1. Introducción
Wastewater is utilized water after being affected by domestic, industrial, and commercial use or polluted by any foreign component. The composition of all wastewater is highly variable due to the different contamination sources, which is why it is so difficult to pinpoint a singular way of pollutant removal [1]. The impact of polluted water is increasingly alarming on earth’s fragile ecosystems as well as its availability for human use [2]. Increasing population and climate change statistics show there is a growing concern about the water crisis that makes water treatment a priority to treat and reuse as effectively and optimized as possible [3]. There are many wastewater treatment technologies used that overall and overlapped can be somewhat effective in the removal process [4].
Flocculation is a process by which colloidal particles come out of suspension to sediment under the form of a floc. Before the actual flocculation process, particles are merely suspended and are not truly dissolved in solution, under the form of a stable colloidal dispersion and will not present natural precipitation [5]. On the other hand, coagulation aims to destabilize and aggregate particles through chemical interactions between the coagulant and colloids, and flocculation to sediment the destabilized particles by causing their aggregation into flocs for precipitation [6].
2. PE flocculation
The stability of a colloid is related to its zeta potential, which involves the particle surface charges and an oppositely charged counter ions adjacent to it, which attracts and stabilize, forming an electrical double layer, these opposite charges systems are called polyelectrolytes (PE) [7]. PEs have remained one of the most attractive resources of scientific research, in recent decades owing to their great importance in advanced technologies and biological applications [8]. A PE is defined as any macromolecular material that has repeating units and dissociates into a highly charged polymeric molecule upon being placed in any ionizing solvent (e.g., H2O), forming either a positively or negatively charged polymeric chain [9].
A PE is characterized by its molecular weight, the nature of the functional group, and the charge density [10]. An important consideration in choosing a PE for a desired process is its potential as a coagulant (by destabilization of the colloid via neutralization) and as a flocculant (by interparticle bridging or charge neutralization). Overall, the charge neutralization principle is the main concept in PE systems. However, the polymeric nature (either in its native state or grafted derivatives) will define the floc formation mechanism, depicted in Figure 1A [11]. Mainly, a PE formed by charge neutralization in a native, linear modality will be dependent on charge accessibility due to steric issues and on the other hand, bridging is charge neutralization by charges present in grafted polymers or branched nature, that can provide greater accessibility to the neutralization process and provide better yields [12].
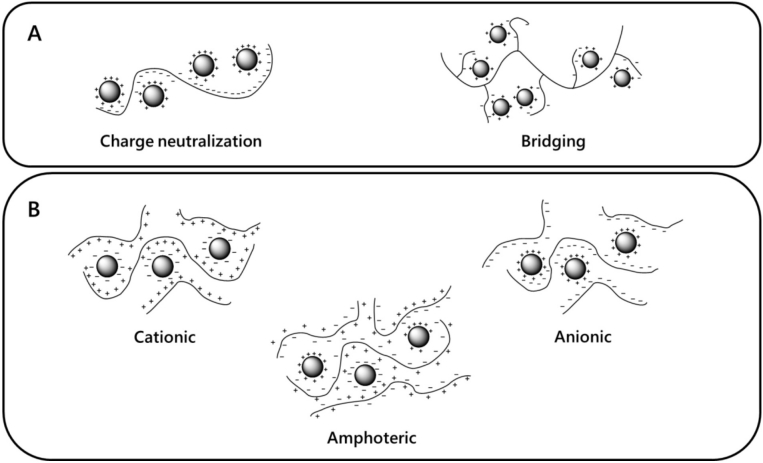
Figure 1 PE floc forming mechanisms. A. Particle neutralization methods dependent of polymer configuration. B. Particle neutralization methods dependent of polymer charge.
