Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease among human population1-3. Characteristic symptomatology involves bradykinesia, tremor, rigidity, and common motor symptoms. With an age increasing prevalence, it affects over 1% of the population over 60 years old4.
Although risk factors for PD are not yet fully understood, more of them have been identified during the past decade. The importance of identifying more risk factors relies on prevention of PD and on raising focus on new mechanisms involved in its pathogenesis that could help obtain a better understanding of the disease and propose new treatment alternatives.
Among newly described risk factors, focus on the link between PD and hematologic disorders has risen, more specifically on the relation between iron deficiency anemia and future PD incidence. As PD, the prevalence of anemia increases as a function of age after the 5th decade5, and more than 10% of older adults suffer from this affection6.
Iron deposition on the substantia nigra of the brain is a hallmark of PD7,8, conversely, the previous evidence has suggested that PD is associated with lower serum iron levels when compared to healthy controls9,10. Studies on animals have shown dopaminergic neurodegeneration in iron deficiency states11, where, on the contrary, other evidences have implied that elevated iron levels lead to reactive oxygen species (ROS) production, which produce further cellular damage and death12. The above-mentioned statements suggest that iron dysregulation plays a role in PD pathogenesis. A study proposed linking iron excess and mitochondria to PD pathogenesis13,14, as its dysfunction has long been implied in the mechanisms of the disease. This suggests that iron level balance is important for adequate brain function as iron is necessary for mitochondria biogenesis15.
In addition to the previously mentioned, a study observed erythrocytes of 30 PD patients and observed changes in their morphology, hypothesizing that the inflammatory signaling molecules involved in PD pathophysiology may affect the hematology system of these patients, which could be used as potential prognostic or diagnostic indicator16. It is important to remark that iron-deficiency and anemia are different events; however, the former could be a possible pathway for the relation of the latter with PD. Two clinical trials have already studied the efficacy, safety, and tolerability of erythropoietin on patients with PD17,18; nevertheless, further studies are needed to corroborate these results.
Some clinical studies evaluating the association between presence of anemia and risk for further PD incidence have been published; however, results have been inconsistent, and no solid conclusion has been reached. Therefore, to further clarify this topic, we performed a systematic review to summarize the available evidence on clinical studies evaluating the relation between anemia and PD.
Material and methods
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. A previous protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020150456.
Eligibility criteria
Prospective or retrospective cohorts and case–control studies performed on humans that directly compared the incidence of PD in anemic population (according to the World Health Organization criteria; hemoglobin [Hb] levels < 12 g/dL in women and < 13 g/dL in men19) versus non-anemic population or evaluated the association between both conditions.
Information sources
A systematic search strategy using a combination of keywords and MesH terms regarding the population, intervention, comparison, and outcomes of interest was developed by an experienced librarian, along with the main researchers of the study. This search was performed in MEDLINE, Scopus, EMBASE, Web of Science, and the COCHRANE Central Register for Clinical Trials (CCRCT). The time lapse considered for our search was from each database inception date until March 2020. In addition, an update was made in November 2021 (see Supplementary Material for search strategy).
Study selection
This process was conducted by four reviewers and included a title/abstract and a full-text screening phase. Before each phase, a pilot study was conducted to ensure adequate inter-rater agreement. Any disagreement that appeared in the tile/abstract phase was passed on to the full-text phase, where disagreements were resolved by consensus or involvement of another reviewer. After concluding each phase, kappa index was calculated to ensure inter-rater reliability (> 0.7). Articles that were included by the reviewers in the full-text phase were included for qualitative analysis.
Data collection process
Data extraction from studies fulfilling validity criteria was conducted using a template form to standardize extraction. The data extracted were as followed: study identification data (author, year of publication, place of study conduction, and study design), population identification (baseline characteristics), and description of intervention and study outcomes (incidence rates for PD in anemic and non-anemic population, and for potential confounding variables adjusted). This process was performed by two researchers in an independent and duplicate manner, where any disagreement was resolved by intervention of a third reviewer. A final version of the data form was approved by all researchers.
