1. Introduction
Important advances regarding construction material durability studies have been noted in recent years. These assessments allow for the identification of material behavior patterns in view of their interaction with the environment, in addition to the determination of other fundamental aspects, such as the fulfillment of the useful life of projects and buildings.
Concrete and mortar are the most applied construction industry materials and the alkali-aggregate reaction (AAR) is noted as one of their various types of degradation. In general, this is a pathological manifestation related to mineralogical and chemical-physical properties, and the result of the combination of these three categories is a damaging expansive effect. This phenomenon exhibits a high degree of complexity, and must be avoided (Junior and Ferro, 2016).
The AAR is one of the most frequent pathological cement structure phenomena and one of the most relevant concerning durability. Briefly, it can be defined as chemical reactions between certain aggregate components and alkaline hydroxides present within the pores of the cementitious matrix. These reactions do not exhibit a pre-established time for their appearance, since they are associated to several factors, such as the amount of alkalis in the matrix susceptible to the reaction, ambient temperature and humidity, aggregate reactivity and, finally, the nature of the material (Figure 1) (Silva, 2007).
More precise definitions can be made by distinguishing among different types of AAR. The alkali-carbonate reaction (ACR) originates from the reaction of the alkaline hydroxides of Portland cement or other sources and aggregates consisting of clayey dolomitic limestone, whereas the alkali-silica reaction (ASR) is based on the reaction of the hydration products of Portland cement and aggregates containing siliceous material (ABNT NBR 15577-1, 2018). For this research, ASR was approached as a general AAR in general.
Cement has a good influence on the occurrence of this manifestation since, when hydrated, it releases alkalis in the matrix, which are accessible through the matrix pores. AAR can also occur due to alkaline minerals from the aggregates, from pozzolans present in the cement composition and even products present in the mixing water used (Rolim, 2010).
Another factor which greatly influences ARR is moisture, which, in turn, may be associated to two functions in deleterious reactions, namely ionizing and transporting alkaline and hydroxyl ions throughout the pores of the cementitious matrix, which can be absorbed by ARR products. In this way, the alkaline silicon gel expands in the presence of water, which can lead to the appearance of cracks. Therefore, greater AAR prevention is required for building components in frequent contact with moisture (Couto, 2008).
It is known that concrete and mortar porosity is a determining factor concerning the chemical resistance of the material. Crystallizing admixtures, also called healing agents, are widely applied in components that maintain frequent contact with water, such as reservoirs, sewage systems and water treatment plants. These products are hydrophilic materials that react easily in the presence of water, generating a crystalline structure through calcium carbonate crystallization. Therefore, it is expected that their application will result in increased material density and reduced water absorption, since the precipitated crystals of this reaction are insoluble. In addition, crystallizing admixtures result in increased hydrated calcium silicate content in the matrix, ensuring better mechanical performance of cementitious materials (Roig-Flores et al., 2015).
Given the above, this article aims to assess the effectiveness of applying different levels of crystallizing admixtures in preventing the alkali-aggregate reaction in mortars. This analysis was performed by means of capillarity and porosity absorption tests, the determination of mortar bar expansion by the accelerated method and mechanical sample performance.
2. Material
2.1. Cement
CPV-ARI cement was used, due to its purity, avoiding any type of change in the aggregate reactivity results. This is one of the cements most prone to the occurrence of AAR, due to its lower slag content and compound fineness. The physical properties of this cement are presented in Table 1, meeting NBR 16697 specifications (ABNT, 2018).
2.2. Fine aggregate
The fine aggregates were used according to Table 2, considering the ideal amounts of each fraction. To be able to meet this relationship, two different fine aggregates were required, obtained within a radius of approximately 170 km from Rio Verde, GO, Brazil.
Table 2 Required particle size of the material for AAR testing.
| Sieve with mesh opening (ABNT NBR NM ISO 3310-1) | Material amount in mass | ||
|---|---|---|---|
| Sieved | Retained | % | G |
| 4.75 mm | 2.36 mm | 10 | 99.0 |
| 2.36 mm | 1.18 mm | 25 | 247.5 |
| 1.18 mm | 600 μm | 25 | 247.5 |
| 600 μm | 300 μm | 25 | 247.5 |
| 300 μm | 150 μm | 15 | 148.5 |
To ensure compliance with the data presented in Table 2, specific mass, unit mass and particle size tests were performed according to ABNT NBR NM 52: 2003, ABNT NBR NM 45: 2006 and ABNT NBR NM 248: 2009 standards, respectively. The results are presented in Table 3.
