1. INTRODUCTION
The rebar of the reinforced concrete structures, typically, are protected from corrosion by a passive layer of oxides formed due the high alkalinity of the concrete, which determines the so-called state of passivation of steel reinforcement. This layer protects indefinitely the steel reinforcement of the corrosion, while the concrete preserve your good quality, no cracks and not have physical or mechanical characteristics changed due to the action of aggressive external agents. The passive protection layer is destabilized by the decrease in the pH of the concrete around the reinforcement to values less than 9, due to the carbonation of concrete, or due the penetration of chloride ions through the porosity of concrete, reaching critical limits, leading to despassivation and start corroding. After the passive layer is broken down and triggered the corrosion process, resistivity and temperature of concrete and the flow of oxygen to the surface of the steel rebar are the main controllers factors of the propagation period of corrosion. (Gjorv; Vennesland; El-Busidy, 1986; Andrade et al., 1990; Castelotte et al., 2001; Francinete; Figueiredo, 1997). The reactions of corrosion can be controlled by several factors, according to illustrate the diagrams of Figure 1. These factors change the polarization characteristics of the reinforcement steel.
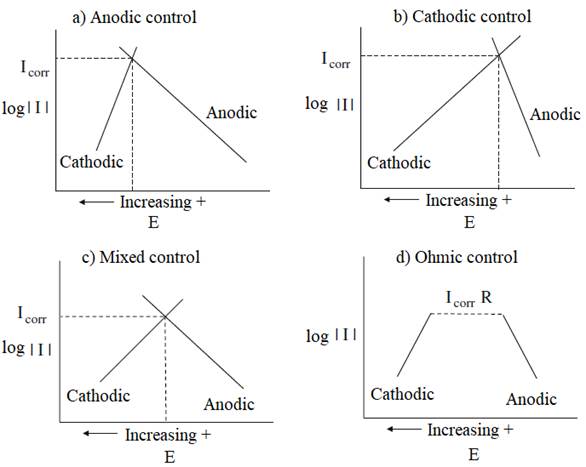
Figure 1 Evans diagram, showing the influence of process of controls anodic, cathodic, mixed and by ohmic resistance about the intensity of corrosion (McCafferty, 2009).
When the polarization occurs mainly at the anode, the corrosion reaction is controlled anodically and the reaction of metal dissolution is diminished. When the resistivity of electrolyte (concrete) is very high, to the point of preventing ion movement, the resulting current is insufficient to polarize the anode and the cathode. In this case, the corrosion reactions are under ohmic resistance control. In practice, the reactions occur in the same intensity at the anode and the cathode and thus has a mixed control. The cathodic control occurs when the oxygen reduction reaction (Equation 1) is constrained by the reduction in oxygen access to the cathodic region, limiting the consumption of electrons from the anode region and, consequently, controlling the kinetics of corrosion.
The presence of oxygen on the surface of the reinforcement steel is essential for reduction reactions occur in the cathodic areas. The oxygen diffusion coefficient in concrete is a concrete property very important and determinant on durability of reinforced concrete structures (Page; Lambert, 1987; Helene, 1993; Hansson, 1993). In some study, the measured oxygen flow is used to predict the durability of the steel reinforcement, based on the relationship between the anodic dissolution, or corrosion, and the amount of oxygen that can be reduced in cathodic areas (Andrade et al., 1990). Kobayashi e Shutton (1991) e Tuutti (1982) studied the influence of the water/cement ratio, the thickness of the covering, the air humidity and the saturation degree of the concrete pores, the presence of mineral additions to cement and concrete curing conditions on the diffusion of oxygen through the concrete.
Restricting the access of oxygen to the steel reinforcement is one of the performance requirements that the coatings applied to the steel reinforcement, or even repair mortars and paints of surface protection, must meet in order to fulfill with efficiency the functions of preservation and restoration of protection and control of reinforcement corrosion.
The measurement of the apparent diffusion coefficient of oxygen (Dap(O2)) through of the concrete covering or through the coatings applied to the steel reinforcement, show the conditions of oxygen supply to the cathodic regions that regulate corrosion kinetics in the anodic regions. Currently, to compose a repair system of concrete structures attacked by corrosion, the technical means has an ample variety of coatings (primers) that are applied on the steel reinforcement. The mechanisms of protection exercised by these coatings can be for barrier, repassivation, inhibition and cathodic protection. In practice usually occurs the joint action of two or more protection mechanisms (Figueiredo, 1994).
