INTRODUCTION
Three-dimensional (3D) printing started as a revolutionizing technology in different fields of healthcare and, at present, has proven to be a useful technology up to a point to become of common use. Clear examples of this are stated in the development of orthotics devices, prostheses, patient-specific implants, Drug Delivery Systems (DDD) [1] [2], realistic anatomic models for education or surgical use [1] [2] [3]; the creation of living cells, drugs, protein molecules, that replicate the tissue anatomy, biology, and physiology [2] [4]. It is forecasted that the 3D printing industry will grow up to 8.4 billion dollars by 2025 [5].
3D printing can be defined as a collection of technologies known as rapid prototyping [1] where the conversion of 3D virtual models (models created through Computer-Aided Design (CAD) or image processing) are converted into physical models without the need for specialized tooling, 3D printing includes several established and experimental manufacturing techniques [1] [3] [5]. It is important to mention that any technique used in 3D printing has its limitations and applications. 3D printing is most commonly known as additive manufacturing (AM) [6]. The most common way of work of a 3D printing machine is laying one thin layer of material that bonds with another thin layer of material [3]. This review aims to identify the latest work and perspectives of 3D printing in healthcare.
MATERIALS AND METHODS
Searches were carried out in PubMed (ncbi.nlm.nih.gov) and Espacenet (worldwide.espacenet.com) in January, 2021. The studies were in English, Spanish, and French, published between 2009 and 2020. No limitation by its publication status was made. Any type of study was included (experimental studies, observational studies, reviews). The following terms were used for the search: "Printing, Three-Dimensional", "Stereolithography", "Health Services", "Medicine", "Biomedical Research", and "Biomedical Engineering". In PubMed, previous terms were used as MeSH terms. Three authors reviewed titles, abstracts, and keywords separately to identify studies appropriate to the topics of prostheses and implants, operative and surgical procedures, musculoskeletal system, tissue engineering, cardiovascular system, education, regenerative medicine, pharmacology, skin, otolaryngology, orthopedics, urology, gastroenterology, medical device regulation and, neurology. After the initial examination, complete texts of identified relevant studies were obtained. Studies were classified according to all authors, and disagreements as to which articles were suitable for each category included in this work were resolved by discussion. Results were arranged in a conventional literature review.
RESULTS AND DISCUSSION
3D printing applied to the area of healthcare has progressed rapidly in the past years. As a result, 6,402 papers (see Figure 1) and 31 patents (see Figure 2) were identified.
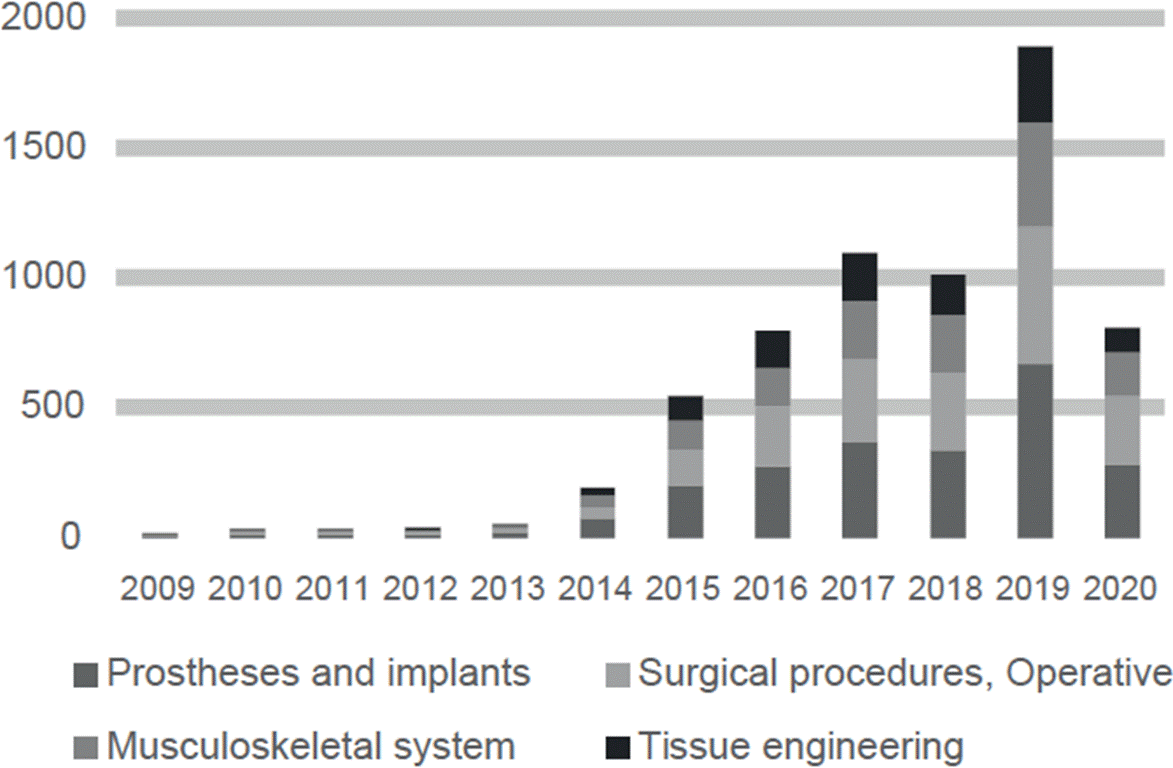
Figure 1 The number of publications per year in PubMed about major 3D printing applications in the healthcare field between 2009 and 2020.
