Introduction
Although native arteriovenous fistula (AVF) is the first option as vascular access for patients with end-stage renal disease, approximately 50% of them fail in the first two years of its creation. In these patients, the main cause of vascular access failure is stenosis of the venous portion of the AVF secondary to an inflammatory process called intimal hyperplasia1,2. The injury of the venous endothelium secondary to flow turbulence plays an important role. When the thickening of the vascular endothelium is excessive, the risk of graft thrombosis increases3,4. The acute phase occurs between 24 h and the first 7 days after manipulation of the venous endothelium. Citokines and growth factors are released after endothelial injury and stimulate smooth muscle cells (SMC) proliferation. Examples are Angiotensin II, bGFG, TGF-B and platelet derived growth factor (PDGF), and inflammatory cytokines such as IL-1, IL-6, IL-8 and TNF-α4-6. Intimal hyperplasia is the cellular and molecular response characterized by a continuous and abnormal proliferation, predominantly of SMC, connective tissue and extracellular matrix, leading to hyperplasia of the intimal layer of the vessel and luminal stenosis7,8. Proliferation cell control is associated with molecular noncoding sequences which regulate several key genes related to this biological function. Intimal hyperplasia includes unstable process of messenger RNAs (mRNAs) inhibition by non-coding sequences microRNas (miRNAs). The catalytic action of miRNAs is complex through RISC and exonucleases (on 3´-UTR site) or repression/desestabilizacion (P-bodies). Several features make miRNAs special regulators of gene expression particularly of proliferative genes associated with Intimal hyperplasia. The knowledge of miRNAs control associated with intimal hyperplasia pathogenesis needs to be studied. This is a complex mechanism because of its combinatorial control on mRNA expanding the available possibilities to the cell by linking gene expression to a combination of different regulators rather than a single regulator. MiRNAs are small 20-25 nucleotide non-coding ribonucleic acids capable of regulating mRNAs post-transcriptionally by inducing their destabilization or translational repression9-11. As post-transcriptional regulators, miRNAs regulate the negative gene expression by binding directly to the 3 untranslated region (3 UTR) of corresponding target mRNA in a specific manner and inducing the RNAm degradation or protein translation repression11. Various miRNAs play an important regulatory role in several cellular processes and can contribute to the initiation, development and progression of several vascular diseases12-14. Some miRNAs with special affinity for structural changes in SMC have been identified. One of the most interesting characteristics of miRNAs is that although they are regulators of several genes, their expression is specific cell and tissue15. An example of the alteration in the expression of miRNAs in the cardiovascular system is in the formation of atheroma plaques, where the down-regulation of miR-145 in SMC has been identified16. In arteries with intimal hyperplasia, down-regulation of miR-145 has been identified as a marker for the formation of intimal hyperplasia. Cheng and Cols. reported that miR-145 is the most abundant miRNA in normal vascular walls and in isolated SMC, representing an important phenotypic regulator. In fact, miR-145 is selectively expressed in SMC of the vascular wall and its expression is negatively regulated in vascular walls with intimal hyperplasia and in SMC in a proliferative state induced by PDGF16. In the study by Peter and Cols, it was reported that intact expression levels of miR-145 are required for SMC to display a quiescent phenotype17. Unfortunately, although there is much data on the expression of miR-145 in arteries, it has not been studied in depth in the venous endothelium, especially in the specific environment of AVFs. Some other miRNAs reported in the literature are miR-146 and miR-155, although there are fewer published data. In recent years, the main functions of miR-146 have been identified, including targets in the innate immune system, vascular and associated with proliferation and migration in cancer18. Regarding the vascular system, the influence of miR-146 was identified in SMC, regulating the expression of Kruppel-like Factor 4 (KLF4) by binding to its 3 UTR region. The silence of miR-146 increases the expression of KLF4 and its activation generates the opposite effect in KLF418,19. The overexpression of miR-146 increases proliferation of SMC and therefore the formation of intimal hyperplasia20. The role of miR-155 has also been studied, which in fact is a critical regulator of inflammatory diseases and has considerable effects on the vascular endothelium, such as atherosclerosis and intimal hyperplasia. Wang et al. studied the effect of miR-155 in a rat AVF model and identified that the miR-155 knockout reduces the expression of inflammatory factors in the outflow vein and prevents intimal hyperplasia21. Among therapeutic maneuvers to prevent dysfunction of vascular grafts secondary to intimal hyperplasia, several strategies have been developed, from surgical to endovascular procedures. Despite these advances, rates of vein graft failure remain quite high22. One of the main reasons for the lack of clear results in the therapeutic field is the lack of knowledge to fully understand the genomic pathways, both in the arterial and venous portion of an AVF with the aim of designing more effective targeted therapeutic strategies. Furthermore, much of the current research on intimal hyperplasia focuses on these processes in arteries leaving veins aside, which is important since veins are structurally and functionally different from arteries. In fact, no studies have been performed comparing the expression of miRNAs in veins and arteries, especially in the context of AVFs. In this study, we evaluated the expression of miR-145, miR-146, and miR-155 in a venous and arterial endothelium exposed to turbulent forces secondary to an AVF.