The knowledge of the composition and properties of primers (coatings) that are directly related to the ability of protection and control of corrosion is important to the overall assessment of the performance of the primers. Such information is also important for the designers of repairing can choose the products most appropriate for a given situation. Therefore, the present study aims to assess the influence of five different types of coatings, specified for protection of steel reinforcement, on the apparent diffusion coefficient of oxygen (Dap(O2)) and on the intensity of corrosion (Icorr), in comparison to a reference coating composed of a cement-sand mortar.
2. EXPERIMENTAL PROGRAM
2.1 Materials and specimens
For the realization of the experiment were casting prismatic mortar specimens in the dimensions 20 mm x 55 mm x 80 mm. The reference mortar was produced with cement/sand ratio of 1/3 and water/cement ratio of 0.50, both in mass. In the mixing water was mixed 3% CaCl2, in relation to the cement mass, to promote the despassivation of steel reinforcement. The cement used was a high early strength. Table 1 shows the chemical and mineralogical composition and physical and mechanical characteristics of Portland cement used to produce the specimens.
Table 1 Mineralogical and chemical composition and physical and mechanical characteristics of employee cement in experiments.
| Chemical Composition | Results (%) |
|---|---|
| CaO | 61,34 |
| SiO2 | 18,32 |
| Al2O3 | 5,43 |
| Fe2O3 | 3,28 |
| SO3 | 3,04 |
| MgO | 1,51 |
| K2O | 1,04 |
| Na2O | 0,15 |
| Cl- | 0,02 |
| P.F. | 3.13 |
| R.I. | 1,92 |
| Mineralogical Composition | Results (%) |
| C3S | 60,54 |
| C2S | 6,85 |
| C4AF | 9,98 |
| C3A | 8,84 |
| Mechanical Characteristics | Results (MPa) |
| Compressive Strength (3 days) | 27,8 |
| Compressive Strength (28 days) | 59,1 |
| Physical Characteristics | Results |
| Initial setting time | 85 minuts |
| Final setting time | 150 minuts |
| Specific weight | 3,15 g/cm3 |
In each specimen were placed two steel reinforcements type CA50 (Brazilian standard denomination) of 6 mm in diameter and 8 cm long, in order to obtain the duplication of results. Table 2 presents the chemical composition of the steel reinforcements used in the experiments.
Table 2 Chemical composition of the steel reinforcement.
| Element | Composition (%) |
|---|---|
| Fe (Iron) | 98,94 |
| C (Carbon) | 0,17 |
| Mn (Manganese) | 0,59 |
| Si (Silicon) | 0,25 |
| P (Phosphorus) | 0,02 |
| S (Sulfur) | 0,03 |
The steel reinforcements (steel bars) went through a cleaning process, in accordance with the ASTM G1 (1999) recommendations, before being immersed in the mortar. This procedure gives to the steel bars identical superficial grade cleaning before being coated. An insulating tape delimited the study area of 5.6 cm2, which was applied protective coating of rebar (primer). Between the two bars of study was placed a graphite bar to act as counter electrode. Figure 2 shows details of the specimen used in the experiments. Table 3 presents the characteristics of coatings (primers) supplied by manufacturers.
Table 3 Characteristics of the primers (coatings) studied provided by manufacturers.
| Primer | Composition | Number
of Components |
Wet
thickness (µm) |
Density
δ (kg/l) |
pH |
|---|---|---|---|---|---|
| 1 | Cement +
thermoplastic polymer + special loads |
2 | 1000 a
2000 (applied in 2 coats) |
1,90 | > 10 |
| 2 | Cement +
thermosetting polymer + inhibitor (Ca(NO2)2) |
3 | 1000 a
2000 (applied in 2 coats) |
2,00 | N.E. |
| 3 | Epoxy + zinc | 1 | 135
µm/demão (applied in 2 coats) |
2,00 | N.E. |
| 4 | Epoxy | 2 | N.E. | N.E. | N.E. |
| 5 | Polymer + lead | 1 | 300 µm (applied in 3 coats) |
1,36 ± 0,05 | 9,4±0,2 |
N.E. (Not specified)
After casting, the specimens was stored in chamber of 100% relative humidity, remaining in this condition for more than 100 days. In the second stage, until the time of the implementation of the measures, the specimens were provided partially submerged, in order to promote the reinforcement corrosion. The measures of oxygen flow (J (O2)) were made when the specimens completed 1 year.