2.3. Admixture
The crystallizing admixture content was defined according to the cement mass of the mortars, and the manufacturer indicates levels ranging from 0.8% to 1.2%. Cardesa and Zephir (2014) used 0.8%, 2.0% and 3.0% and Takagi, Lima and Helene (2012), 2.5%. Both groups of researchers obtained satisfactory results in terms of mixture crystallization and waterproofing. Therefore, 1% and 2% were adopted in the present study. Tables 4 and 5 display the additive data provided by the manufacturer.
Table 4 Characteristics of the crystallizing admixture presented in the technical sheet available on the manufacturer's website.
| Characteristic | Corresponding value | Unit |
|---|---|---|
| PH | 10 – 13 | % |
| Fusion point | 1000 | °C |
| AspectSpecific mass | Solid gray powder 1.1 | -g/cm3 |
| Odor and odor limit | Cement characteristic | - |
Table 5 Chemical composition of the admixture provided by the manufacturer.
| Chemical name | CAS N° | % |
|---|---|---|
| Portland cement | 65997-15-1 | 65 to 80 |
| CTS-15-1* | Industrial secret | 10 to 30 |
| CTS-15-2* | Industrial secret | 5 to 10 |
| Calcium and magnesium hydroxide (CaMg(OH)4) | 39445-23-3 | 1.5 to 6 |
| Magnesium and calcium hydroxide oxide (Ca(Mg(OH)2O) | 58398-71-3 | 1.5 to 6 |
| Calcium hydroxide | 1305-62-0 | 1 to 2 |
| * Industrial secret - The exact percentage (concentration) of the composition was retained as an industrial secret. | ||
3. Methods
The investigation method followed the step flow chart presented in Figure 2. All the steps described below are intended to meet the requirements of the NBR 15577-4 standard (ABNT, 2018).
3.1. Trace determination
To determine mortar expansion by the accelerated method, the trace is composed of 1 part of cement, 2.25 parts of aggregate and a water/cement ratio (w/c) of 0.47. To mold three mortar bars, whose specific aggregate (d) mass is greater than or equal to 2.45 g/cm³, 440 grams of cement and 990 grams of aggregate must be adopted.
The tests were carried out according to Figure 2, and the traces were defined according to Table 6, with a reference mixture (AR) without additive and two mixtures (AA1 and AA2) containing 1% and 2% crystallizing admixture contents. During the mixture production mixtures, the admixture was previously diluted in the mixing water, as specified by the manufacturer.
3.2. Fresh tests
The tests regarding mortar specific mass and incorporated air content were carried out according to the NBR 13278 standard (ABNT, 2005). Incorporated air was determined by using the results of the specific mass obtained through the relation between the theoretical and estimated masses.
Mixture consistencies were evaluated applying NBR 13276 standard recommendations (ABNT, 2016).
3.3. Specimen molding and curing
Cylindrical and prismatic specimens were molded in accordance with NBR 7215 (ABNT, 2019) and NBR 13729 (ABNT, 2005) standards, respectively. The prismatic specimens (30x25x285mm) for expansion assessments were molded according to the NBR 15577-4 standard (ABNT, 2018), in two densified layers with 20 strokes each. For the accelerated AAR testing, at least three mortar bars are required for each aggregate. The bars were cured in a humid chamber at 23 °C for 24 hours, with their faces protected from splashes. After this period, the mortars were removed from the molds and placed in a thermoregulatory bath containing a sodium hydroxide solution. This last step is an integral part of the test procedure in the hardened state.
3.4. Hardened state tests
As established by NBR 15577-4 (ABNT, 2018), the mortar molds were immersed for 30 days in a NaOH solution (sodium hydroxide - 1.0 N), as displayed in Figure 3. During this period, eight specimen dimension readings were obtained, with one mandatory at 16 days and the other at 30 days after molding.
The compressive strength test was performed with cylindrical specimens according to the NBR 7215 standard (ABNT, 2019), while the flexural tensile strength test was performed according to the NBR 13279 standard (ABNT, 2005), using prismatic test pieces, both under a hydraulic press, as shown in Figure 4.
The capillarity absorption (in grams per square centimeter), void indices and porosity absorption (both in percentage) tests were performed according to NBR 15259 (ABNT, 2005) and NBR 9778 (ABNT, 2005) standards, respectively. These tests were carried out to evaluate the crystallizing effect of the admixture and associate it to a possible delay in mortar AAR.
3.5. Result analysis
All data concerning the accelerated AAR test were analyzed and compared following the tables provided by the NBR 15577-1 standard (ABNT, 2018), which determined potential aggregate reactivity, degree of occurrence risk, consequences, and reactivity classification (Figure 5). The other tests were carried out to expand and complement the approach of admixture effect assessments concerning mortar performance and behavior.