Quality assessment in individual studies
Three reviewers working independently and in duplicate assessed the methodological quality of each study included in the review using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies and Case–Control Studies20. This scale evaluates three domains: selection, comparability, and outcome. For our study, a maximum of eight stars was considered as maximum score for Cohort Studies, whereas a maximum of nine stars for case–control studies, indicating this greater quality. Concerning cohort studies, the last question of the “Outcome” section with regard to the adequacy of follow-up did not apply to the included studies due to their retrospective nature.
Outcomes’ measure
A qualitative synthesis is provided, where the characteristics and findings from each study are explained and summarized. Categorical data were reported as frequency and percentage, whereas numeric data as mean and standard deviation. Incidence of PD was reported for each group divided by sex and/or in total population when available. Risk of developing PD is reported in total anemic population or/and divided by sex as unadjusted hazard ratios (HR) with their respective confidence intervals (95% C.I.). HR for PD adjusted for confounders was also reported when available.
Statistical analysis
When enough data were available (more than one study reporting on the outcome), random effect meta-analysis was performed to estimate exposure’s effect on PD incidence. p < 0.10 for the test of heterogeneity across studies and > 50% for the measure of inconsistency (I2) was considered as high heterogeneity. The primary analysis used an inverse-weighted variance random-effect meta-analysis to account for the uncertainty in the location of the mean of different effects between studies. When events were evaluated, we used a modified Mantel-Haenszel meta-analysis with Peto’s method. Meta-analyses were performed using R (Version 4.0).
Results
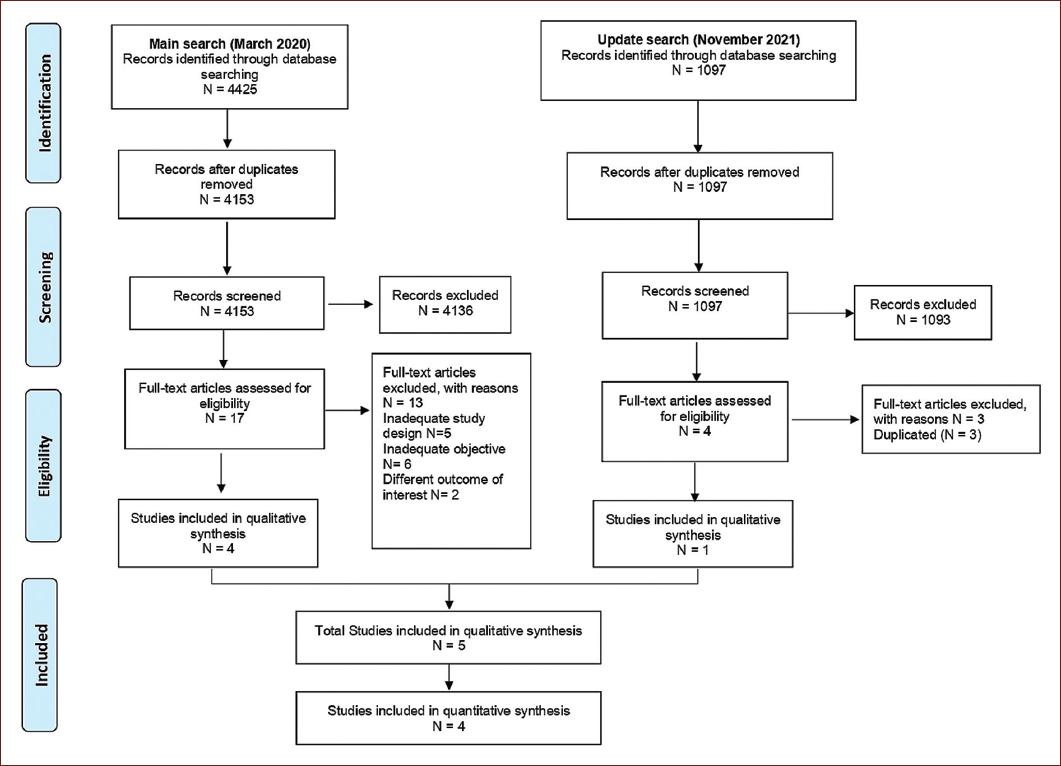
Initially, database search identified 4153 references. Of these, 4136 studies were excluded, because they did not meet the inclusion criteria. Subsequently, 17 full-text articles were reviewed for possible eligibility and 13 were excluded for different reasons, mainly as they did not assess the intervention of interest. At the update, from March 2020 to November 2021, data search identified 1097 references, and 1093 were excluded at the abstract screening. Afterward, four full-text articles were assessed, and three of them were excluded for being duplicated. Finally, five studies met the inclusion criteria and were included in our final qualitative synthesis; three of them were retrospective cohorts; and two were case–control studies21-25. The complete study selection process is depicted in Figure 1.