2.2 Experimental methodologies and evaluations
The oxygen flow through a material is influenced by your thick and interconnectivity of your porous network. In this sense, were made measurements of the thickness of each coat and the total thickness of the wet applied coatings (fresh state), employing a fresh film thickness gauge on a glass plate, as shown in Figure 3. The dry thickness of the primers, the estimate of the size of the pores and your interconnectivity were evaluated by means of magnifying glass, optical microscopy and scanning electron microscopy (SEM). The magnifying glass with increased 4 times was employed to identify surface defects of dry films. These evaluations were also important to detect by comparing possible changes existing surface after the end of the tests and rupture of the specimens. When on the surface were detected imperfections, with suspicion that could have continuity and reach the steel reinforcement, made use of the stereomicroscopic to observe and photograph the defects with more details. At various times, to enter through the defect or surface porosity of the second coat, it was possible to identify the presence of primer from the first coat, reaching the conclusion that the pore no continuity. In this sense it is evident the importance of number of coats to the coating adhere your barrier function. Microscopy allowed estimating pore size and the thickness of hardened coatings, identify elements and semi quantitative composition and observe the presence of resin within the porosity, in order to interrupt the continuity of the pores. While the thickness and porosity of coatings are associated with barrier protection mechanism of the steel rebar, the high pH value of coatings is key to activate the repassivation protection mechanism (FIGUEIREDO, 1994). The pH of the coatings was measured with equipment having glass and calomel electrodes combined with pH range 0 to 14. Ph measurements were obtained 15 minutes after mixing of components of coatings.
The corrosion intensity (Icorr) was measured by Polarization Resistance technique, where a polarization of ( 10 mV around of the corrosion potential (Ecorr) was applied and the ohmic drop of the covering was compensated by means of the positive feedback of the potenciostat between the working electrode (reinforcement steel) and the reference electrode (calomel electrode saturated). Intensity changes resulting from the application of potential difference were determined with at a sweep rate of 10mV/min. Corrosion intensity (Icorr) was calculated using the equation of STERN and GEARY (1957).
To determine the oxygen flow to the surface of the steel rebar inside of the specimen, was measured the cathodic current (Icat) at a constant potential-750 mV relative to the saturated calomel electrode (ECS). At this level of potential the only reaction possible is the cathodic reduction of oxygen (GJORV et al., 1986; ANDRADE et al., 1990). Cathodic intensity (Icat) was measured when the cathodic current versus time curve reached the so-called steady state. After 24 hours of test it was possible to verify that all the reinforcement coated found their stationary states, as shown in Figure 4.
With the value of the Icat as steady-state applied to Faraday's law to get the oxygen flow J(O2)) until the reinforcement steel.
onde,
J(O2) |
( oxygen flow in mol/second; |
Icat |
( cathodic current intensity in the steady-state in amper (A) |
n |
( number of electrons consumed (4) |
F |
( Faraday constant (96500 coulomb/mol) |
From the oxygen flow (J(O2)), and using the first Fick's law, it has been calculated the apparent diffusion coefficient of oxygen (Dap(O2)).
Onde:
Dap(O2) |
( apparent diffusion coefficient of oxygen in cm2/s; |
J(O2) |
( oxygen flow in mol/s; |
e |
( thickness of the covering in cm (0,7 cm); |
S |
( study area in cm2 (5,6 cm2); |
Co |
( oxygen concentration in a saturated solution of Ca(OH)2 in mol/cm3 (1,06 x 10-6 mol/cm3, in accordance with PAGE, LAMBERT, 1987). |
3. RESULTS AND DISCUSSION
Figure 5 shows examples of images obtained by optical and scanning electron microscopy (SEM). During the attainments and evaluations of the images was possible to estimate the thickness and pore size of dry coatings applied on the reinforcement steel, as well as assess the interconnectivity of the pores. The results are in Table 4. In Table 4 are also the thicknesses of the fresh film applied on each coat, total fresh film thickness and the pH of the coatings (primers).