Hence, flocculation takes place through hydrophobic or hydrophilic interactions (mainly hydrogen bonds or electrostatic interactions), and any compression of the double layer, can be induced by increasing the ionic strength, which should enhance stabilization by allowing closer particle approach and as the flocculant adsorbs several electrically charged sites, it doesn’t cover the whole area of the colloidal particles. Flocculation takes place through hydrophobic or hydrogen bonds, and any compression of the double layer that can be induced by increases in ionic strength should enhance bridging by allowing closer particle approach interactions, leading to increased stability [13].
The repeating unit charge of the PE polymers, is neutralized by oppositely charged smaller counter ions that tend to preserve the electro neutrality, this principle will confer electrostatic interaction with pollutants charge dependent for the removal capability. If any PE solution contains a positively charged electrolyte, it would interact with negatively charged ions, and similarly, negatively charged materials with positively charged ions [14]. This principle also works in amphoteric polymers with the added benefit of interacting with both positive and negative particles and strengthening the PE with interactions polymer-particle and polymer-polymer joined by electrostatic attraction and can confer the flocculant with great diversity, [15] as can be observed in Figure 1B.
The pH is also an important parameter to be considered when selecting a PE for a particular application [11]. Usually, sensitivity to pH occurs with cationic polymers in which quaternary ammonium groups are dominant, and with anionic polymers containing hydroxyl or carboxylate groups. Flocculants with high carboxyl or amine groups density and availability confer stronger pH dependence and will define its efficiency [16].
Flocculation requires gentle mixing and the use of a high molecular weight polymeric flocculant [17]. The flocculant adsorbs to the small flocs and facilitates the bridging of gaps between flocs, bringing particles closer together, which creates the effective range for Van Der Waals attraction forces to reduce the energy barrier for flocculation and form loosely packed flocs. Subsequently, aggregation, binding, and strengthening of flocs occur until visibly suspended bigger flocs form, which usually precipitates in such a state [18].
By concept, PE flocculation is very effective but it can present some challenges. First, it generates sludge that can represent a separation, elimination, and disposition problem, [19] and second, flocculant traces remain in the treated water [20] and commercial flocculants are reported to be toxic, [21] which can make treated water discharge problematic without disrupting the ecosystem or contaminating water for human use [22]. A possible solution to avoid toxic flocculant traces and sludge toxicity, is using biodegradable, biocompatible polymers as flocculants, such as polysaccharides and their derivatives.
Polysaccharides are biocompatible, biodegradable natural polymers formed of monosaccharide units that are joined together by glycosidic linkages [23]. In its disposition, they can be linear, branched structures or linear structures with short branches regularly spaced, irregularly spaced, or in clusters [24].
Some important polysaccharides are chitosan, alginate, chitin, cellulose, various gums, carrageenan, chondroitin sulfate, pullulan, starch, among many others (Figure 2); [25] each one, shares the glycosidic link between different sugar rings, but in the sugar diversity and interaction lies their differences and properties. Usually, polysaccharides classify according to their charge due to different sugar atoms that confer partial charges in neutral environments because of their functional groups, which can rule their environmental interaction and, in consequence, the interaction with molecules aiming to remove, [26] e.g. amine groups in chitosan confer a positive charge at a neutral pH (ca 7) and hydroxyl groups or carboxylate groups confer a negative charge, each of them, complexing with their corresponding particles with neutralizing charges, producing the flocculation process [27].
As mentioned before, the PE capacity and strength depends on charge availability and distribution, molecular weight and graft o branching conformation to allow the bridging process. However, the charge can be induced or modified by modifying the polymer native chain. For example, there are some anionic polysaccharide derivatives (not in the native state), that can be recognized as cationic, which can be induced by adding a quaternary nitrogen or a free amine group to the polysaccharide chain [28]. A wide variety of grafts have been used in polysaccharides, aiming to modulate the polymer charge and in consequence, its affinity to a targeted pollutant (Table 1).