Study characteristics
Three retrospective cohorts were included; all of them retrieved their data from healthcare databases in Israel, Korea, and Taiwan and compared PD incidence between anemic population and a control group. Mean follow-up varied between across them, ranging from 5.0 to 8.8 years and mean age of the studied population ranged between the fifth and sixth decade. Only one cohort (Rozani et al.) had an almost balanced population based on gender (47.4% men and 52.6% female). Two case-control studies were included; both compared anemia diagnoses between a group of patients with PD and a group of matched healthy controls; one retrieved its data from a previous epidemiological project from US, while the other employed a Korean national healthcare cohort. From the five studies, two of them reported patient comorbidities such as hypertension, diabetes mellitus, and hyperlipidemia (Table 1).
Table 1 Baseline characteristics of included studies: (a) cohort studies and (b) case-control studies
| A. Cohort studies | NOS score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | Population number | Follow- up (years) | Age (mean, SD) | Female (n, %) | Hypertension (n, %) | Diabetes Mellitus (n, %) | Hyperlipidemia (n, %) | |
| Rozani et al., 2019 |
Israel | 474,129* | 8.8 ± 3.6 | Male: 48.7 (9.3) Female: 47.7 (9.7) |
249,181 (52.6) |
NR | NR | NR | 8 |
| Cho et al., 2020 |
Korea | AN: 217,086 CO: 2,024,033 |
5.0 | AN: 59.6 (9.4) CO: 57.17 (7.2) |
AN: 129,962 (59.9) CO: 699,075 (34.6) |
AN: 72,438 (33.4) CO: 704,394 (34.8) |
AN: 25,174 (11.6) CO: 247,706 (12.3) |
AN: 40,984 (18.9) CO: 468,511 (23.2) |
8 |
| Hong et al., 2016 |
Taiwan |
AN: 86,334 CO: 86,334 |
6.6 | AN: 56.4 (11.5) CO: 56.4 (11.5) |
AN: 65,526 (75) CO: 65,526 (75) |
AN: 26,018 (30.1) CO: 26,036 (30.2) |
AN: 13,362 (15.5) CO: 26,037 (30.2) |
AN: 17,994 (20.8) CO: 18,001 (20.9) |
8 |
| B. Case-control studies | |||||||||
| Author, year | Country | Population number | Age (mean, range) (years) | Female (n, %) | Median duration of enrollment (years) | NOS score | |||
| Savica et al., 2009 |
United States of America | Cases: 196 Controls: 196 |
71 (41-97) |
75 (38.3)* | 38 | 8 | |||
| Kim et al., 2021 |
Korea | Cases: 5844 Controls: 23,376 |
NR | 3094 (52.9) |
13 | 8 | |||
*Total population; AN: anemic population, CO: control population; NR: not reported; NOS: Newcastle-Ottawa scale.