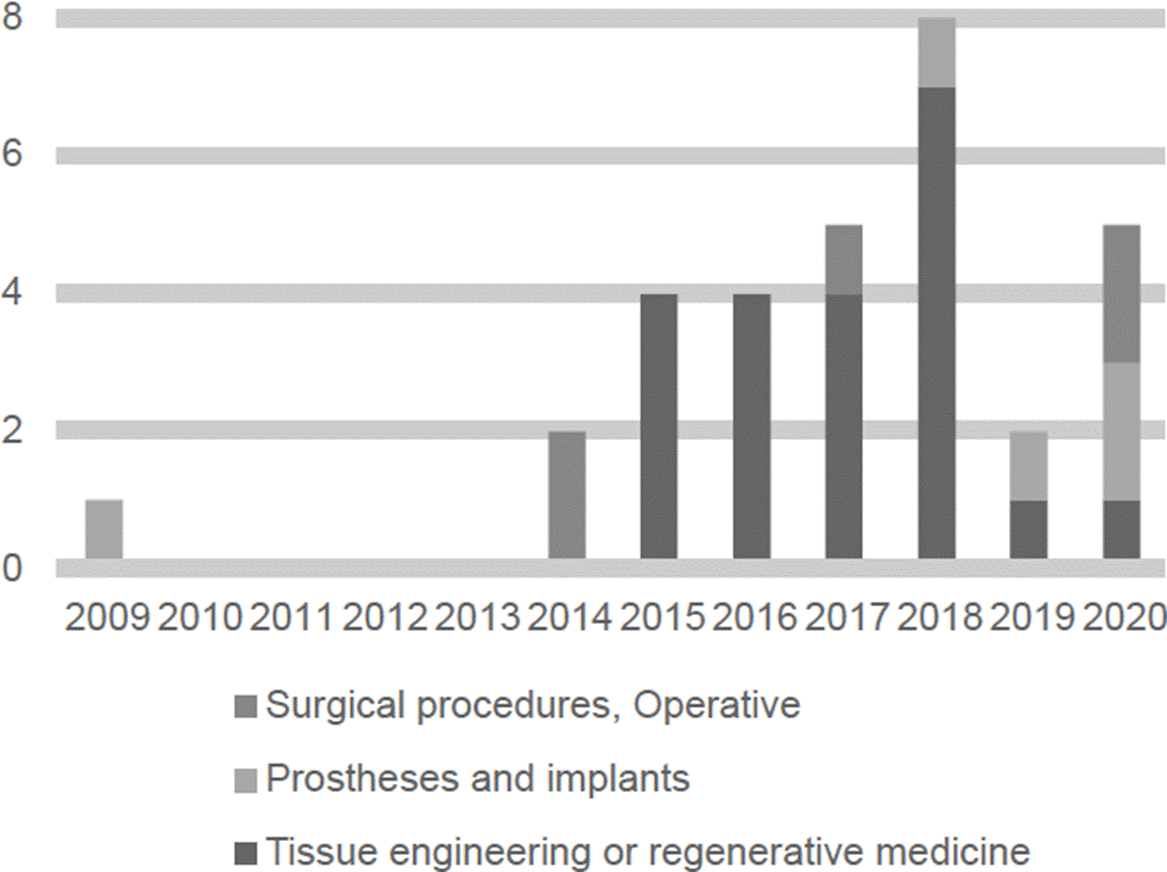
Figure 2 The number of patents per year in Espacenet about major 3D printing applications in the healthcare field between 2009 and 2020.
Major fields of publication of papers about 3D printing in healthcare include prostheses and implants, surgical procedures, and research about the musculoskeletal system, see Figure 3. Major fields of publication of patents about 3D printing in healthcare include tissue engineering and regenerative medicine, prostheses and implants and, operative and surgical procedures, see Figure 4. In the next sections, a detailed and selective review of major applications among the advantages and disadvantages of 3D printing in healthcare is presented.