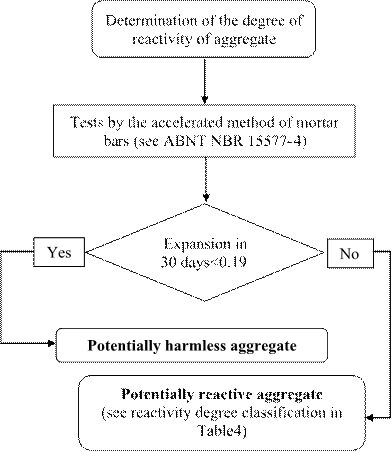
Figure 5 Procedure for determining the aggregate reactivity degree as established by the NBR 15577-1 standard (ABNT, 2018).
4. Results and discussion
4.1. Fresh assays
Table 7 presents the results of the tests carried out with the fresh mixtures. The healing additive leads to fresh mortar effects, where increasing admixture content increases the specific mass and spreading, while decreasing incorporated air content.
Table 7 Specific mass, spreading and incorporated air content results for fresh.
| Trace | Specific mass | Spreading | Incorporated air content |
|---|---|---|---|
| AR | 2216.87 kg/m3 | 267 mm | 1.05 % |
| AA1 | 2226.51 kg/m3 | 278 mm | 0.34 % |
| AA2 | 2228.92 kg/m3 | 283 mm | 0.09 % |
The mixtures registered non-expressive specific mass increases which, in turn, can be explained by the fineness of the admixture. The finer grains of the admixture fill interstitial voids and increase mixture cohesion by up to 0.5%.
Although the admixture is very similar to cement, spreading did not decrease, and, instead, increased fluidity was observed. This is due to the different chemicals present in the mixture, which can act as a water-reducing plasticizer. The spreading increase indicates that the admixture can reduce the water/cement ratio to a certain consistency, as reported by Cardesa and Zephir (2014). Some researchers, such as Moreira (2016) and Takagi, Lima and Helene (2012), identified loss of consistency with the use of similar admixtures, although not the same product in utilized herein. Manufacturers can use different chemicals in the production of crystallizing admixtures and, in the case of the product assessed in the present study, consistency was improved.
Regarding both, incorporated air content and specific mass, the fineness of the material is responsible for reducing the air content with increasing amounts of admixture. This reduction in the fresh state can contribute to reductions in the void index, capillarity, and porosity of the hardened mixtures.
4.2 Hardened assays
The hardened assay results are presented in Figures 6 to 13. Figure 6 presents the results regarding the compressive strength of the tested mortars. Increasing additive resulted in increased compressive strength, as the AA2 mixture obtained the best performance, followed by AA1 and AR, respectively.
This behavior is similar to that observed by Takagi, Lima and Helene (2012), Cardesa and Zephir (2014) and García-Vera, Tenza-Abril, Saval and Lanzón (2019), who reported that concretes mortars containins 1% or more crystallizing admixtures under the cement mass displayed increased compressive strength when compared to mixtures containing no admixtures.
At seven days, an approximation of the resistance results of the three mixtures is observed, due to the short period of time elapsed since the mortar production for the admixture reaction, since their reactivity is dependent on cement hydration reactions. However, at 28 days, a noticeable resistance gain of the admixture containing samples compared to the reference mixture is observed. Mortar AA2 reached a resistance 24.9% higher than AR, while AA1 reached a value around 17.5% higher than AR. As no information between the mixtures was altered other than the use of the admixture, resistance gain is solely due to the admixture use.
Figure 7 presents the results related to the flexural tensile strength tests, where a performance gain is verified with the use of the crystallizing product, similarly to what was noted in the compressive strengths assays. The AA2 mixture once again achieved the best result, reaching a value approximately 12.8% higher than AR, while AA1 presented a resistance 11.3% higher than the reference.
The gain in tensile strength when flexing mortars containing admixtures is explained by the crystallizing action of this product, which results in larger massive cross section areas, thus increasing the material's carrying capacity. This gain in tensile strength, even if not expressive, was also reported by Moreira (2016) when evaluating mixtures containing 0.8% of crystallizing admixtures.
According to the admixture manufacturer, this material is capable of filling cracks and voids of up to 0.5 mm. Figure 8 presents microscopic images under a 35x magnification of the cross sections of the specimens containing (Figure 8a) and not containing (Figure 8b) the admixture. Figure 8b also displays a portion of the material of a different color from the standard without the admixture, indicative of the healing power of this chemical.