Incidence and risk of Parkinson disease
RISK FOR PD AMONG ANEMIC POPULATION
Cho et al. and Hong et al. both reported an adjusted multivariate analysis assessing PD risk among population who had a diagnosis of anemia, the former reported a decreased PD risk among this population (HR = 0.89 95% CI = 0.81-0.99), whereas contrasting results were reported on the latter (HR = 1.36 95% CI = 1.22-1.52). Rozani et al. analysis was stratified by gender; therefore, general PD risk was not reported (Table 2a). Moreover, in the case–control study performed by Savica et al., greater odds for having a diagnosis of anemia were associated with the presence of PD when compared to healthy controls (unadjusted OR = 2.00, 95% CI = 1.31-3.06). This was supported in the study by Kim et al., with an adjusted OR for the total population of 1.09, 95% CI = 1.01-1.18 (Table 2b).
Table 2 Parkinson’s disease incidence among included studies: (a) cohort studies and (b) Case-control studies
| A. Cohort studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year | PD incidence in male group | HR (95% CI) | aHR (95% CI) | PD incidence in female group | HR (95% CI) | aHR (95% CI) | PD incidence in total population | HR (95% CI) | aHR (95% CI) |
| Rozani et al., 2019 |
AN: 1.2%Ω CO: 0.6%Ω |
1.19 (1.04-1.37) |
NR | AN: 0.3%Ω CO: 0.4%Ω |
1.02 (0.95-1.09) |
NR | NR | NR | NR |
| Cho et al., 2020 |
AN: 236 (0.3%) CO: 2,258 (0.2%) |
1.57 (1.37-1.79) |
0.88 (0.77-1.02) |
AN: 219 (0.1%) CO: 1,131 (0.1%) |
1.04 (0.91-1.21) |
0.91 (0.78-1.05) |
AN: 455 (0.2%) CO: 3,389 (0.16%) |
1.24 (1.13-1.37) |
0.89 (0.81-0.99) |
| Hong et al., 2016 |
NR | NR | NR | NR | NR | NR | NR | NR | 1.36 (1.22-1.52) |
| B. Case-control studies | |||||||||
| Author, year | Incidence of anemia in cases | Incidence of anemia in controls | OR total population (95% CI) | OR (95% CI) in male group | OR (95% CI) in female group | aOR total population (95% CI) | |||
| Savica et al., 2009 |
86 (43.9%) | 54 (27.6%) | 2.00 (1.31-3.06) |
1.52 (0.88-2.64) |
2.91 (1.47-5.77) |
2.17 (1.40-3.37)* 1.74 (1.02-2.97)** |
|||
| Kim et al., 2021 |
1090 (18.7%) | 3944 (16.9%) |
1.13 (1.05-1.23) |
< 70
years: 1.44 (1.22-1.70) > 70 years: 1.14 (0.97-1.34) |
< 70 years:
1.05 (0.92-1.21) > 70 years: 1.04 (0.90-1.20) |
1.09 (1.01-1.18)*** | |||
HR: Hazard ratio, 95% CI: 95% confidence interval, aHR: adjusted Hazard ratio, NR: not reported, Ωabsolute incidence not reported.
OR: odds ratio, 95% CI: 95% confidence interval, aOR: adjusted odds ratio.
*adjusted for smoking.
**adjusted for pesticide exposure.
***adjusted for obesity, smoking, blood glucose, head trauma and alcohol exposure.
GENDER STRATIFIED RISK FOR PD
In their unadjusted analysis, both Rozani et al. and Cho et al. found a greater risk for PD among male population with diagnosis of anemia (HR = 1.19 95% CI = 1.04-1.37; HR = 1.57 95% CI = 1.37-1.79); however, in their adjusted analysis, Cho et al. found no associated risk for PD among them (HR = 0.88 95% CI = 0.77-1.02). Rozani et al. did not report an adjusted analysis. Regarding female population with diagnosis of anemia, no higher risk of PD incidence was observed in neither of these studies (Table 2).