Table 1 Different grafts functionalized on to polysaccharides
| Graft charge | Reactive used |
| Cationic grafts | 3-chlorohydroxypropyl-trimethylammonium chloride |
| Acrylamide | |
| Azobisisobutamidine hydrochloride | |
| Methacryloxyethyl trimethyl ammonium chloride | |
| Acryloyloxyethyltrimethylammonium chloride | |
| Dimethyl diallyl ammonium chloride | |
| [2-(methacryloyloxy) ethyl] trimethyl ammonium chloride | |
| Amino thiourea | |
| Anionic grafts | Monochloroacetic acid |
| Itaconic acid | |
| 2-acrylamido-2-methyl-1-propanesulfonic acid | |
| Sodium xanthate | |
| Starch | |
| Dextran | |
| Ammonium dithiocarbamate | |
| Sodium silicate | |
| Neutral grafts | Polyacrylpiperidine |
| Poly(N-vinylcaprolactam) | |
| Cinnamic acid | |
| Dodecylamine |
3. PE flocculation by cationic polysaccharides
Positively charged polymers can be used for PE flocculation and are widely used for this application. Small particles, as well as cells, carry negative surface charges that hinder aggregation and settling [29], so high weight cationic polymeric particles can act as effective flocculants. Usually, nitrogen groups are responsible for a positive charge conferred to a molecule, therefor chitosan (CS) is one of the few native polysaccharides with the capacity to exhibit a positive charge, even though its pH dependent.
CS is a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine (conferring the positive charge) and N-acetyl-D-glucosamine [30]. CS is obtained from the exoskeleton of crustaceans and has attracted widespread attention due to abundant resources, low cost, and availability [31]. Another appealing remark is its unique structure and the diversity of active groups within the molecule, which has good environmental compatibility and modification reproducibility [32]. It is a natural basic polysaccharide, and the molecular segment contains a large number of active functional groups such as the amine group, hydroxyl group, and N-acetyl group, which have strong physical or chemical adsorption capacity for a variety of pollutants such as heavy metal ions, dyes or biological agents to mention some [33]. In addition, CS has been reported to be ecofriendly [34], biodegradable [35], good chelation behavior [36] and non-toxic [37]. However, due to the solubility of chitosan in acid conditions, its application in wastewater treatment can be complicated in standard conditions or a pure state. For these reasons, better results have been reported in acidic working condition [38].
The PE flocculation mechanism is mainly thru CS’s amine and hydroxyl groups. Therefore, the better chitin’s deacetylation rate is, the better the flocculation process occurs [39], even more so if these groups are used to functionalize it with more active versatile molecules solving the solubility issue mentioned before, and increasing the flocculation efficiency [40]. CS is one of the most used natural polymers because of its amine group, facile functionalization in mild conditions and biocompatibility, making the flocculation process green and environmentally friendly [41].
CS has been reported to remove a wide variety of pollutants [42], some of them are inorganic compounds and suspended solids, such as kaolin or clay, removed by the complexation their negative charge with CS protonated amine group’s positive charge, at a neutral or slightly acidic pH, which makes CS and CS based materials effective for waterbodies flocculation [43]. CS has also been reported to remove organic compounds exploiting its hydrophobic nature at neutral pHs such as antibiotics [44] and dyes [45]. For example, acrylamide is a very common positive charged graft functionalized onto CS to provide property, specifically because, it maintains the cationic characteristic adding a larger chain to enhance the bridging capability.
Even though, the PE flocculation process is fairly simple, the reality and practice of it, is a complex process due to the wide variety of pollutant scenarios, and to make the polysaccharide used multifunctional and more efficient, they are grafted with complementary opposite charges producing a zwitterionic polymeric chain and providing diverse anchoring points that, as mentioned before, can neutralize the particle, to remove it and also stabilize itself to promote a more, efficient, fast, and versatile flocculation [46]. The amphiphilic properties can be induced in cationic or anionic polymers depending on the graft added, this can modulate the range of particles the flocculant can remove as well as the pH window [47].