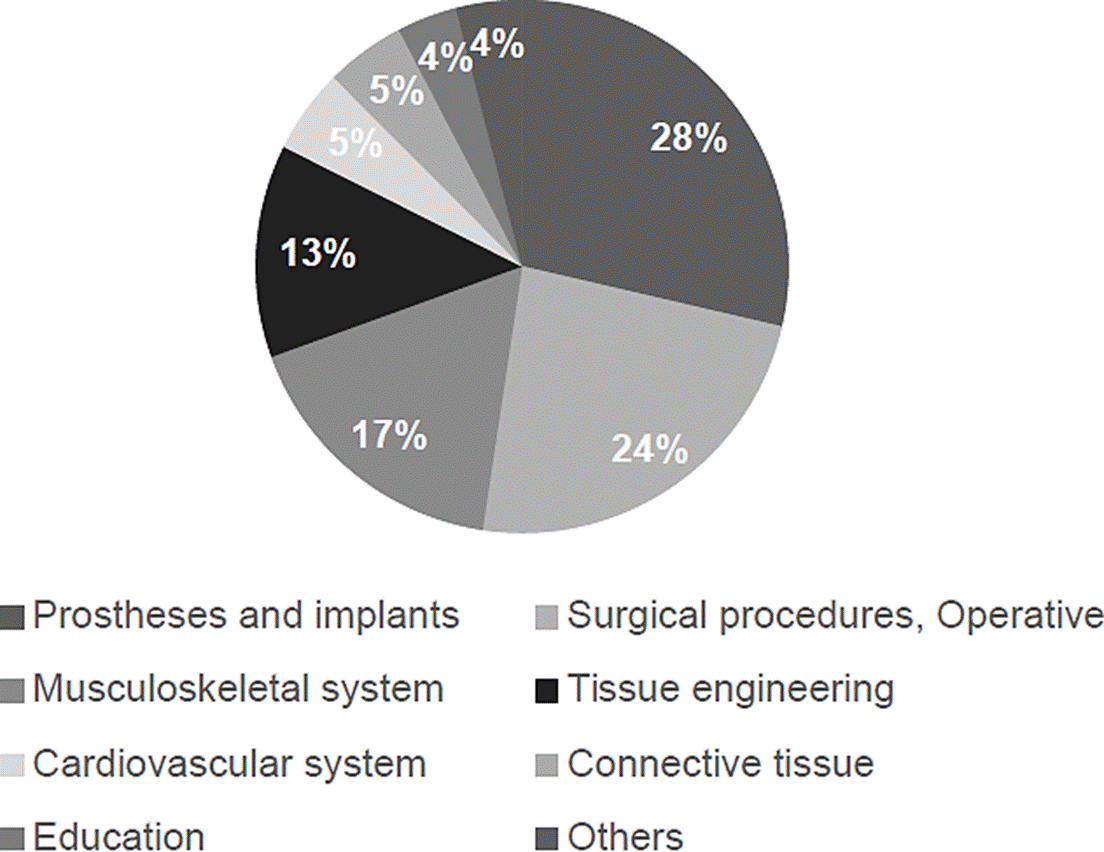
Figure 3 Major fields of publication of papers about 3D printing in healthcare are based on the number of publications per year in PubMed between 2009 and 2020.
Advantages of 3D printing
It can be said that the main advantage of 3D printing is the personalization of a 3D model that solves a patient-specific problem at a low-cost burden [6] [7]. However, 3D printing technologies also enhance or complement surgical training, research, minimally invasive diagnosis, and disease models [7] [8] [9] [10] [11] [12] [13].
The main advantage of 3D printing is that it can be used in different applications by only changing the printing technique and material. i.e., in tissue engineering using 3D printing techniques, it is commonly the use of hydrogels because hydrogels allow good permeability of nutrients and facilitate cell growth [14]. The use of materials such as metal is used in applications where high mechanical resistance is needed. In applications such as prosthetics or orthotics, materials using metal can be used. In contrast, in applications such as tissue engineering, materials such as bioceramics and bioglass are used since these materials allow the permeability of nutrients [14].
One of the biggest roadblocks in 3D printing technology is that there is no well-established method of the process that validates the quality check in developing 3-D printed medical devices [8] [15].
The main disadvantage in 3D printing is found in its manufacturing, where the two challenges are the negative effects of the blemishes situated in the inner part of the 3D model at sub-millimeter scale, which can result from the feed supply that is damaged or machine failures and the effect of the component’s placement amid the print on the execution of the material [2].
Regarding the printing technologies, there are still some limitations such as: narrow color range in stereolithography printing (STL), low resolution in fused deposition modeling (FDM), and poor quality of the surface finish in selective laser melting technique (SLM) [16] [17]. Although, inkjet bioprinting represents an optimal printing technology in terms of costs and performance, the printed structures may have an irregular and rough shape due to the hydrogels used as bio-inks [16] [18]. Additionally, the 3D printed material should have specific properties such as flexibility, adaptation, and significant biocompatibility to ensure sufficient vascularization and maturation of the printed tissue [18] [19].
3D printing in prosthetics and implants
3D printing in prosthetics can be divided into two types: internal prosthetics and implants and external prosthetics.
Orthoses and splints could be found in external prosthetics. 3D printing has shown various advantages, such as sustainable development and costs reduction. This is particularly relevant in marginalized communities [7] [10] [15] [20]. The most common technique used is additive manufacturing (AM). The main advantage of 3D printing is efficiency since this model can be modeled using CAD design, allowing to suit the prosthetics perfectly to human anatomy [11].