Table 4 Results of evaluations with fresh thickness gauge, magnifying glass, optical microscopy, SEM and pH.
| Primer | Wet thickness (µm) | Dry thickness (µm) |
Estimated pore size (µm) |
Pore connectivity |
pH | |||
|---|---|---|---|---|---|---|---|---|
| 1ª coat | 2ª coat | 3ª coat | Total | |||||
| Refer | - | - | - | - | 7000 (*) | 1000
(***) |
Existence of
connectivity |
13,15 |
| 1 | 550 | 550 | - | 1100 | 1000 | ≤ 250 | Frequently
interrupted by the presence of resin and overlapping of coats |
12,53 |
| 2 | 700 | 650 | - | 1350 | 800 | ≤ 100 | Frequently
interrupted by the overlapping of coats |
11,47 |
| 3 | 175 | 175 | - | 350 | 330 | ≤ 50 | Despite the
low porosity, low presence of resin and high zinc allow a lot of connectivity between the pores |
8,48 |
| 4 | 350 | - | - | 350 | 500 (**) | ≤ 40 | Without
connectivity |
10,91 |
| 5 | 100 | 100 | 100 | 300 | 250 | ≤ 20 | High presence of small pores with possibility of connections |
8,31 |
* The reference mortar was also on the primers
** Greater than the newly applied thickness because the observed area was situated between two ribs, where there is an accumulation of epoxy resin.
*** Air pores
The values of cathodic current (Icat), corrosion intensity (Icorr), oxygen flow (J(O2)) and apparent diffusion coefficient of oxygen (Dap(O2)) obtained in experimental evaluation, are presented in Table 5 and Figure 6.
Table 5 Results of Icorr, Icat, J(O2) e Dap(O2).
| Primer | Icorr (µA) | Icat (µA) | J(O2) (mol/s) | Dap(O2) (cm2/s) |
|---|---|---|---|---|
| Refer. | 8,000 | 6,800 | 1,76 x 10-11 | 2,07 x 10-6 |
| 1 | 0,004 | 0,030 | 8,00 x 10-13 | 9,00 x 10-9 |
| 2 | 1,200 | 1,500 | 3,90 x 10-12 | 4,60 x 10-7 |
| 3 | 35,000 | 27,990 | 7,25 x 10-11 | 8,55 x 10-6 |
| 4 | 0,008 | 0,013 | 3,00 x 10-13 | 4,00 x 10-9 |
| 5 | 0,250 | 3,010 | 7,80 x 10-12 | 9,20 x 10-7 |
Observing Figure 6 it is possible to note that the less-active rebar, protected with primers 1, 2, 4 and 5, the Icat proved greater than the Icorr. This means that in the reinforcement coated with primers of largest barrier effect the anodic dissolution reactions are controlled, while in the cathodic regions of these rebars, due to introduction of a potential-750 mV (ECS) and the presence of some dissolved oxygen in the vicinity of the rebars, the oxygen reduction reactions end up happening. The reinforcement steel protected with the reference mortar and primer 3, after one year of exposure to chlorides, recorded Icorr values indicative of that were in the process of corrosion. In this case, the high values of Icat registered may indicate that was taking place, also, the reduction of iron oxides presents on the surface of these reinforcements. Figure 6 shows that there are still major differences between the primers studied with regard to its characteristics of permeability to oxygen. The primers 1, 2 and represent barrier protection systems and showed less permeable to oxygen than others under the conditions tested. The assessments regarding the porosity and pore connectivity shown in Table 4 support and help in the understanding of the smallest values of Dap(O2), especially with regard to the primer 4 based in epoxy resin.
The values found for the flow and diffusion coefficient, relative to the reference (cement mortar and sand), were of the same order of magnitude of the found by other authors, as can be seen in Table 6.