To accomplish this, CS has also been modified with carboxylate and ammonium groups, this confers a negative and positive charge at a neutral pH range, which would not be possible with native CS [48]. To continue with the example given above, CS-acrylamide can be further modified with an anionic graft to confer this amphoteric property. There are several CS evaluations with this pattern, e. g. CS-acrylamide was modified with itaconic acid to remove crystal violet and clay with up with great removal efficiencies [49], or with sulfonate groups to remove dissolved and natural organic matter (DOM and NOM) with up to 95% efficiency [50], or with sodium xanthate to remove Cr and Ni with up to 99% removal efficiencies [51]. Monochloroacetic acid is used to produce carboxymethyl CS, it is commonly used because it works as an anionic graft, and has been used to remove turbidity [52] or oil [53]. A wide variety of grafts have been used, searching these multifunctional properties, some of them with remarkable results, exhibited in table 2.
Table 2 Recent results (2019-2023) in studies about diverse polysaccharides’ optimal removal efficiencies of common pollutants. Note: Turbidity results include TSS, clay and kaolin.
| Polysaccharide | Pollutant removed |
Optimal removal efficiency Reference |
|---|---|---|
| CS | Dye | 81%,49 97%,58 98.7%,59 91.9%60 |
| Turbidity | 95.8%,52 90%61, 91.1%,62 95%,63 93%49 |
|
| Orthophosphate | 93.4%,52 | |
| Organic matter | 95%50 | |
| Heavy metals (Ni, Cr, Pb, Cd, Cu) | 94.7%,51 99.3%,51 95.2%,64 95.7%,64 83.9%,65 93.3%66 90.2%58 |
|
| Oil | 91%,67 | |
| Antibiotics | 84.7%,44 | |
| COD | 83.9%,65 97%62 | |
| Drugs | 95%,68 | |
| Algae | 90%,69 99%,70 99.3%71 |
|
| Chlorophyl a | 97%62 | |
| CH | Antibiotics | 95.5%57 |
| Turbidity | 95%,72 94%73 | |
| AG | Heavy metals (Pb, Cu, Cr) | 92.3%,74 96%,75 97.2%,76 90%,77 80%77 |
| Humic acid | 95.1%,74 | |
| Turbidity | 97.6%,78 90%,79 95%,80 90%81 |
|
| Dye | 98.4%,78 73.5%82 89.8%83 |
|
| Bisphenol A | 88.6%76 | |
| Xanthan gum | Iron Oxide | 98%84 |
| Turbidity | 96.5%85 | |
| Dye | 93.8%,86 99.7%,87 99%88 |
|
| Guar gum | Turbidity | 80%,89 90%90 |
| Fenugreek gum | Arsenicals | 90%91 |
| Turbidity | 92.7%,92 88.1%93 95.9%94 |
|
| Dextran | Turbidity | 96%,95 98.2%96 |
| Cellulose | Dye | 91.76%,97 87.5%98 99%,99 80%100 |
| Algae | 95%,101 | |
| Turbidity | 99.8%,102 99.7%103 |
|
| Starch | Heavy metals (Ni, Cu | 90%,104 99.2%105 |
| Turbidity | 92.7%,106 95.4%107 |
Chitin (CH) is a long-chain polymer of N-acetylglucosamine, it’s the second most abundant polysaccharide in nature (behind only cellulose) [54]. CH has a nitrogen in the acetylamine group of the glucosamine, this can confer a positive charge to the polysaccharide structure. However, this cationic nature is limited to a very acidic pH (ca. 2) [55], which limits the interaction with negative charge pollutants in a native state. There are reports of CH used as a flocculant in suspended solid removal with efficiency up to 85% [56] or ciprofloxacin with up to 95% removal efficiency [57].
4. PE flocculation by anionic polysaccharides
Anionic polysaccharides are the most common due to the functional groups in their structures. As mentioned before, hydroxyl, carboxylates, and sulfate groups confer negative charge and, however pH dependent, these are the most common functional groups in polysaccharides. Therefore, the vast majority of polysaccharides are anionic in nature [26]. Anionic polysaccharides are even more widely researched due to multiple reactive functional groups, which can facilitate the modification of the polymeric chain’s nature.