Internal prostheses and implants are mainly used in orthopedics. Prostheses and implants must mimic the mechanical properties of bone to maximize its performance in the human body [10]. The selective laser melting (SLM) technique is a 3D printed technique used to produce internal prostheses and implants. Another technique is selective electron beam melting (SEBM) created by the Swedish company Arcam AB [9]. Both processes work by using high energy beams to disintegrate transversal forms into plies of metal powder melding powder particles into a new structure. The major difference between SEBM and SLM techniques is their source of power, the former uses electrons, and the later a laser [9]. Enumerated additional requisites of internal prostheses and implants are being biocompatible, allowing cell attachment and expansion, and possess satisfactory porosity [9] [21] [22] [23] [24] [25].
Patents in the field are related to manufacturing devices based on multiple robotic arms and sprayers, manufacturing methods based on the reconstruction of 3D images for facial prosthesis, development of new materials for bone implants, and guiding systems for colocation of dental implants made by stereolithography such as three-dimensional printing devices [26] [27] [28] [29] [30].
3D printing in surgical procedures
Surgical procedures request high-quality procedural outcomes in combination with optimal safety outcomes [6]. 3D printing brought into the surgical field as a tool for simulating all surgical steps ahead of time results in a better comprehension of complex underlying anomalies. This can enhance better diagnostics and assist in surgical planning [6] [7]. For example, in craniofacial and maxillofacial surgery where its success has been demonstrated [7]. The key components for surgical 3D printing are (1) analysis (critical thinking for problem-solving solutions), (2) planning (endpoint goals are fixed to formulate a surgical plan), (3) virtual surgery (multiple strategies can be run to determine the most optimal approach for surgery), (4) implant design and production (biocompatible implants can be printed as an additional complement in surgery), and (5) postoperative analysis (the accuracy of the results can be compared using computed tomography) [6]. 3D models regularly take a day or longer to create. This means that 3D models can be used for surgical procedures where long-term planning is involved.
Success stories of application in surgical training include endoscopic ear surgery (the 3D printed models help the surgeons to reduce time), comparison of planning with 3D imaging techniques vs. 3D printing (3D printing allowed surgeons to create better surgical strategies), endoscopic endonasal (where surgeon accelerate their learning process) [6].
Patents in the field are related to methods for fabrication and collocation of 3D printed materials for facial bone fractures and knee joint replacement [27] [31] [32] [33][34].
3D printing in regenerative medicine
3D printing in regenerative medicine is recognized as an emerging technology that allows the fabrication of biomimetic tissues and organs by cultivating cells on scaffolds. 3D printing in regenerative medicine is made using CAD and computer-aided manufacturing (CAM) methods [18] [35] [36]. Fabricated tissues and organs could be used for medical transplantation in regenerative medicine or drug testing.
To differentiate the specific application of 3D printing within regenerative medicine, it is commonly referred in the literature as 3D bioprinting. As it can be imagined, 3D printing in regenerative medicine has specific challenges, such as the preservation of biological functions of cells and to mimic the architectures and mechanical properties of biological tissues [19]. The 3D printing technology in the field of regenerative medicine had advanced rapidly since its beginning in 2005, when the first cell printing was performed [36].
Patents in the field are related to the fabrication of encapsulated bodies of cells [37] [38] [39], preparation and application of new materials useful as scaffolds [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54], or new bioinks with improved printability characteristics [55].
Bioprinting, biofabrication and bioassembly
Bioprinting in regenerative medicine has been used in literature as a general term, which refers to any biofabrication process. However, Moroni et al. have recommended a classification to distinguish each term [56]. Biofabrication is the production by bioprinting or bioassembly of products that are biologically functional using living cells, biomolecules, or biomaterials [56]. Consequently, bioprinting is only one approach to biofabrication. Bioassembly, which is the other major approach of biofabrication, is the process of biofabrication by the assembly of pre-formed cell-containing building blocks [56].
Bioprinting techniques
The most widely used bioprinting techniques are inkjet-based printing, laser-based printing, extrusion-based printing, and stereolithography STL [18] [19].
Inkjet-based printers dispense cells or biomaterials as liquid droplets. The first inkjet-based printer was a modification of a commercial desktop inkjet printer where ink cartridge was substituted by a suspension of cells [19]. Inkjet based printers use thermal, piezoelectric, or electromechanical valve mechanisms to print heads to generate the droplets. Thermal printers apply heat to the print head to increase pressure and generate droplets. Piezoelectric printers apply a wave of pressure to split out a droplet of ink. Electromechanical valves printers open and close electromechanical valves to generate droplets. Advantages of inkjet printers are low cost, versatility in managing a wide range of cells and biomaterials with low viscosity (<10 centipoises), high resolution (20-100 micro-meters), high speed (1-10,000 droplets per second), precise control of droplets parameters such as volume (1-300 pico-liters), pressure and temperature [19]. However, one disadvantage of inkjet-based printers is stress, either thermal o mechanical induced to cells, limitation to use high viscosity cells and biomaterials which could result in obstruction of projecting spouts, the high variability of droplet volume, early condensation, and random dispersion of cells within dispensed volume [19].