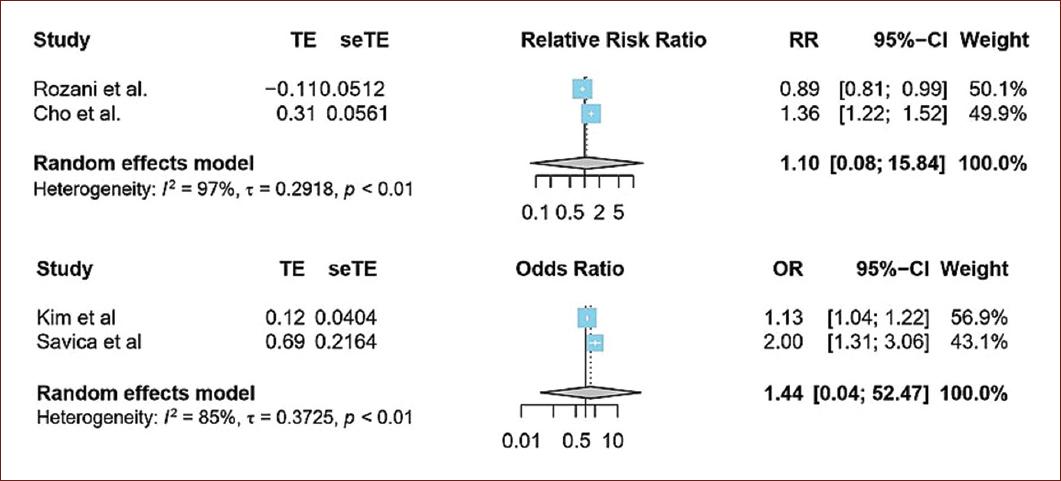
SUBGROUP ANALYSIS
To perform the subgroup analysis, the cohorts and case-controls studies were pooled in a separate way. In the cohort studies, the pooled relative risk of anemia and PD was 1.10; 95% CI = 0.08-15.84, and it is presented in figure 2a. On the other hand, the pooled OR of the case–control studies was 1.44; 95% CI = 0.04-52.47 (Fig. 2b). High heterogeneity was observed among included studies.
Certainty of evidence
All studies, both cohort and case-control, were classified as high-quality studies according to Newcastle-Ottawa Quality Assessment Scale (Table 1).
Discussion
In this systematic review, we aimed to assess the currently available clinical evidence which evaluated a possible association between a diagnosis of anemia and PD. We found three retrospective cohorts that reported PD risk associated to presence of anemia and two case-control studies that reported odds of having anemia when PD was present. Among the two cohorts who reported PD risk among general population with anemia diagnosis, results were contrasting. Furthermore, when analyzed by gender, unadjusted analyses revealed higher risk for PD among male anemic population; however, the only adjusted analysis, which was reported by Cho et al. did not support this finding. No higher risk for PD was observed among female anemic population. In the case–control studies, individuals with PD had higher odds of having diagnosed anemia than patients without PD diagnosis21,25. Overall, the pooled relative risk observed in the included studies suggested a not significant increased risk for PD in anemic populations.
Analyzing the implications of the included studies, two cohorts and one case-control study proposed anemia to be a prodromal marker for PD. This, as it is acknowledged that this disease begins years before dopaminergic degeneration in the substantia nigra26. Thus, the presence of anemia may be originated by the inflammatory, oxidative stress changes in the prodromal stages of PD27,28. Moreover, we suggest a bidirectional model, in which anemic conditions might further contribute to PD related to oxidative stress, inflammation, and iron dysregulation. Nonetheless, when considering the study by Cho et al., the authors concluded a protective effect of anemia in future PD incidence, contradicting the conclusions of the other studies. However, one important aspect that must be considered to interpret these conclusions is the definition of exposure in these included studies.