Alginate (AG), on its own, doesn’t have a good flocculating property, but using its PE nature it can improve, e.g. with calcium ions can remove up to 40% suspended solids [108], or with CS to remove up to 100% turbidity and 90% COD [109]. As mentioned before, the charge neutralization in the PE process is key to the flocculation to occur, however, small counterions, such as calcium, would ionically crosslink AG more tightly with less space for particle removal; however, it can leave space for particle adhesion and improves the removal process due to surface interaction [110]. AG can also be modified to induce an amphiphilic properties, ca. using 3-chloro-2-hydroxypropyltrimethyl ammonium chloride, modified AG containing both cationic groups -N+(CH3)3 joined with the anionic groups -COO- from the native state will be obtained and can hold positive and negative charges, this was evaluated in its capacity to remove positive and negative charged particles (Pb2+ and humic acid) with removals up to 95% [74]. These amphiphilic flocculants exhibit the potential to flocculate both organic and inorganic pollutants and, because of the PE condition of charges, reduce the flocculant dosage to achieve removal efficiencies close total removal. AG has also been grafted with acrylamide as a co-polymer system to develop this ability and increase floc viscosity [111, 112, 113], with protamine to remove quartz sand and kaolin [80], or with amino thiourea to remove heavy metals [75]. For that reason, we can determine AG as a versatile flocculant when functionalized, especially with opposite charge grafts, this would confer diversity in its removal efficiency, that, considering AG’s chain flexibility, the charges have availability to neutralize pollutants.
Gums are naturally occurring polysaccharides with a lot of hydroxyls that can form hydrogen bonds, this confers a high viscosity or solid state at room temperature [114]. This property gives extra functionality to the material. However, there are many different gums, such as cashew, xanthan, gellan, and fenugreek gum, among many others [89]. In gums, the isoelectric point, hence the working pH, is very important to ensure charge interaction. In the flocculation process, gums have been used in a native state or conjugated to achieve better results, as it represents an anionic polymer. Namely, xanthan gum was used in a native state to remove iron oxide using the combined isoelectric points, achieving a flocculation efficiency of up to 98% with a work pH window of 2-9 [84]. In this regard, guar gums have been used in the native state as well to remove a variety of pollutants such as kaolinite [89], Pb2+ [115], bentonite [116] among others. Gums used in a native state represent a green flocculation and can help flocculate a certain type of pollutant. As some examples in the native state, xanthan gum was used to remove kaolinite and calcium carbonate with 95 and 76% efficiency, respectively [85], guar gum to remove the oil with up to 90% efficiency [117], or fenugreek gum to remove suspended solids with efficiency [93, 118], and COD [119]. However, gums can also be modified special to provide bridging capacity of amphiphilic properties, e.g. guar gum has been modified with itaconic acid to remove kaolin and coal with 88 and 81% efficiency respectively, this is an anionic graft that would increase the negative charges surface, improve bridging capacity and, consequently, increase its interaction with the particles to remove [120]. On the other hand, gums can be modified by the amide groups, to increase the flocculation properties manly because of charge availability, diversity, and working pH and can be modulated with the flocculant dosage not explored in this review. One common example, as used with other polysaccharides, acrylamide was use to modified fenugreek gum [94], tamarind seed gum [121], ghatti gum [122], cashew gum [123] to mention some that were used as a way to remove kaolin or dye with promising results.
Dextran (DX) consists of α-1,6 glycosidic linkages between glucose monomers, with branches from α-1,3 linkages. Therefore, it has a branched structure that provides a good charge surface and spaces for particle interaction (bridging), which makes an excellent characteristic for flocculation [124]. Still, it is used grafted to improve particle interaction, e.g. grafted with acrylamide to remove turbidity with efficiency up to 96% [125], with ammonium groups to explore the PE amphoteric nature to remove both inorganic or organic contaminants with remarkable results [126], or even in a star-like arrangement to remove kaolinite with up to 90% efficiency [127].