Extrusion based printers extrude strands of cells and biomaterials by applying constant pressure through a nozzle by pneumatic or mechanical systems. The advantages of inkjet printers are their versatility to manage a wide range of cells and biomaterials, especially with high viscosity, precise control of nozzle pressure, and nozzle moving speed [19]. One disadvantage of extrusion-based printers is the shear stress imposed on cells. The survival rate of cells after extrusion-based printing is 40-86% lower than inkjet-based printing [19].
Laser-based printers eject droplets of cells and biomaterials from a ribbon structure into a receiving substrate propelled by the high pressure produced by the energy absorption of the laser by the ribbon structure. The ribbon structure is composed of one energy-absorbing layer made of glass coated with nanofilms of gold or titanium and a second layer with a suspension of cells and materials [18]. Advantages of laser-based printers are their versatility in managing a wide range of viscosity of cells and biomaterials (1-300 mili-Pascals per second), high density of cell deposition (<108 cells per mili-liter), high resolution (one cell per drop), high speed and visualization of cells during deposition [19].
STL uses a laser of UV light to solidify sections of a photo-sensitive precursor solution layer by layer. The advantages of stereolithography are high resolution (micro-meters). Disadvantages are using only photo-sensitive materials and damage induced to cells and biomaterials for UV exposure [19].
Bio-inks
Bio-inks are compositions of cells and biomaterials, such as supporting materials and growth factors. Bioinks are mainly composed of hydrogels, polymers with high water content, and cross-links like extracellular matrix. Bio-ink components could be natural such as agarose, alginate, chitosan, collagen, fibrin, gelatin, gel, and gum, or artificial such as hyaluronic acid, pluronic, polyethylene glycol (PEG), and polycaprolactone (PCL) [36]. Desirable properties of a bioink are mechanical stiffness, structural stability, biodegradability, thermic stability, biocompatibility and tissue induction and printability [36] [57]. The mechanical stiffness of bio-ink should be similar to the mechanical stiffness of healthy tissue, which is important to support loads and maintain the shape of the implant within the body [36]. Structural stability and biodegradation denote the maintenance of the structure during printing with a duration of the implant enough to allow tissue remodeling and integration of the cells into the target tissue [36]. Thermal stability relates to the property of the bio-ink to remain intact at a relatively high temperature, for example, polymers are solid at 37°C [57]. Biocompatibility and tissue induction are related to the biological activity and adherence in agreement with cell-matrix interactions of the target tissue [36]. Printability is related to the viscosity and homogeneity of the solution to allow extrusion while maintaining structure after deposition and avoiding high shear stress on cells [36].
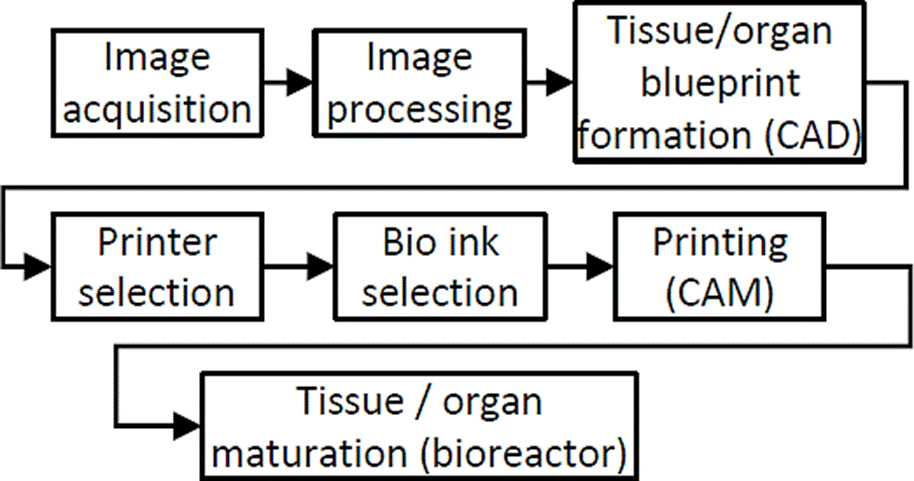
3D Bioprinting process
The 3D bioprinting process is like a 3D printing process with some particularities, see Figure 5. The bioprinting process is initiated by the formation of a tissue or organ blueprint using CAD based on digital images coming from imaging modalities such as Magnetic resonance imaging (MRI) or computed tomography (CT) and the selection of suitable materials according to histological structure, composition, and tissue and organ topology [18]. The bioprinting process continues using CAM with the selection of the appropriate printer and bio-ink [18]. Finally, the bioprinted tissue or organ is maturated in a bioreactor to mimic in vivo environment of the target tissue [18].