Table 6 Dap(O2) values for cement mortar obtained by various authors.
| Author | Dap(O2) (cm2/s) |
|---|---|
| Gjorv et al (1986) | 1,3 x 10-6 a 3,4 x 10-6* |
| Andrade et al (1990) | 2,44 x 10-6** |
| Kobayashi et al (1991) | 084 x 10-6 |
| Hansson (1993) | 2,36 x 10-6** |
| Figueiredo (1994) | 2,07 x 10-6 |
* variation in function of thickness of the covering
** calculated from data from the authors
The area used for the calculation of the Dap (O2), presented in Table 5, was 5.6 cm2, so the total area under study. It is important to note, however, that this may not be correct, since the barrier effect exercised by certain coatings reduces the area that effectively is in contact with the electrolyte, reducing, thus, the area where it would be possible take place the oxygen reduction reaction on the rebar. When there are identical situations (same metal type, same polarization imposed (-750 mV, ECS), same environment (reference cement mortar) and same ambient conditions) for all specimens, it is expected that the oxygen diffusion coefficient calculated is always the same. The differences found, therefore, are probably due to differences in areas where oxygen reduction takes place, which, for your time, depend on the greater or lesser barrier effect exerted by each primer. Based on the above, it is possible deduce the equations 4, 5 and 6 that can be applied to calculate the effective oxygen reduction areas of each case and to compare with the reference.
where:
Drefer (O2) |
( is the diffusion coefficient of oxygen of the reference mortar; |
Arefer |
( is the reference area of the study (5,6 cm2); |
Dprimer x (O2) |
( is the oxygen diffusion coefficient of primer studied.; |
Aprimer x |
( is the effective area of oxygen reduction related to the primer studied in cm2; |
Jrefer |
( is the oxygen flow of the reference cement mortar, in mol/s; |
Jprimer x |
( is the oxygen flow of the studied primer, in mol/s. |
Table 7 presents the effective area values (Aprimer x) for each primer studied by using the values of J(O2) presented in Table 6 and applying Equation 6.
Table 7 Effective area values calculated.
| Primer | Refer. | Primer 1 | Primer 2 | Primer 3 | Primer 4 | Primer 5 |
|---|---|---|---|---|---|---|
| Aprimer x (cm2) |
5,6 | 0,03 | 1,24 | 23,13 | 0,01 | 2,49 |
The values shown in Table 7 indicate that, with the exception of primer 3, all other exercised at the time of measurement of Icat, barrier effect superior to the reference mortar. The value obtained for the effective area of the primer 3 probably is due to the registration of the reduction of oxygen on the surface of the zinc particles present in this primer.
Due to corrosion of zinc, both Icorr and Icat show higher values than reference because the reactions of anodic oxidation and cathodic reduction get along on the surface of the reinforcement steel and the zinc particles. In this case, the primer 3 would be exercising a cathodic protection mechanism and not by barrier.
The values of J(O2) and Dap(O2) presented in Figure 6, as well as the values of Aprimer x, shown in Table 7 indicate that the primer 4 (epoxy-based polymer coating) represented the largest barrier to the oxygen diffusion.
As time goes by test, primer can deteriorate. Thus, the calculation of the effective area of contact between the electrolyte and the reinforcement steel (Aprimer x) can be used as a parameter to track the evolution of primer deterioration over time, if the area obtained to increase each test.
4. FINAL CONSIDERATIONS
The primers can protect the reinforcement for passivation, inhibition, cathodic protection and barrier. However, hardly a primer protects the reinforcement steel, along all the time, through a single mechanism. In this study it was found that the majority of primers studied was exercising barrier effect more than reference mortar. The results obtained with the technique employed in this work, show that there are significant differences between the primers with regard to its characteristics of oxygen permeability, and those who represent barrier protection system are less permeable to oxygen under the conditions tested. The value of the oxygen diffusion coefficient obtained in this paper for reference cement mortar are in accordance with the results of other researchers, demonstrating the feasibility of the methodology used to measure the diffusion of oxygen. The electrochemical technique employed in this work allows to track the performance of the primer with the time, watching if there is damage to the primer or not through the monitoring of the effective area of oxygen reduction (Aprimer x) on the reinforcement steel.











 nueva página del texto (beta)
nueva página del texto (beta)