Cellulose (CL) is a polysaccharide consisting of a linear chain of several hundred to various thousands of β(1→4) linked D-glucose units and the main structural component of green plants and algae, making it the most abundant polysaccharide [128]. Because of its broad availability, renewability, sustainability, and surface modification potential, cellulose is regarded as one important polymer for flocculant production and modification, however, its limitation lays on its solubility, that restricts the ability to use it in a native state [129]. CL has also been modified to obtain carboxymethyl and acrylamide CL to remove dye [99], bivalent metals [130], with ammonium groups to remove turbidity with up to 99% efficiency [102], or with citric acid to remove dyes or oil with up to 87% efficiency [131].
Starch (ST) consist of numerous glucose units joined by glycosidic bonds. It consists of two types of molecules: linear and helical amylose and branched amylopectin [132]. On its own, ST has a low flocculation power, but despite of that, grafted, it can potentiate its ability to remove diverse contaminants. To explore ST’s amphiphilic capability, it has been grafted with polyacrylamide, and ammonium groups to remove kaolin and sodium humate up to a 95 and 99% efficiency [133], with acrylic acid to remove bivalent metals with up to 90% efficiency [104], or triazine to remove dyes in highly saline effluents because of its high pH sensitivity and capability to be reused 3 times and continue exhibiting flocculation capability with efficiencies up to 85% [134]. This last capability is a very rare property to be evaluated in polysaccharides giving a whole new spectrum to study on these materials, that can save resources and increase cost-benefit characteristics.
In general, polysaccharides are greatly versatile polymers that, as shown in table 2 can be very effective against a variety of pollutants and are widely used in the flocculation process, even more so in a coordinated PE complex system. Most of the anionic state polymers coincide in charge, functional groups, reactivity, and solubility, yet the abundance in usage depends a lot on availability and costs. For instance, hyaluronic acid [135], pullulans [136], or chondroitin sulfate [137] are less available and more expensive so there is a gap found in research and interest in using these as flocculants, however efficient they may be [138]. On the other hand, sulfated carrageenan (CG) [139] and agar (AR) [140] are more commonly used as emulsifiers than flocculants due to their high gelling and thickening capabilities, there for, most of the research focuses on these applications. Mostly, polysaccharide cost depends on availability, for example, CL is the most abundant polysaccharide in the planet, seconded by CH, CS’s precursor making them the most viable and cost effective resources for high scale flocculation applications [141].
5. Conclusion
Flocculation by polysaccharide-based PE is very versatile, mainly because of the polymer diversity and the almost infinite possibilities of combinations that can be achieved and studied with the native charges plus the grafted charges to obtain stable potential (either positive, negative, or both) to remove a variety of contaminants (heavy metal, COD, suspended solids such as kaolin or clay, dyes, and organic molecules or particles) with removal efficiencies from 80 to 99% which represent remarkable results. The polysaccharide’s flocculation power is directly affected by the grafted group and the charge density considering the diverse polysaccharide-graft options since they are very versatile flocculants for diverse pollutants removal. However, the operational challenge is the polysaccharides’ charge limitations, the pH dependency, and the affinity to ions that can interfere with their removal power, thus making it hard to apply in large scale, there for, there is a gap of opportunity to research scalability and efficiency in real, in situ situations. The research tendency reveals that adding to the degradability and biocompatibility of polysaccharides-based flocculants, they can offer efficient flocculation processes without toxic flocculant traces remaining or toxic mud formed. In other words, they represent excellent candidates for safer wastewater or bodies of water low cost and efficient treatment without the inorganic flocculant complications.











 nueva página del texto (beta)
nueva página del texto (beta)