3D printing in research
According to [5], 3D printing opens new opportunities for scientific research activities. 3D printing allows the creation of phantoms that can help understand physiological processes that are not yet fully comprehended alongside a better understanding of complex pathologies. e.g., hemodynamics can be investigated by velocity-encoded MRI or by optical flow measurements in transparent models.
3D printing in medical education and training
3D printing has proven its value in medical training. As stated in [5] [6] [58], 3D printed models can have a very high value in educational use instead of just images. 3D sections representing a patient with its body structures (bone and vascular) and soft tissues can be printed. To create a 3D model first, an imaging modality is chosen; it can be either through computed tomography or magnetic resonance imaging. From multiple images, volumetric data is acquired. This set of images are put together, and after the noise is smoothed out, a 3D virtual model is created, ready to be printed [2] [3]. The accuracy of these models is enough due to the image processing techniques. Within an educational setting, results show that 3D printing gives a better understanding of anatomy compared to 2D images [6].
3D printing in pharmacology
Patient-specific DDD is the most common technological development in pharmacology [59]. At present, the FDA has approved 3D printed DDD in disintegrating dosage forms such as mini-tablets and films that are suitable for children [60].
3D DDD offers favorable circumstances regarding individualized medication conveyance and financial perspective since this is a cheaper alternative to traditional dosage solutions [12] [15] [20] [59] [60] [61]. To print 3D, DDD 5 techniques will be reviewed.
Stereolithography (STL) in pharmacology is the most commonly used technique. Some examples of materials fabricated using this technique are topical patches and microneedles [59]. This technique has the disadvantage of short dosage delivery [13] [59].
Selective laser sintering is used to build DDD using polymers such as Nylon, poly-L-lactic acid (PLLA), and PCL [59]. This technique gives potential outcomes for controlling the porosity and the medication discharge energy of the 3D printed structures. The fundamental limitations are the absence of reasonable cost printers [2] [13] [59].
Fused filament fabrication (FFF) or fused deposition modeling (FDM) is the most common technique in the area of pharmacology. The adaptability of FFF permits assembling drug conveyance with different geometries and additionally changed medication discharge profiles for patient-explicit medicines at high reproducibility [13] [59].
A pressure-assisted microsyringe is the newest strategy for DDD. Pressure-assisted microsyringe produces DDD from films of poly-lactide-co-glycolide (PLGA) glues, hydrogels, or viscous polymers. However, the amount of medication is limited by the rheological characteristics of the materials [59].
3D printing applications
3D printing applications Neurologic Applications
When medical personnel performs neurological surgeries (i.e., Cerebral aneurysm surgery), they can encounter intricate anatomical structures that cannot be seen externally. In order to observe complex anatomical structures, it is commonly used two and three-dimensional images obtained through CT or magnetic resonance imaging MRI [62]. Although images allow complex anatomical structures to be visualized, they do not allow medical personnel to appreciate these structures physically. By using imaging techniques, 3D printed anatomical forms can be constructed, which allow preoperative planning.
Preoperative planning brings a few advantages, such as reducing surgery time and decreasing the risk of injuring a patient [63]. Neurosurgical models can be printed through FDM, STL, selective laser scintigraphy, and photopolymerization technologies [64]. According to [65] Acrylonitrile Butadiene Styrene (ABS), plastic, and white resin are validated materials to be used in surgical training. Current patents in this field cover the methodology of creating the 3D replica of patient anatomy [66].
3D printing technology can also be used for printing instruments and implants that correspond to individual patient anatomy [67]. In the case of 3D printed implants, advanced materials such as stem cells are used. These methods are considered for either creating new nervous tissue or enhancing the innervation of tissue-engineered constructs [57]. These tissues have basic cellular phenotypes, and previous research shows that their functionality lasts for over two weeks after printing [16]. Current patents using steam cells relate to using inkjet printers to place viable cells in a three-dimensional architecture [68] [69].
3D printing applications -Cardiovascular Applications
Cardiovascular disease is one of the most severe illnesses that endanger human health, therefore, the best treatment must be applied [70]. 3D printing offers the possibility of delivering the best diagnostic and cardiovascular treatment since 3D printing has been used for customized individual printing of cardiovascular models, surgery planning, and simulation of intravascular surgery, resulting in improving the success rate of cardiovascular surgeries or treatments [16] [71] [72]. For printing 3D cardiovascular models, photopolymer inkjet printers are recommended since they retain a high resolution and can print complex structures [70] [73]. The polymeric materials perform the best in terms of quality for cardiovascular models and training tools [70]. The current challenge of 3D printing in the cardiovascular field is bio-printing materials used to create functional tissues (stent, valve, tissue-engineered scaffold) that mimic the hearth's physiological function [16] [70] [74] [75] [76]. The biomaterials used for tissue are mainly based on hydrogels since it is a very versatile material. The hydrogel material can adjust its cross-linking density, molecular bonding, and swelling degree to customize its mechanical property [70]. The future research of 3D printing cardiovascular disease will focus on bioprinting that can transport blood nutrients [70]. Like in 3D printing in neurologic applications, the methodology for creating a 3D printed cardiovascular model is founded in U.S. Pat. No. 20150025666A1 and its application is being applied at the Children National Medical Center. In 2015 the Guangzhou Hongchang registered a technique for printing a small-caliber bioartificial blood vessel that can be used for coronary artery bypass grafting, hemodialysis, and cerebrovascular replacement (C. N. Pat. No. 104771783A).
