Articles
Improved Knoevenagel Condensation Protocol for the Synthesis of Cyanoacrylates and their Anticancer Activity
-
Publication dates-
March 11, 2024
Jan-Mar , 2023
- Article in PDF
- Article in XML
- Automatic translation
- Send this article by e-mail
- Share this article +
Abstract.
DIPEAc (diisopropylethylammonium acetate) has been used as a catalyst in the Knoevenagel condensation of aldehydes and ethyl cyanoacetate to produce cyanoacrylate with high yields. The reaction's key characteristics are a shorter reaction time, a large substrate scope, the viability of different functional groups, ease of work-up, and high yields, which provide environmental benefits. Furthermore, the anticancer activity of the synthesized series of cyanoacrylate derivatives was tested against A549, HT-29, and HepG2 cell lines.
Keywords::
Ethyl cyanoacetate, diisopropylethylammonium acetate (DIPEAc), cyanoacrylate, Knoevenagel condensation, anticancer activity
Introduction
In recent decades, the development of homogeneous catalyzed transformations has played a critical role in organic synthesis. Ionic liquids have been used as catalysts or reagents in homogeneous medium in organic synthesis in recent years [1-3]. Ionic liquids have created numerous opportunities for their use in the synthesis of key motifs, which has generated tremendous interest from an academic, industrial, and specifically in the synthesis of pharmaceutical compounds [4]. Ionic liquid solubility can be altered by modifying cations and anions to improve catalytic activity and solubility differences in an aqueous medium and less polar solvent system. The ionic liquid catalyst/reagent can be separated from the reaction at the end and reused or regenerated [5-8].
-
1Adv. Synth. Catal, 2007
-
3Tetrahedron Lett, 2008
-
4Chem. Eur. J, 2010
-
5J. Org. Chem, 2006
-
8J. Chem. Thermodyn, 2021
Room temperature ionic liquid supported (ILs) catalysts are used in homogeneous organic synthesis to achieve higher catalytic activity and selectivity than a homogeneous parent catalytic system. Advantages of ionic liquids, which have been designed to be a soluble polar organic solvent by incorporating a nonpolar organic solvent that can be recovered and reused. Ionic liquids have a number of advantages. 1) ionic character and high polarity 2) ionic liquid supported catalysts outperform solid supported catalysts in terms of loading capacity. 3) excellent selectivity.
In organic synthesis, the Knoevenagel condensation reaction is the most powerful tool for forming a C-C bond. Emil Knoevenagel developed a valuable method [9] for Knoevenagel condensation between formaldehyde and diethylmalonate with diethylamine as a catalyst in 1894. Later several improvements have been reported by different research groups such as piperdine [10], L-Proline [11], β-alanine [12], TBAB [13], [MeHMTA]BF4 [14], resin poly (propylene imine)dendrimer [15], nano-Fe3O4 [16], 2,4,6-trichloro-1,3,5- triazine (TCT) [17], [bmim(NTf2)] [18], Al2O3-SiO2 [19] and other conditions [20].
-
9Ber. Dtsch. Chem. Ges, 1894
-
10J. Am. Chem. Soc, 1929
-
11J. Mol. Catal. A: Chem, 2006
-
12Chin. J. Chem, 2012
-
13ARKIVOC, 2007
-
14Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem, 2015
-
15Tetrahedron Lett, 2014
-
16J. RSC Adv, 2017
-
17RSC Adv, 2014
-
18J. Braz. Chem. Soc, 2012
-
19Journal of Chemistry, 2013
-
20Green Chem. Lett. Rev, 2020
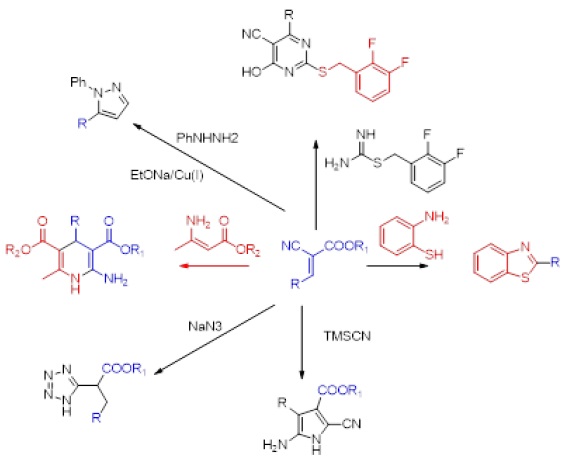
On the other hand, cyanoacrylate is having several applications, which undergoes several transformations with different reagents to produce variety of biologically active scaffolds, including 1,5- disubstituted pyrazoles [21], substituted tetrazoles [22], 2-amino dihydropyridine derivatives [23], 2-amino pyrrole derivatives [24], pyrimidine-5-carbonitrile [25], benzothiazole derivatives [26] (Sheme 1).