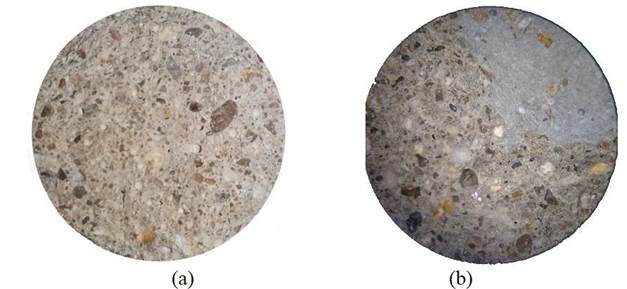
Figure 8 Microscopic image of the cross section of the specimen without the admixture (a) and containing 2% of the admixture (b), both after rupture at 28 days. Images under 35x magnification.
Bearing in mind that the specimen contained some internal irregularities, possibly caused during its production, the admixture reacted and filled the small internal voids located inside the specimens. Its distribution was homogeneous, although crystallization occurs exclusively in the mass voids and, for this reason, a region was formed resulting from the admixture action, displayed in Figure 8. No fine aggregate concentrations are found in the “healed” area, which, in accordance to data presented by the Japan Concrete Institute at JCI-TC075B (2009), corroborate the idea of the crystallizing effect.
Figures 9 and 10 display the results obtained through the porosity water absorption test and void indices performed according to the requirements of the NBR 9778 standard (ABNT, 2005). The presence of the healing admixture reduces both mixture porosity water absorption and void indices.
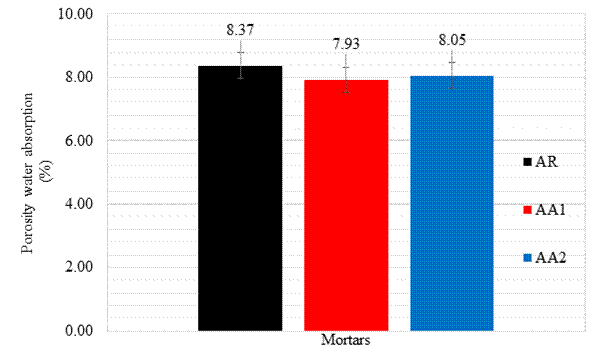
Figure 9 Porosity water absorption test or pressure water absorption (%) results in mortar specimens immersed in water at 28 days.
This test, because it is carried out with specimens under pressure, identifies the open and closed porosity and the total void indices of the mortars. The specimen containing 1% of the admixture attained the best results, attesting that the range of contents indicated by the manufacturer is adequate. By increasing the admixture content to a value higher than the indicated range, a new increase is observed both in porosity and in the void index. However, even though the results of mortars containing the admixture are better than the reference mixture, the difference between them is not significant, with the largest reduction in porosity and void rate of around 5.3%.
Figures 11 and 12 display the results obtained in the absorption and capillarity coefficient tests, performed according to the NBR 15259 standard (ABNT, 2005). Capillarity absorption is defined by the cross-sectional area being measured in constant contact with water, in grams per square centimeter. The addition of the healing admixture above 1% considerably reduces mortar capillarity absorption.
The capillarity values, given the result shown in Figure 11, can reduce by approximately 20%, again attesting the sealing power of the admixture. Similar results were reported by Takagi, Lima and Helene (2012) and Pazderka and Hájková (2016), both using 2% admixture.
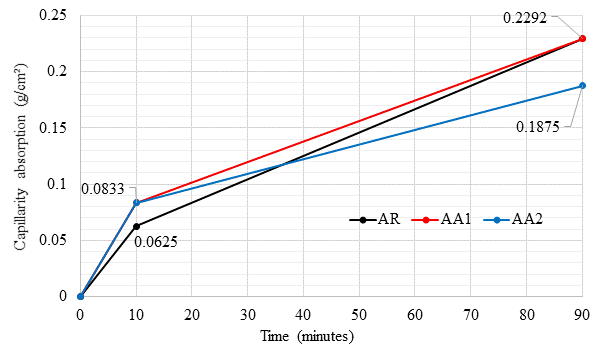
Figure 11 Water absorption capillarity (%) test results in a mortar specimen in contact with a water layer at a constant level of 5mm.
The capillarity coefficient refers to the slope of the line that passes through the representative points of readings taken at 10 and 90 minutes, calculated by subtracting the masses recorded in these determinations.
The higher the admixture content, the lower the capillarity coefficient and, consequently, the lower the capillarity absorption for a fraction of time. As in the previous data, the AA2 mixture presented the best results, reaching a coefficient about 37.5% lower than the RA, while AA1 reached a reduction of 12.7% compared to the RA.