The previously mentioned is rather important as most studies define the exposure (presence of anemia) in heterogeneous ways (Table 3). Rozani et al. calculated the mean annual hemoglobin levels of each included individual, with a mean number of hemoglobin tests of 6 ± 3 per individual during the entire follow-up, where if the mean of one annual Hb measurement was below the threshold for anemia, exposure was considered positive. Moreover, Hong et al. required at least two diagnostic claims for anemia or iron supplementary therapy during a time lapse of 7 years. Savica et al. study required a diagnosis of anemia ever mentioned, or hemoglobin levels under anemic values according to the World Health Organization persisting over a 6-week period to exclude blood loss related values. Compared to these exposure criteria, Cho et al. and Kim et al. used a one-time hemoglobin result under anemic conditions to define anemia, which raises questions on the validity of the exposure’s effect on the measured outcome, as a one-time reported result might not reflect its actual effect. In this manner, the former studies, which reported an increased risk for PD incidence and used multiple hemoglobin or diagnostic claims, might reflect better the exposure’s effect on the measured outcome. However, a limitation that must be highlighted in the study by Rozani et al. is the lack of adjustment for confounding variables, as the other cohort studies and case–control studies did perform. This is important as PD is influenced by various factors that have been observed in meta-analysis and observational studies such as diabetes mellitus, hypertension, pesticide exposure, and smoking29-32.
Table 3 Definition of anemia exposure for each included study
| Study | Anemia definition |
|---|---|
| Cho et al. 2020 |
One-time result at baseline (Hb < 13g/dL M, < 12 g/dL W) |
| Rozani et al. 2019 |
Mean annual Hb classified as anemia (Hb < 13g/dL M, <12 g/dL W) |
| Hong et al. 2016 |
At least two diagnostic claims for anemia or
iron supplementary medication in a 7-year period. |
| Savica et al. 2009 |
1) A diagnosis of anemia ever mentioned in medical
records or, 2) Hb < 13 g/dL M, < 12 g/dL W persisting over a 6-week period to exclude acute blood related anemia. |
| Kim et al. 2021 |
The most recent hemoglobin concentration before the diagnosis of PD (index date), Hb < 13 g/dL in M and < 12 g/dL in W |
Hb: hemoglobin, M: men, W: women.
PD clinical characteristics, progression and risk factors follow sex-specific differences33. As an example, in this review, we found that Savica et al. reported anemia to be more associated to PD among female population, whereas lifestyle and occupational risk factors were more related to the disease in male population34. As PD, anemia also has gender-dependent differences as its prevalence is higher among female population35,36; nonetheless, no increased risk for PD was observed for female anemic population in neither of our included cohort studies. Whether gender-related differences are present in the relation of anemia and future PD incidence is a non-clarified issue to date. In this review, no conclusive evidence regarding the abovementioned was reached.
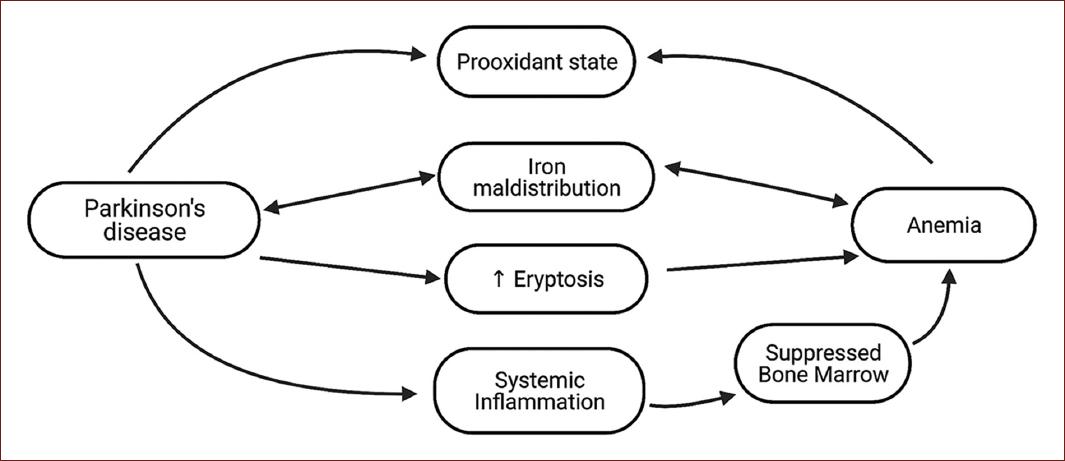
The possible pathological pathways that may explain anemia association with PD are inflammation, oxidative stress, and iron metabolism (Fig. 3). The previous evidence has assessed hemoglobin and iron levels on patients with PD, showing inconsistent results, as some studies have shown hemoglobin levels to decreases as PD progresses, but others exhibited no difference in neither hemoglobin nor iron levels compared to controls37-39. Nonetheless, as systemic inflammation has been recognized to contribute to PD pathogenesis40, and inflammatory cytokines have been shown to suppress bone marrow and inhibit erythropoietin-induced erythrocyte maturation41, this could possibly explain the low red blood cell count and its relation with future PD.