-
21Asian. J. Chem, 2020
-
22Mol. Diversity, 2017
-
23J. Chem. Crystallogr, 2007
-
24J. Org. Chem, 2018
-
25J. Bioorg. Med. Chem. Lett, 2014
-
26Lett. Drug Des. Discovery, 2013
Thumbnail
Scheme 1
Applications of cyanoacrylates.
Applications of cyanoacrylates.
However, numerous methods for Knoevenagel condensation are available in the literature, with several drawbacks such as strong acidic, hazardous, and expensive reagents, toxic nature, longer reaction time, and lower yields. Many researchers have worked seriously to develop an efficient cyanoacrylate synthesis using Knoevenagel condensation, which is still needed.
In this paper based on our research programme [27,28], we report an efficient Knoevenagel condensation between ethyl cyanoacetate and aromatic aldehyde in the presence of diisopropylethylammonium acetate (DIPEAc) for the formation of cyanoacrylates in good to excellent yields. Diisopropylethyl ammonium acetate (DIPEAc) was used as an ionic liquid catalyst for various synthetic applications, including the synthesis of Biginelli dihydropyrimidines and dihydrothiophenes by Gill et al. [29,30]
-
27Asian. J. Chem, 2016
-
28ACS Omega, 2019
-
29ACS Omega, 2020
-
30Chemistry Select, 2020
Experimental
Materials and methods
All the reagents and solvents used in the synthesis are laboratory grade. The purity of the compounds was checked by TLC (silica gel 60 F254), which were purchased from merck Inc and visualized under UV light. Melting points are analyzed for all synthesized analogues by open tube capillary tube by using Meltemp equipment. The mass spectrums were obtained on Agilent (1100 series) instrument. The Perkin Elmer FT-IR spectrometer was used for IR spectra.
1HNMR &13CNMR were recorded with BRUKER-400 MHz spectrometer using CDCl3/DMSO solvent. All the chemical shifts were reported in δ (ppm), using tetramethylsilane (TMS) as internal standard. Multiplicities are recorded by the following abbreviation: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; bs broad singlet; bd, broad doublet; J, coupling constant (hertz).
General procedure for synthesis of Cyano acrylate derivatives
Diisopropylethylammonium acetate (0.1 mmol) was added to a mixture of aromatic aldehydes 1a-1l (1 mmol), ethylcyanoacetoacetate 2 (1 mmol) in hexane (10 ml) and heated at 65-70 °C. After 3-6 hours, the progress of reaction was monitored by TLC (hexane:Ethylacteate, 8:2). After completion of the reaction, was cooled to 40-45 °C. Separated the layers and bottom (product) layer was concentrated under vacuum, resulting material was purified by suitable solvents to give desired products are given below (3a-3l).
Ethyl-2-cyano-3-phenylacrylate (3a). [1] solid (91 %) mp 48-51 °C; IR (KBr) Cm-1: 2982, 2220, 1716, 1600, 1440; 1HNMR (400 MHz, CDCl3): δ1.41 (t, J= 7.4 Hz, 3H), 4.36-4.41 (q, J = 7.4 Hz, 2H), 7.50-7.60 (m, 3H), 8.02 (d, J= 7.4 Hz, 2H), 8.40 (s, 1H); 13CNMR (100 MHz, CDCl3): δ14.2, 62.8, 103.0, 115.5, 130.1, 131.1, 131.5, 133.4, 155.1, 162.5.Anal Calc for C12H11NO2: C, 71.63; H, 5.51; N, 6.96 %; found:C, 71.59; H, 5.48 ; N, 6.84 %.