Materials and Methods
For the animal model two groups were included, of 3 animals each. In the experimental group, an aorto-caval fistula was performed. Sham surgery was performed in the other group. For this purpose, we used the surgical technique of the experimental model developed by Yamamoto et al.23, where an AVF is made by a puncture between the infrarenal vena cava and the abdominal aorta. Due to the availability of our bioterium, we used as an animal model Wistar Rat, Female, between 200 and 300 g. This project was carried out with the support of the Department of Surgery of the School of Medicine, at the National Autonomous University of Mexico. The study was accepted by the animal ethics committee (Research Division, Number 098) and based on the Official Mexican Standard 062-ZOO, regarding the use and care of laboratory animals, which is equivalent to the Guide for the Care and Use of Laboratory Animals24. For anesthetic management, we used 4% isoflurane with 0.8 l/min of oxygen in a plexiglass box through an isoflurane vaporizer. The anesthesia was conducted with 2% Isoflurane using a silicone mask. An exploratory laparotomy was performed to obtain retroperitoneal access to the vena cava and abdominal aorta. A clamp was placed in the aorta and a direct puncture was performed with a 22 G needle (this size was chosen due to the weight and size of the Wistar Rat, to make it proportional to the model described by Yamamoto et al.23). Direct compression was made over the puncture and patency of the aorto-caval fistula was verified. In the post-operative period, the vascular spectrum of the AVF was analyzed to verify its permeability by ultrasound. A daily evaluation was carried out during the first 7 days. Euthanasia was performed on the 7th day after the AVF. The venous portion of the fistula was carefully harvested (first 2 mm to the puncture). Samples were obtained during surgery procedure and immediately shipped in liquid nitrogen. Finally, samples were stored until used at −80ºC. The procedure for extraction and purification of total RNA from tissue (up to 5 mg tissue) was done using RNeasy Plus Micro Kit (Cat. 74034) from rat tissues. With the RNeasy Micro procedure, all RNA molecules longer than 200 nucleotides were purified. RNA samples were eluted with 25 μL of RNase-free water. The quality and concentration of each RNA sample was evaluated with RNA integrity number (RIN) > 6 using the Agilent Bioanalyzer 2100. RNA samples were chosen for analysis only when quality, integrity and concentration were achieved. After isolation and purification of total RNA, we made the reverse transcription and amplification using a polymerase chain reaction (PCR). We used small TaqMan RNA assays to detect and quantify mature miR-145, miR-146 and miR-155. To quantify the expression levels of miRNAs, we normalized endogenous control genes. We used DataAssit software (7500 Fast Real-Time PCR System/Software v2.0.6) to calculate the relative quantification of gene expression by means of the ΔΔCT-1 comparison. Significant statistical differences of intergenic sequences expression were obtained as p-value (*).
Results
The use of the experimental model was carried out without complications. In the exploratory analysis, we found a difference in the expression of miRNAs in the fistula versus control contrast within the first 7 days (Fig. 1). We observed a downregulation of miR-145 in the vein (0.233 ± 0.3, p = 0.0030), and artery (0.33 ± 0.4, p = 0.0630) of the arterio-venous fistula compared with the control vein (0.766 ± 0.15, p = 0.0110) and control artery (0.873 ± 0.3, p = 0.0600). We observed an upregulation of miR-146 in the vein (29 ± 0.25, p = 0.0256), and artery (34.66± 0.35, p = 0.0345) of the arterio-venous fistula compared with the control vein (5.43 ± 0.16, p = 0.0123) and control artery (3.8± 0.12, p = 0.0650). Finally, we observed an upregulation of miR-155 in the vein (20 ± 0.39, p = 0.0231), and artery (22.66 ± 0.49, p = 0.0234) of the arterio-venous fistula compared with the control vein (2.74 ± 0.2, p = 0.0367) and control artery (3.43 ± 0.2, p = 0.0767) (Table 1).