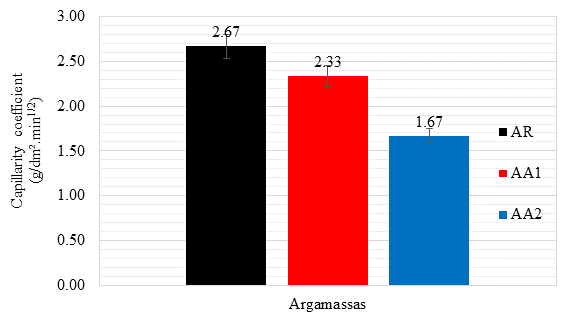
Figure 12 Capillarity coefficients (g/dm².min1/2) of the mortars, determined with readings taken at 10 and 90 minutes.
Figure 13 presents the results regarding the expansion determinations during the accelerated ARR mortars tests according to the NBR 15577-4 standard (ABNT, 2018). A high initial expansion, at 6 days, was observed in mortars containing the crystallizing admixture.
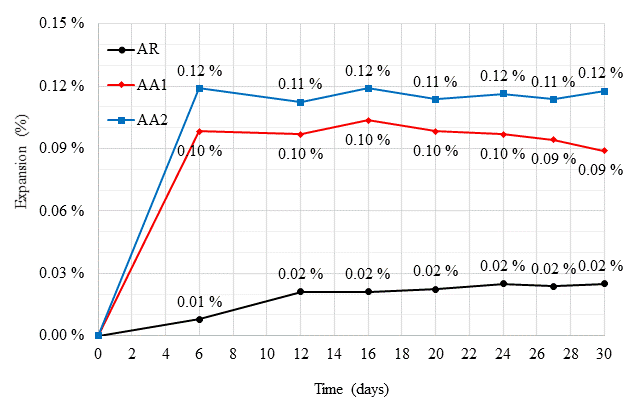
Figure 13 Expansion (%) of mortar specimens during accelerated AAR tests recorded for 30 days after molding
This result indicates that the alkaline products present in the admixture composition, described in Table 5, may have led to the evolution of the expansions in the first week, considering that an evident initial volumetric evolution increase is noted with increasing admixture contents.
The differences between mortar variations are significant, where AA2 underwent an initial expansion at 6 days about 1100% higher than RA, while AA1 exhibited a 900% increase in expansion compared to RA. However, after this 6-day period, expansion ceased, specimen dimensions stabilized and, consequently, percentage expansions decreased as a function of time, to 500% and 350%, respectively.
The expansions were clearly caused by the admixture, as the AR mortar exhibited much lower values. The chemical admixture composition indicates that the reactions may have been caused by magnesium, although further research focusing on microstructural assessments is required.
Regardless, the admixture seems to display an activation time of approximately one week, starting void and pore sealing and controlling volumetric mortar variations beginning at the moment of expansion stabilization.
It is important to note that these readings were recorded on small-sized mortar prismatic specimens. If applied on a large scale, the use of the admixture may require some attention to the limits set by the manufacturer, in order to avoid further inconveniences.
The use of products that inhibit alkali-aggregate reactions, such as pozzolanic materials, is indicated, as they will inhibit initial expansions and prevent damage to concrete or mortar components in the early stages, allowing admixture constituents to seal pores and voids. However, admixture behavior analyses in conjunction with pozzolanic materials, such as active silica, should be evaluated and remains as a suggestion for future research.
Finally, regarding the potential reactivity classification of the aggregate, considering the limits established in the NBR 15577-1 standard (ABNT, 2018), the material proved to be potentially harmless grade R0, i.e. the mortar bar expansion at 30 days was less than 0.19%. Although the differences between the mortar expansions are high, the degree of risk related to this classification is negligible, and no mitigation action due to ARR is necessary, depending on the aggregate.
5. Conclusions
The crystallizing admixture used herein, did not cooperate in the first days regarding the inhibition of the alkali-aggregate reaction. On the contrary, the presence of alkaline products in the admixture composition increased initial expansion until the admixture reacted with cement hydration products. Therefore, the use of ARR-inhibiting materials, such as pozzolans, is indicated in parallel with healing admixtures of similar chemical composition.
Nevertheless, the admixture use was quite satisfactory concerning mechanical performance, water absorption and void indices when present at 1% and 2%. Admixture use resulted in compression and flexion traction resistance gains, in addition to decreased void indices and porosity absorption. However, its greatest asset lies in the significant reduction of capillarity coefficients and absorption, indicated for elements in constant contact with water, such as reservoirs and basic sanitation systems.











 texto en
texto en 