-
1Adv. Synth. Catal, 2007
Ethyl-2-cyano-3-(2-nitrophenyl)acrylate (3b). [3] solid (90 %) mp 119-123 °C; IR (KBr) Cm-1: 2986, 2218, 1718, 1582, 1485; 1HNMR (400 MHz, CDCl3): δ= 1.42 (t, J= 7.4 Hz, 3H), 4.39-4.45 (q, J = 7.4 Hz, 2H), 7.70- 7.74 (m, 1H), 7.80-7.87 (m, 2H), 8.28 (d, J= 7.4 Hz, 2H), 8.72 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 14.1, 63.1, 106.7, 113.7, 125.3, 128.1, 130.5, 132.1, 134.4, 147.3, 152.9, 161.0;Anal Calc for C12H10N2O4: C, 58.54; H, 4.09; N, 11.38 %; found:C, 58.50; H, 3.99; N, 11.32 %.
-
3Tetrahedron Lett, 2008
Ethyl-2-cyano-3-(3-nitrophenyl)acrylate (3c). [3] solid (91 %) mp 129-132 °C; IR (KBr) Cm-1: 2929, 2252, 1738, 1600, 727; 1HNMR (400 MHz, CDCl3): δ= 1.42 (t, J= 7.4 Hz, 3H), 4.40-4.45 (m, 2H), 7.72-7.76 (m, 1H), 8.32 (s, 1H), 8.39-8.42 (m, 2H), 8.70 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 13.5, 62.7, 106.3, 113.9, 125.3, 126.4, 130.0, 132.5, 134.6, 148.2, 151.2, 160.9;Anal Calc for C12H10N2O4: C,58.54; H,4.09; N,11.38 %; found:C, 58.51 ; H, 4.01; N, 11.29 %.
-
3Tetrahedron Lett, 2008
Ethyl-2-cyano-3-(4-nitrophenyl)acrylate (3d). [3] solid (93 %) mp 161-165 °C; IR (KBr) Cm-1: 2856, 2196, 1738, 1519, 854; 1HNMR (400 MHz, CDCl3): δ= 1.32 (t, J= 7.2 Hz, 3H), 4.32-4.38 (m, 2H), 8.23-8.26 (m, 2H), 8.42-8.40 (m, 2H), 8.56 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 14.4, 63.2, 107.1, 115.4, 124.6, 132.1, 137.7, 149.7, 153.1, 161.6; Anal Calc for C12H10N2O4: C,58.54; H,4.09, ; N,11.38 %; found:C, 58.48; H, 4.03; N, 11.35 %.
-
3Tetrahedron Lett, 2008
Ethyl-2-cyano-3-(2-Chlorophenyl)acrylate (3e). [1,2] solid (88 %) mp 45-48 °C; IR (KBr) Cm-1: 2981, 1716, 1688, 1441, 755; 1HNMR (400 MHz, CDCl3): δ= 1.41 (t, J= 7.2 Hz, 3H), 4.38-4.43 (m, 2H), 7.41-7.51 (m, 3H), 8.22-8.24 (m, 1H), 8.68 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 14.0, 62.9, 106.1, 114.8, 127.4, 129.8, 130.3, 136.4, 151.2, 161.8; Anal Calc for C12H10ClNO2: C,61.16; H,4.28, ; N,5.94 %; found:C, 61.09 ; H, 4.23; N, 5.89 %.
-
1Adv. Synth. Catal, 2007
-
2Chem. Lett, 2007
Ethyl-2-cyano-3-(4-Chlorophenyl)acrylate (3f). [1] solid (94 %) mp 88-90 °C; IR (KBr) Cm-1: 2986, 2218, 1718, 1582, 1485, 828; 1HNMR (400 MHz, CDCl3): δ= 1.40 (t, J= 7.2 Hz, 3H), 4.36-4.41 (m, 2H), 7.48(d, J= 7.2 Hz, 2H), 7.93 (d, J= 7.2 Hz, 2H), 8.19 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 14.1, 62.8, 103.5, 115.2, 129.6, 129.9, 139.5, 153.3, 162.1; Anal Calc for C12H10ClNO2: C,61.16; H,4.28, ; N,5.94 %; found:C, 61.08; H, 4.19; N, 5.87 %.
-
1Adv. Synth. Catal, 2007
Ethyl-2-cyano-3-(4-methoxyphenyl)acrylate (3g). [1,2] solid (96 %) mp 77-80 °C; IR (KBr) Cm-1: 2988, 2213, 1712, 1583, 1177; 1HNMR (400 MHz, CDCl3): δ= 1.37 (t, J= 7.2 Hz, 3H), 3.87 (s, 3H), 4.32-4.37 (m, 2H), 6.97 (d, J= 7.2 Hz, 2H), 7.98 (d, J= 7.2 Hz, 2H), 8.15 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 14.1, 55.8, 62.4, 99.4, 114.7, 116.2, 124.3, 133.6, 154.3, 163.1, 163.7; Anal Calc for C13H13NO3: C,67.52; H,5.67 ; N,6.06 %; found:C, 67.49; H, 5.61; N, 6.03 %.