Musculoskeletal Applications
Muscles, ligaments, and connective tissues are used on the body to transfer force and facilitate joint movements. Muscles, ligaments, and connective tissue might get broken or hurt due to trauma or tumors [77]. Muscle, ligaments, and connective tissue treatment refer to tissue transplantation, which its disadvantage is found in the host tissue mechanical properties [77]. 3D printing using tissue engineering techniques has the purpose of muscle reconstruction [18]. Hydrogels are a common material used in 3D printing tissue engineering techniques. Hydrogels are designed to act as an artificial extracellular matrix and give living cells an environment to grow [14]. Other materials like bioceramics (hydroxyapatite), calcium phosphate, and bioglass have been used for bone regeneration because they are porous, and like hydrogels, facilitate cell growth [14]. Common 3D printing techniques for tissue engineering are stereolithography, selective laser sintering, FDM. Previous research has shown that these 3D cell printed muscles exhibit bioelectrical mimetic functionality and structural characteristics [35] [16].
Bones and Cartilage Applications
The bones are tissues with the ability to self-regenerate and self-repair, but diseases like cancer, infection, trauma, and congenital deformities can prevent bone regenerates or repairs. To treat bone diseases, the transplantation of artificial bone substitutes promotes bone healing by osteogenesis [77]. Bone substitute transplantation treatment has many disadvantages, such as transplant rejection and transmission of diseases. Like in musculoskeletal 3D applications, 3D printing is used in bones and cartilage, tissue engineering using 3D printing techniques, create scaffolds in combinations of cells, materials to improve or replace biological tissues. The 3D printed scaffolds must use biocompatible materials connected by a porous 3D connected matrix to deliver nutrients [18] [77] [78]. A common technique used for creating bone tissue is the hyaluronic acid bio-inks [35]. A material that has proven to be useful for 3D printing bone applications is the polyethylene glycol (PEG) hydrogel, which is mechanically strong, and porous matrixes can be created with this material [35] [57] [77].
The articular cartilage is a smooth surface. The cartilage surface provides support and facilitates joint movements. Since the articular cartilage is avascular and is subjected to external forces, when it degenerates, it is common to see injuries that can evolve in a disease like joint arthritis [14]. Tissue engineering using 3D printing techniques, and using hydrogels materials, have the purpose of creating cartilage. Currently, the main problem in 3D printing for articular cartilage is a strong form of the inferior 3D printed cartilage tissue [77].
Gastroenterology Applications
Stem cells are also employed in the field of gastroenterology for the 3D bioprinting of liver tissue, in the form of microstructures that show hepatocyte-like phenotypes and high cell viability [16] [71]. This represents a great advantage in the process of liver transplants since there is currently a limited number of donors. Furthermore, the production of hepatic tissue allows a personalized study about the condition of the liver [71]. As seen in other areas, further applications of 3D bioprinting include planning and guidance during surgical operations of the liver for improved screening of the hepatic structures and for medical education, since the 3D bioprinting of these tissues is a preferable alternative to the use of cadavers in terms of costs and sociocultural issues [79]. Patents in this field are related to the methods for 3D printing of liver tissue [80] and modeling methods for pancreatic surgical planning [45].
Dermatology Applications
Applications in the field of dermatology include the fabrication of adipose tissue and skin tissue with its equivalents (sweat glands and hair follicles). In this field, the great advantage of the 3D bioprinted tissues is that they are quickly obtained while having enough accuracy as the natural ones [18] [35]. Stem cells originating from human adipose tissue are used to create 3D grafts that are complex enough to be compared with the natural adipose tissue. Moreover, the 3D printed adipose tissue has the optimal conditions to be used for transplants and allows a personalized study about the condition of the adipose tissue. A great process in the development of skin tissue has been achieved by using collagen matrices embedded with fibroblasts and keratinocytes as bio-inks. This ensures that there is sufficient vasculature to support the tissue since a poor vascularization may have adverse effects on the 3D bioprinted tissue, including necrosis [16]. For the reproduction of sweat glands, a combination of components taken from the tissue is used, and its initial experiments have been successful [18] [35]. Patents on this field specify the preparation methods for 3D printed skin [44] [81].