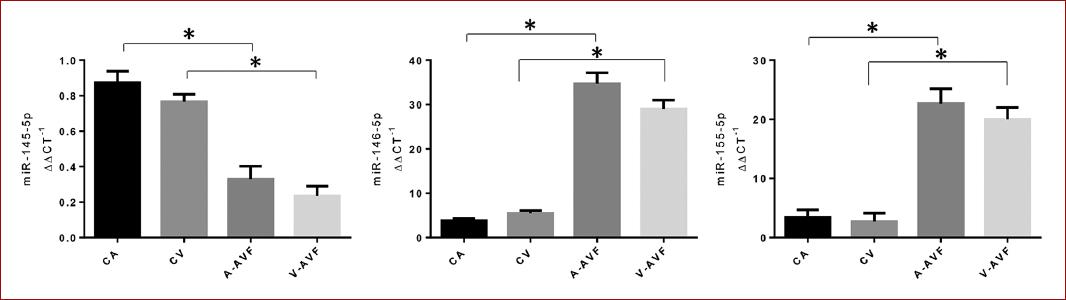
Figure 1 Relative expression of miR-145, miR-146 and miR-155. A downregulation of miR-145 can be observed both in vein and artery compared to their respective control. Likewise, an upregulation of miR-146 and miR-155 is observed both in vein and artery compared to the control. Control artery (CA), control vein (CV), arteriovenous fistula artery (AVF-A), arteriovenous fistula vein (AVF-V).
Table 1 Relative expression (RE) levels of miR-145, miR-146 and miR-155 in the experimental model of arteriovenous fistula compared with the control
| Experimental model | miR-145 (ΔΔCT-1) | p-value | RE | miR-146 ΔΔ(CT-1) | p-value | RE | miR-155 (ΔΔCT-1) | p-value | RE |
|---|---|---|---|---|---|---|---|---|---|
| Control artery | 0.873 ± 0.3 | 0.0600 | UP | 3.8 ± 0.12 | 0.0650 | Down | 3.43 ± 0.2 | 0.0767 | Down |
| Control vein | 0.766 ± 0.15 | 0.0110 | UP | 5.43 ± 0.16 | 0.0123 | Down | 2.74 ± 0.2 | 0.0367 | Down |
| Artery of AVF | 0.33 ± 0.4 | 0.0630 | Down | 34.66 ± 0.35 | 0.0345 | UP | 22.66 ± 0.49 | 0.0234 | Up |
| Vein of AVF | 0.233 ± 0.3 | 0.0030 | Down | 29 ± 0.25 | 0.0256 | Up | 20 ± 0.39 | 0.0231 | Up |
AVF: arteriovenous fistula.
Discussion
Veins are used as a vascular graft in AVF due to their effectiveness and patency. Despite being considered the best vascular graft, they are not without complications. Veins, whose physiological hemodynamic environment is characterized by blood flow with low longitudinal and radial stress, when used as a vascular graft in the arterial system, show remodeling of their vascular structure. In other words, the graft has a process of adaptation to high pressure blood flow, arterializing, that is, increasing the amount of extracellular matrix and SMC under the venous endothelium, a process known as intimal hyperplasia. Because intimal hyperplasia is the main cause of venous graft failure, various treatments have been proposed, ranging from innovations in open and endovascular surgery, pharmacological treatments, to gene therapies, the results of which continue to be controversial. One of the proposals for the absence of conclusive results in this therapeutic field is the lack of knowledge to fully understand the molecular and genomic pathways that regulate the initiation and progression of intimal hyperplasia with the aim of designing more effective targeted therapeutic strategies. In recent years, the regulation of SMC have been identified as a critical event in the development of intimal hyperplasia. The venous endothelium normally produces factors that maintain homeostasis and inhibit their proliferation. SMC have external stimulus-dependent plasticity and two main types of cells have been described20. There is a quiescent, contractile and differentiated phenotype whose structure is essentially composed of alpha-actin and myosin, which has a low proliferation and constitutes the main structure of the middle layer in vessels25. On the other hand, a proliferative phenotype of SMC has been described, which are key cells in the development of intimal hyperplasia26. There are mechanisms that can produce this change from a contractile phenotype to a synthetic one, especially associated with a vascular lesion. Interestingly, miRNAs can regulate this sequence of events20. SMC are an essential element in blood vessels since they constitute structural support, confer elasticity, and regulate vascular tone (vasodilation and vasoconstriction). However, if this architecture is altered after a stimulus such as a vascular lesion, there are modifications in this physiological and structural balance, which is evidenced as intimal hyperplasia20,25. Due to their regulatory functions, miRNAs are essential modulators for important cellular functions such as cell differentiation, contraction, migration, proliferation, and apoptosis. It is to be expected that the expression of miRNAs is altered in the main pathological processes involving the cardiovascular system, including intimal hyperplasia27. One of the most interesting characteristics of miRNAs is that although they are regulators of several genes; their expression is specific in cells and tissues28,29. An example in blood vessels is miR-145, miR-146, and miR-155, of which its regulatory effect has been documented in SMC therefore in the formation of intimal hyperplasia20. In our study, we found a downward expression of miR-145 in the vein of the model with fistula, compared to the control, in the first 7 