-
1Adv. Synth. Catal, 2007
-
2Chem. Lett, 2007
Ethyl-2-cyano-3-(4-benzyloxyphenyl)acrylate (3h). [5] solid (93 %) mp 146-149 °C; IR (KBr) Cm-1: 2922, 2218, 1714, 1586, 1177, 903; 1HNMR (400 MHz, CDCl3): δ= 1.33 (t, J= 8.0 Hz, 3H), 4.34-4.39 (m, 2H), 5.15 (s, 2H), 7.04-7.08 (m, 2H), 7.34-7.45 (m, 5H), 8.01 (d, J= 4.0 Hz, 2H), 8.17 (s, 1H);13CNMR (100 MHz, CDCl3): δ= 14.1, 62.4, 70.3, 99.4, 115.5, 116.1, 124.5, 127.4, 128.3, 128.7, 133.6, 135.7, 154.3, 162.8; Anal Calc for C19H17NO3: C,74.25; H,5.58 ; N,4.56 %; found:C, 74.18; H, 5.51; N, 4.53 %.
-
5J. Org. Chem, 2006
(4E)-Ethyl-2-cyano-5-phenylpenta-2,4-dienoate (3i). [6] solid (96 %) mp 113-115 °C; IR (KBr) Cm-1: 2981, 2248, 1737, 1580, 1449, 1236; 1HNMR (400 MHz, CDCl3): δ= 1.28 (t, J= 7.2 Hz, 3H), 4.24-4.30 (m, 2H), 7.18-7.25 (m, 1H), 7.46-7.48 (m, 3H), 7.69-7.72 (m, 3H), 8.17 (d, J= 7.2 Hz, 1H);13CNMR (100 MHz, CDCl3): δ= 14.4, 62.4, 103.7, 115.0, 123.0, 129.1, 129.6, 131.7, 135.0, 150.5, 156.3, 162.2; Anal Calc for C14H13NO2: C,73.99; H,5.77 ; N,6.16 %; found:C, 73.91; H, 5.63; N, 6.09 %.
-
6J. Mol. Catal. A: Chem, 2004
Ethyl-2-cyano-3-p-tolylacrylate (3j). [1, 2] solid (92 %) mp 88-92 °C; IR (KBr) Cm-1: 2983, 2251, 1742, 1600, 1445; 1HNMR (400 MHz, CDCl3): δ= 1.39 (t, J= 7.4 Hz, 3H), 2.46 (s, 3H), 4.34-4.40 (m, 2H), 7.18-7.30 (d, J= 8.0 Hz, 1H), 7.86 (d, J= 8.0 Hz, 1H), 8.22 (s, 1H); 13CNMR (100 MHz, CDCl3): δ= 13.8, 21.4, 62.0, 101.1, 115.3, 128.5, 129.5, 130.8, 144.2, 154.5, 162.3. Anal Calc for C13H13NO2: C,72.54; H,6.09 ; N,6.51 %; found:C, 72.38; H, 6.04; N, 6.39 %.
-
1Adv. Synth. Catal, 2007
-
2Chem. Lett, 2007
Ethyl-2-cyano-3-(furan-2-yl)acrylate (3k). [3] solid (90 %) mp 83-85 °C; IR (KBr) Cm-1: 2984, 2252, 1741, 1613, 1462; 1HNMR (400 MHz, CDCl3): δ= 1.38 (t, J= 7.2 Hz, 3H), 4.33-4.38 (m, 2H), 6.66-6.67 (m, 1H), 7.39-7.40 (m, 1H), 7.75-7.76 (m, 1H), 8.01 (s, 1H) ;13CNMR (100 MHz, CDCl3): δ= 14.1, 62.5, 98.7, 113.8, 115.2, 121.5, 139.4, 148.1, 148.7, 162.5; Anal Calc for C10H9NO3: C,62.82; H,4.74 ; N,7.33 %; found:C, 62.63; H, 4.56; N, 7.09 %.