Orthopedic Applications
The obtention of 3D printed models to compensate for the loss of bone and cartilage is the main goal within this area by using the inkjet bioprinting technology [18] [82]. With hydrogels as bioinks, the time needed for the creation of the bone tissue is reduced, and its mechanical properties are enhanced. To ensure vascularization, the pores of the printed structures are around 300 µm [82]. These models are also used in pharmacokinetic studies and for the research of bone-related diseases [82]. Several patents are found in this field related to the methods for bone and cartilage printing [26] [31] [34] [43] [47] [50].
Otolaryngology Applications
One of the clearest examples of the 3D printing relevance in the field of otorhinolaryngology is the production of hearing aids, as most of the ones available on the market are printed in 3D [83]. Patents in this field include the preparation and manufacturing methods of bones with applications in hospitals [53] [69] [84] [85]. Furthermore, as with the above-mentioned applications, 3D printing is also used for surgical education and training since, by providing accurate and complex models, reconstructive surgeries have proven to have improved outcomes and have even been carried out in a reduced period of time [83]. The most relevant 3D-printed models/simulators used for surgical education and training in the field of otolaryngology are [17] [83]:
Urology Applications
Applications of 3D-print in urology include education and surgical planning [86] [88]. Printed models include kidney, liver, prostate, ureters, kidney tumors, and renal pelvicalyceal systems [87] [89]. The materials used for this go from silicone, wax, or polymers for the kidney models and polyvinyl alcohol hydrogels and 3D-printed injection molds for renal systems. These models are used for renal transplantation and treatment of renal masses with suspected renal cell carcinoma. This is possible since the materials used in 3D printing share similar characteristics with the organ in terms of shape, elasticity, and mechanical strength [87]. Renal pelvicalyceal systems are used in the treatment of nephrolithiasis. Other applications of 3D-printing in urology are the creation of equipment such as stents and trochars [86].
Nutriology Applications
Within food science, 3D-printing has been proposed as a method to obtain personalized functionalized foods [90]. Functional foods (FF) are considered as “foods that supply nutrients and offer potential health benefits that can enhance the well-being of people” [90]. The aim is to offer customized foods that, according to the needs of the user, are supplemented with proteins, sugars, vitamins, and minerals. Examples of ingredients used in 3D-printing are chocolate, pasta, and pork pure, which have been used to produce pizza and enriched cookies [90].
Radiology Applications
As well as in other fields, applications of 3D-print in radiology include education and surgical planning [91]. Specifically, within this field, 3D-printing has been used in vascular radiology with the aim to reduce operating times and complications [91]. The production of those 3D printed models follows four steps: image acquisition, image segmentation, creation of a 3D model, and 3D printing [92].
The modality used for the image acquisition in DICOM format (being CT the most common one) depends on the characteristics of the target [93]. The segmentation consists of the extraction of regions of interest (ROI) for its 3D reconstruction based on the target tissue and pathophysiology [93]. It has been proposed that artificial intelligence can be applied to improve segmentation and also a fusion of multiple imaging modalities [94].
3D printing regulations
In America, the Food and Drug Administration (FDA) has emitted, through several centers, guidance documents and regulations to be followed according to the type of application [95]:
The Center for Devices and Radiological Health (CRDH) supervises medical device applications.
The Center for Biologics Evaluation and Research controls Biological applications.
The Center for Drug Evaluation and Research supervises pharmacology applications.
Along with these regulations, the FDA also offers descriptions of the 3D printing processes and learning resources for its application in biomedical sciences. The goal is to accelerate the manufacturing process in 3D printing and ensure the correct implementation of this technology.
3D printing challenges
The use of 3D printing involves several challenges for its implementation in the healthcare sector. According to a survey made on 700 professional users, the biggest challenges are related to the expenses of the equipment and production, the requirements for its posterior processing, and the short supply of materials [96].
A solution for the cost-related challenges could be the optimization of the manufacturing processes. Another challenge is the resistance to change the guidelines and processes in the healthcare sector [12].
Further challenges are described below. Within the printing process to obtain blueprints of tissues and organs of complex anatomies is a challenge [97]. Also, during manufacturing replication of tortuous and thin structures [97], removing negative material from cavities [98] are other challenges. Another specific challenge for regenerative medicine is the integration of tissues and various cell types and the assurance of long-term functionality [4].
Specific challenges for applying 3D printing in pharmacology include regulatory aspects such as assurance of safety and quality of materials and final products [60]. Ensuring the proper disposal of printed parts when they are no longer required is an additional regulatory challenge.
These and other challenges need to be addressed to ensure advanced and application of 3D printing in healthcare.
CONCLUSIONS
3D printing started as a revolutionizing technology in the different fields of healthcare and, it has demonstrated to be a useful technology up to the point of becoming of common use. This brief review aimed to identify the latest work rapidly and perspectives of 3D printing in healthcare. Major applications among the advantages and disadvantages of 3D printing in healthcare were presented. Three-dimensional printing still has challenges that need to be tackled to ensure the progress and application of 3D printing in healthcare.











 nueva página del texto (beta)
nueva página del texto (beta)