days. This pattern was also observed in the arterial portion, which has not been evaluated in detail in the literature. This can have important implications because the fistula must be considered globally, not separately the venous and arterial segments. In addition, diagnostic and therapeutic efforts should also be directed to the treatment of the venous and arterial portion. Interestingly, the negative regulation of miR-145 has been associated with the phenotypic transformation from a quiescent state to a proliferative state30, and this was corroborated by Zhang who reported that the overexpression of miR-145 increased the expression of marker genes of SMC differentiation such as α-actin, myosin heavy chain, and calponin. In contrast, the levels of these genes decreased in SMC treated with a miR-145 inhibitor, 2OMe-miR-145. In addition, in the SMC of arteries that underwent angioplasty or that were exposed to a PDGF concentration of 20 ng/ml, the expression of miR-145 was significantly decreased31. In our study, an up regulation of miR-146a and miR-155 was observed in the first 7 days after the initial stimulus of intimal hyperplasia in an AVF, both in the venous portion and in the arterial portion. Furthermore, when comparing the results, we found an overexpression of both miRNAs in the arterial portion compared to the venous portion. miR-146 is involved in SMC proliferation through its target gene KLF419. miR-146 expression was elevated in an experiment performed in rat carotids, using a balloon injury model. In this study, miR-146 inhibition attenuated SMC proliferation and neointima formation in carotid arteries32. However, the role of miR-146 in the development of neointimal lesions associated with venous graft failure in AVFs has not been described in detail. KLF4 is a transcription factor encoded in the KLF4 gene and is made up of three zinc finger motifs within their carboxyl terminal sequences33. It has an important function to maintain the integrity of the vascular wall and is associated with processes of differentiation, proliferation, and cell growth34. It also has important structural functions associated with the functioning of SMC, which has been related to the regulation through myocardin and serum response factor20. The expression of KLF4 is regulated by transcriptional and post-transcriptional pathways, with regard to the vascular system there is an important regulation through miRNAs34. This represents a potential area of opportunity, because by developing therapeutic targets aimed at reestablishing or regulating the levels of these factors, it could potentially be possible to attenuate or regulate the intimal hyperplasia process. Regarding miR-155, it has been identified that it increases the production of intimal hyperplasia and that it induces inflammation through macrophages. This biological effect stimulates the proliferation of SMC by integrating oxidative and inflammatory stress signals. As we have mentioned, this biological effect has been studied more deeply in arteries, where there is an increase in intimal hyperplasia and secondary atherosclerosis. In our AVF model, we also found upregulation of miR-155 both in the arterial and venous portions in a model that favors the formation of intimal hyperplasia, so there may be an association with mechanisms that stimulate SMC proliferation. As we observed in the results of our study, the regulation of miRNAs on genes with structural functions in the wall of the blood vessels is very important. In the specific environment of venous intimal hyperplasia, in-depth knowledge about the miRNAs with effects on the endothelium and their interaction with SMC, as well as the target genes with structural functions on the vein wall is essential. One of the main importances of this is the development of potential therapeutic targets. The main limitation of our study is that we only studied the expression of miR-145, miR-146, and miR-155 in the earliest phase of the development of intimal hyperplasia (first 7 days).
Conclusion
We found alterations in the expression of miR-145, miR-146, and miR-155 comparing to AVF versus control, both in the arterial and venous portions in an experimental model of AVF through a puncture between the inferior vena cava and the infrarenal aorta. These miRNAs are involved in genes with structural processes such as differentiation, migration, and inflammation.











 nueva página del texto (beta)
nueva página del texto (beta)