-
3Tetrahedron Lett, 2008
Ethyl-2-cyano-3-(thiophen-2-yl)acrylate (3l)- [4] solid (91 %) mp 93-96 °C; IR (KBr) Cm-1: 3085, 2919, 2217, 1715, 1596, 1216; 1HNMR (400 MHz, CDCl3): δ= 1.39 (t, J= 7.2 Hz, 3H), 4.39-4.34(m, 2H), 7.22-7.26 (m, 1H), 7.78-7.84 (m, 2H), 8.35 (s, 1H) ;13CNMR (100 MHz, CDCl3): δ= 14.1, 62.5, 99.3, 115.7, 128.5, 135.1, 137.1, 146.6, 162.6; Anal Calc for C10H9NO2S: C,57.95; H,4.38 ; N,6.76; S, 15.47 %; found:C, 57.88; H, 4.31; N, 6.59; S 15.36 %.
-
4Chem. Eur. J, 2010
Mechanism
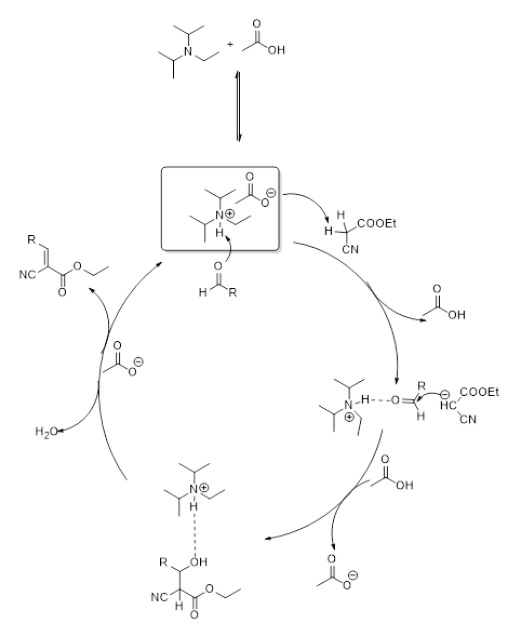
Scheme 2 proposes a plausible mechanistic pathway based on controlled experiments and previous literature reports. In step 1, the DIPEAc catalyst deprotonates a proton from the active methylene compound to form carbanion. The nucleophilic attack of a carbanion on the carbonyl carbon of an aldehyde to form a C-C bond with an alcohol compound. The anion of DIPEAc attacks alcohol to produce the dehydrated product cyanoacrylates. In this reaction mechanism, the counter anions play an important role in activating the nucleophile of the active methylene compound to produce carbanion, while the cation acts as an activator for the electrophiles of the carbonyl compound by increasing C=O bond polarity via hydrogen bond formation. Finally, the Knoevenagel condensed product is obtained by dehydrating or expelling water molecules from the structure.
Thumbnail
Scheme 2
Plausible mechanistic pathway.
Plausible mechanistic pathway.
The synthesized compounds (3a-3l) were tested for cytotoxic activity against human cancer cell lines A549 (Human lung adenocarcinoma cell line), HepG2 (Human liver cancer cell line), and HT-29 (Human colorectal adenocarcinoma cell line) using our previously followed protocol [31], and IC50 values were calculated using a linear regression equation from dose response data obtained at different concentrations. Doxorubicin (CAS 23214-92-8) was chosen as the reference standard medication due to its well-known therapeutic efficacy in a wide range of malignancies. The cyanoacrylates (3a-l) synthesised were discovered to have a 10-65 % inhibitory capacity against the proliferation of cancer cell lines. A dose-response study was conducted on chemicals that inhibited cancer cell growth by 50 % or more, and their IC50 values were examined. A comparative data of the IC50 values for the cyano acrylate are presented in Table 1.
Table 1
Results of anticancer activity on selected cell lines using different concentration (0-200 µg/mL).
