous N. glauca. Although no mechanical contact with pollinators is assumed in the case of N. glauca, pollinating moths possibly perch on the flowers of C. nocturnum. In addition, tabular cells are present in flowers of species where the pollinator does not land on the corolla, namely, passerine-pollinated ornithophilous G. robusta, chiropterophilous A. americana and M. ( paradisiaca, and autogamous S. media. Furthermore, species pollinated by larger animals (bats and passerine birds) have thicker corollas than other species. Female flowers of the parasitic genus Cytinus have thick corollas as well, which probably serves to protect the ground-level inflorescences. Because differences in tissue turgidity and mesophyll thickness may exist in dichogamous and unisexual taxa (e.g., Aristolochia and Cytinus, respectively), we recommend that future studies of such species analyze their sexes/sexual phases separately. Finally, our findings indicate that the presence of a particular pollination type does not imply, as widely believed in this type of works, absence of contact between the floral epidermal surface and certain pollinating animals (e.g., moths and bats).
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.3415











 nueva página del texto (beta)
nueva página del texto (beta)