Considering oxidative stress, some evidence has shown that red blood cells of PD patients lack superoxide dismutase, an enzyme responsible for counteracting oxidative stress, and are as a result susceptible to stress-induced damage42,43. Regarding iron-deficiency anemia, a study showed that patients with this condition have a higher oxidant and a lower antioxidant activity compared to controls, which normalizes to control levels after iron treatment44. In addition, a review described the pro-oxidant shift that occurs in anemic conditions, which is further worsened by a weakened antioxidant capacity in this environment45. Thus, anemia might contribute to the oxidant conditions that are needed for PD pathogenesis, whereas red blood cells’ changes in PD patients might support eryptosis16, showing a hypothetical bidirectional association.
Considering the oxidant properties of iron46 and that its deposition in the substantia nigra has been observed in PD patients7, its metabolism could be another element in the relationship between anemia and future PD incidence. Contrary to the hypothesis that arises when considering the oxidant properties of iron, where anemia and low serum iron levels should apparently contribute to a decreased risk for PD, evidence has shown that increased levels of serum iron were associated with a reduced risk for future PD9,47. The latter could be supported considering that iron is a cofactor for the enzyme L-tyrosine hydroxylase, which is responsible for dopamine synthesis48. Animal models of iron deficiency have shown altered dopamine metabolism and function49,50, which could explain the protective effect of increase serum iron levels on PD incidence. Moreover, an iron maldistribution process could also explain the increased deposition in substantia nigra but the low serum iron levels51, which might affect erythropoiesis, exhibiting a possible link between anemia and PD incidence.
Finally, another element linking both pathologies is red cell distribution width (RDW), which is commonly increased in iron-deficiency anemia as sign of anisocytosis52. A study showed patients with PD have increased RDW levels compared to controls, whereas another correlated RDW to PD progression53,54. The former compared hemoglobin and white blood count to controls, and no difference except RDW was shown. Although RDW has been shown to be an inflammatory marker55,56, its increase in PD patients could be related to an iron maldistribution process and could demonstrate that patients with PD experience disruption in erythrocyte cell line, possibly linking iron metabolism, and PD.
This study has some limitations. The high heterogeneity of the pooled studies limits the interpretation of the pooled analysis. Moreover, available evidence regarding our research questions was scarce and this could seriously limit our findings. Another consideration is that patients with decreases in hemoglobin values not reaching anemic WHO cutoffs might have inflammatory and iron maldistribution processes that might contribute to PD and could not be evaluated.
Conclusion
A non-significantly increased pooled risk for PD was observed in the included cohort and case–control studies. The pathophysiological mechanisms related to iron maldistribution, oxidative stress, and systemic inflammation might explain this observed tendency. Prospective studies with various hemoglobin measurements under anemic values during follow-up and control of confounding variables are needed to corroborate and further confirm the idea of anemia as prodromal marker of PD.











 nueva página del texto (beta)
nueva página del texto (beta)