Results of anticancer activity on selected cell lines using different concentration (0-200 µg/mL).
| S. No. | Compound | Activity IC 50 (µg/mL) | ||
| A549 | H epG2 | H T -29 | ||
| 1 | 3a | >200 | >200 | 179.5± 16.4 |
| 2 | 3b | >200 | >200 | 130.8± 22.5 |
| 3 | 3c | 175.8± 16.3 | 192.5± 17.1 | 169.4± 10.3 |
| 4 | 3d | 183.4± 14.3 | >200 | 172.7± 15.1 |
| 5 | 3e | 169.6 ± 11.2 | >200 | 131.6± 9.7 |
| 6 | 3f | >200 | 171.3± 13.4 | 170.7± 15.3 |
| 7 | 3g | >200 | 178.5± 20.7 | >200 |
| 8 | 3h | >200 | 163.5± 8.7 | 179.5± 18.1 |
| 9 | 3i | 77.78± 5.8 | 158.5± 14.2 | 117.6± 10.4 |
| 10 | 3j | >200 | 170.1± 12.5 | >200 |
| 11 | 3k | >200 | >200 | >200 |
| 12 | 3l | >200 | 184± 13.8 | >200 |
| Reference | Doxorubicin | 1.1 ± 0.4 | 0.8 ± 0.3 | 1.3 ± 0.6 |
Cinnamyl (3i) and 4-methoxyphenyl (3g), 4-benzyloxyphenyl (3h), and 4-chlorophenyl (3f) compounds were discovered to have good cytotoxic activity against HT-29 and HepG2 cancer cell lines. All para substituted phenyl (3g, 3h, 3f) demonstrated increased anticancer activity. The compounds 3i and 3e demonstrated more potent cancer activity, with IC50 values of 77.78 and 169.6 µg/mL, respectively. Similarly, the compounds with phenyl (3a), 2-nitrophenyl (3b), 4-nitrophenyl (3d), and 2-chlorophenyl (3e) substitutions demonstrated improved cytotoxicity against HT-29 and HepG2 cancer cell lines. However, substitution on the para-position increased activity more than substitution on the ortho- and meta-position. In respect of colorectal cancer cell line HT-29 and liver cancer cell line HepG2, cinnamyl analogue 3i proved to be the most potent compound with superior anticancer activity, suggesting that conjugated cyanoacrylate is a good platform for further optimization to develop an anti-cancer activity. It is anticipated that SAR study may help in designing future analogs for promising anticancer molecule.
In conclusion, we established an efficient Knoevenagel condensation protocol for the synthesis of cyanoacrylates with a wide range of substrates to demonstrate the protocol's generality. We believe that this methodology, which provides superior benefits for the construction of cyanoacrylates using commercially available and inexpensive materials, will gain widespread acceptance in the synthetic organic chemistry, medicinal, and pharmaceutical industries. The anti-cancer activity of the synthesized compounds was tested on the cancer cell lines A549, HepG2, and HT29. The compounds 3i and 3e demonstrated more potent cancer activity, with IC50 values of 77.78 and 169.6 g/mL for various cancer cell lines, highlighting the role of substituent position in the activity of the compounds. These potential compounds and analogues could be further optimized and developed as a novel class of anti-cancer agents.
Results and conclusions
We began with benzaldehyde (1 mmol) and ethylcyanoacetate (1 mmol) as model substrates to achieve optimal conditions for Knoevenagel reaction. The reaction carried out in MDC without a catalyst yielded 5 % product at reflux temperature and there was no further progress in the reaction with increased time. We tested by changing the catalyst, temperature, and solvents, and the results are summarized in Table 2. We tested various catalysts, including triethylamine, piperdine, piperdine acetate, triethylamine hydrochloride, phenylalanine, and diisopropylethyl ammonium acetate, to obtain the preferred product (DIPEAc). We repeated the same reaction with tetraethylammonium tetrafluoroborate and tetrabutylammonium chloride as catalysts and obtained 62 % and 57 % of the desired product, respectively. Among the tested conditions, the product was obtained in excellent yield in the presence of diisopropylethyl ammonium acetate (DIPEAc).
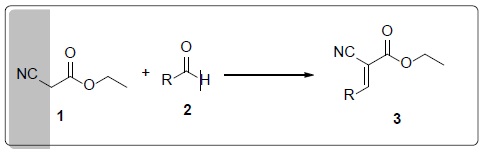
Thumbnail
Scheme 3
Optimization of reaction condition.
Optimization of reaction condition.
Table 2
Comparison of different catalyst efficiency for the synthesis.
Comparison of different catalyst efficiency for the synthesis.
| S. No. | Catalyst | medium | Time | Yield of compound 3 |
| 1 | Without catalyst | MDC | 12h | 5 % |
| 2 | Triethylamine | MDC | 12h | 60% |
| 2 | Triethylamine hydrochloride | MDC | 10 h | 64% |
| 3 | Piperidine | MDC | 8h | 72% |
| 4 | Piperdine acetate | MDC | 10h | 58% |
| 5 | Phenylalanine | THF | 12h | 52% |
| 6 | Diisopropylethyl ammonium acetate | MDC | 8 h | 76% |
| 7 | Diisopropylethyl ammonium acetate | Hexane | 3h | 91% |
| 8 | Tetraethylammonium tetrafluoroborate | Hexane | 7h | 62% |
| 9 | Tetrabutylammonium chloride | Hexane | 7h | 57% |
Inspired by this preliminary success, we carried out the reaction with varying amounts of DIPEAc ranging from 2 % to 20 %. (Table 3). At reflux temperature, 10% DIPEAc yields 91 % of the 3a. Increasing the amount of DIPEAc did not improve the reaction yield.
Table 3
Comparison of different amount catalyst.
Comparison of different amount catalyst.
| S. No. | Catalyst quantity | Time | Yield |
| 1 | 2% | 12 h | 55% |
| 2 | 5% | 10 h | 78% |
| 3 | 10% | 3 h | 91% |
| 4 | 15% | 3h | 91% |
| 5 | 20% | 3h | 90% |
So, 10 mol % DIPEAc was tested in various solvents, including MDC, hexane, ethanol, methanol, DMF, acetonitrile, THF, and diisopropyl ether. In hexane, the reaction proceeds smoothly and with a high yield. At reflux temperature, the Diisopropyl ether reaction proceeds with a lower yield than the MDC, ethanol, methanol, DMF, acetonitrile, and THF reactions. Based on the above optimization, we have reached a final concentration of 10 mol % DIPEAc in hexane solvent at reflux temperature, yielding the desired product with 91 % yield.
Similar reaction conditions were successfully used to produce high yields of various substituted cyanoacrylates (3a-o) (Table 4). With various substituted aromatic aldehydes (2a-2o), electron releasing groups such as -OMe, -OBn, and 4-Me formed corresponding cyanoacrylates with excellent yields of 96 %, 93 %, and 92 %, respectively. Under these optimized conditions, halogen conataing (-Cl) aromatic aldehyde performed well (Table 4). Electron withdrawing groups and hetero aryl aldehydes provide up to 95 % yield of cyanoacrylates. With the ideal condition in hand, we applied the same conditions to various aldehydes, including cinnamaldehydes, to produce the desired product up to 90 % yield (Table 4).
Table 4
Substrate scope of Cyanoacrylate.
Substrate scope of Cyanoacrylate.
| S. No. | Aldehyde | product | Yield (%) |
| 1 | 91 | ||
| 2 |
|
90 | |
| 3 | 91 | ||
| 4 | 93 | ||
| 5 | 88 | ||
| 6 | 94 | ||
| 7 | 96 | ||
| 8 | 93 | ||
| 9 | 96 | ||
| 10 | 92 | ||
| 11 | 90 | ||
| 12 | 91 |
References
- 1. Lombardo, M.; Pasi, F.; Easwar, S.; Trombini, C. Adv. Synth. Catal. 2007, 349, 2061-2065. Links
- 2. Zhou, L.; Wang, L. Chem. Lett. 2007, 36, 628-629. Links
- 3. Siyutkin D.E.; Kucherenko A.S.; Struchkova M.I.; Zlotin S.G. Tetrahedron Lett. 2008, 49, 1212-1216. Links
- 4. Ni, B.; Headley, A.D. Chem. Eur. J. 2010, 16, 4426-4436. Links
- 5. . Handy, S.T. J. Org. Chem. 2006, 71, 4659-4662. Links
- 6. Anjaiah, S.; Chandrasekhar, S.; Gree, R. J. Mol. Catal. A: Chem .. 2004, 214, 103-106. Links
- 7. Vijaya Durga, T.; Rambabu, A.; Srinivas Reddy, M.; Hari Babu, B. Asian. J. Chem. 2017, 29, 1313-1316. Links
- 8. Sowjanya, P.; Srinivasa Rao, V.; Srinivas Reddy, M.; Md Nayeem, S.K.; Haribabu, B. J. Chem. Thermodyn.. 2021, 154, 106330. Links
- 9. Knoevenagel, E. Ber. Dtsch. Chem. Ges. 1894, 27, 2345-2346. Links
- 10. Rodionow, W. M. J. Am. Chem. Soc. 1929, 51, 847-852. Links
- 11. Wang, Y; Shang, Z.; Wu, T.; Fan, J.; Chen, J.; J. Mol. Catal. A: Chem. 2006, 253, 212-221. Links
- 12. Zhu, L.; Lei, N.; Miao, Z.; Sheng, C.; Zhuang, C.; Yao, J.; Zhang, W. Chin. J. Chem. 2012, 30, 139-143. Links
- 13. Gupta, M.; Wakhloo, B.P. ARKIVOC, 2007, 94-98. Links
- 14. Sanjoy, K.; .Nimalini, M; Warjeet, S.L. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2015, 54B, 1157-1161. Links
- 15. Rajesh Krihnan, G.P.; Sreekumar, K. Tetrahedron Lett. 2014, 55, 2352-2354. Links
- 16. Zhang, Q.; Ma, X.M.; Wei, H.X.; Zhao, X.; Luo, J. RSC Adv. 2017, 7, 53861-53870. Links
- 17. Wan, J.P.; Jing, Y.; Liu, Y.; Sheng, S.; RSC Adv. 2014, 4, 63997-64000. Links
- 18. de Paula, B.R.S.; Zampieri, D.S.; Zukerman-Schpector, J.; Tiekink, E.R.T.;. Rodrigues, J.A.R; . Moran, P. J.S. J. Braz. Chem. Soc. 2012, 23 (5), 825-830. Links
- 19. Kolahdoozan, M.; Kalbasi, R.J.; Shahzeidi, Z.S.; Zamani, F. Journal of Chemistry. 2013, https://doi.org/10.1155/2013/496837. Links
- 20. Beurden, K.V; de Koning, S.; Molendijk, D.; Schijndel, J.V. Green Chem. Lett. Rev. 2020, 13, 349-364. Links
- 21. Ashok, S.P.; Aravind, S.B.; Devkate, S.S. Asian. J. Chem. 2020, 32, 575-579. Links
- 22. Ruger, N.; Fassauer, G.M.; Bock, C.; Emmrich, T.; Bodtke, A.; Link, A. Mol. Diversity. 2017, 21(1), 9-27. Links
- 23. Naveen, S.; Lakshmi, S.; Dinesh, M.; Alpesh, P.; Anamik, S.; Sridhar, M.A.; Prasad, J.S. J. Chem. Crystallogr. 2007, 37, 733-738. Links
- 24. Guchhait, S.K.; Sisodiya, S.;. Saini, M; Shah, Y.V..;Kumar, G; Daniel, D.P.; Hura, N.; Chaudhary, V. J. Org. Chem. 2018, 83, 5807-5815. Links
- 25. (a)Porter, D.W.; Bardley, M.; Brown, Z.; Charlton, S.J.; Cox, B.; Hunt, P.; Janus, D.; Lewis, S.; Oakey, P.; Connor, D.O.; Reilly, J.; Smith, N.; Press, N. J. Bioorg. Med. Chem. Lett. 2014, 24, 3285-3290. (b)Youseff, M.; Mohamed, H.M.;. Czezowski, C; Ata, A.; Abd-El-Aziz, A.S. Heterocycles. 2006, 68(2), 347-355. Links
- 26. Surendarnathareddy, O.; Surya Narayana, Ch.V.; . Sharmila, N; Ramana, G.V.; Anuradha, V.; Hari Babu, B. Lett. Drug Des. Discovery. 2013, 10, 699-705. Links
- 27. Ramesh, N.; Gangadhara Rao, M.; Tirumala, M.; Uma, M.V.; Hari Babu, B. Asian. J. Chem ., 2016, 28, 1321-1324. Links
- 28. Jadhav, C.K.; Nipate, A.S.; Chate, A.V.; Songire, V.D.;. Patil, A.P; Gill, C.H. ACS Omega, 2019, 4, 22313-22324. Links
- 29. Jadhav, C.K.; Nipate, A.S.; Chate, A.V.; Dofe, V.S.; Sangishetti, J.N.; Khedkar, V.M.; Gill, C.H. ACS Omega. 2020, 45, 29055-29067. Links
- 30. Surendarnathareddy, O.; Baby Ramana, M.; Vijaya Durga, T.; Basavaiah, C.; Rajya Lakshmi, C.; Mokesh, R.G.; Vijaya, K.; Hari Babu, B. Chemistry Select. 2020, 5, 8194-8197. Links