Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.59 no.1 Ciudad de México ene./mar. 2015
Article
Preparation of (+)- and (-)- β-phenyl- and β-(4-chlorophenyl)-γ-butyrolactones: Key Intermediates in the Synthesis of β-phenyl-GABA and Baclofen
Jazmín Gómez-Gutiérrez, Myriam Meléndez-Rodríguez,* Óscar R. Suárez-Castillo, Luis E. Castelán-Duarte, Manuel J. Fragoso-Vázquez, Erick López-Vázquez, and Maricruz Sánchez-Zavala
Área Académica de Química, Universidad Autónoma del Estado de Hidalgo, Mineral de la Reforma, Hidalgo, 42184 México. melendez@uaeh.edu.mx
Received December 13th, 2013
Accepted August 21st, 2014
Abstract
The preparation of β-phenyl- and β-(4-chlorophenyl)-γ-butyrolactones ( ± )-3 and ( ± )-4 and their resolution to the corresponding (+)-(S)-3, (-)-(R)-3 and (+)-(S)-4, (-)-(R)-4 through formation, flash column chromatography separation and subsequent hydrolysis of diastereoisomeric 4-hydroxybutyramides (2'R,3S)-5, (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6 is described. The absolute configuration assignment of enantiopure 3 and 4 was supported by X-ray crystallographic structures of (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6.
Key words: Phenyl-γ-butyrolactones, Resolution, Diastereoisomeric 4-hydroxybutyramides.
Resumen
Se describe la preparación de las β-fenil- y β-(4-clorofenil)-γ-butirolactonas (±)-3 y (±)-4 y su resolución en (+)-(S)-3, (-)-(R)-3 y (+)-(S)-4, (-)-(R)-4 mediante la formación, separación por cromatografía en columna rápida y posterior hidrólisis de las 4-hidroxibutiramidas diastereoisoméricas (2'R,3S)-5, (2'R,3R)-5, (2'R,3S)-6 y (2'R,3R)-6. La asignación de la configuración absoluta de las lactonas enantiopuras 3 y 4 se confirmó mediante cristalografía de rayos-X de (2'R,3R)-5, (2'R,3S)-6 y (2'R,3R)-6.
Palabras clave: Fenil-γ-butirolactonas, resolución, 4-hidroxibutiramidas diastereoisoméricas.
Introduction
Enantiopure compounds are important in the chemical and pharmaceutical sciences due to their particular properties, and especially because one enantiomer usually behaves differently than its counterpart when interacting with a biological system [1, 2]. Thus, the absolute configuration of such compounds must be known. In particular, separation of optical isomers by formation of diastereomeric derivatives could offer advantages on their absolute configuration assignment, especially if they can be crystallized and monitored by physical techniques such as NMR spectroscopy or X-ray crystallographic analysis.
The 4-amino-3-phenylbutanoic [3] (β-phenyl-GABA, 1) and 4-amino-3-(4-chlorophenyl)butanoic acids [4] (baclofen, 2) (Fig. 1), which are lipophilic analogues of γ-aminobutyric acid (GABA), show different pharmacological activities. Compound 1 is a mood elevator and tranquillizer [5], while 2 is widely used as a muscle relaxant [6]. The biological activity of these compounds resides on the (-)-(R)-enantiomer [3,4], however baclofen is commercialized in its racemic form (Lioresal® and Baclon®). Due to the increasing demand of enantiomerically pure drugs for the pharmaceutical industry, the development of new approaches to obtain compounds such as 1 and 2 in enantiomerically pure form is an important target.

Examples of synthesis of (±)-2 [7], enantiomeric and chemoenzymatic synthesis of (R)-1 , (S)-1, (R)-2 and (S)-2, and the resolution of racemates of 1 and 2 have been previously reported [3a,6,8]. Besides, it is worth noting that some approaches for the enantiomeric synthesis of (-)-2 are based on the use of the enantiopure (-)-(R)-β-(4-chlorophenyl)-γ-butyrolactone (-)-(R)-4 as the key building block involving a few steps [9].
Due to the important pharmacological activities of γ-amino acids such as 1 and 2 and knowing that lactone 4 is an intermediate in the synthesis of 2, herein we report the preparation of the enantiopure lactones (+)-(S)- and (-)-(R)-3 and (+)-(S)- and (-)-(R)4 through the reaction of racemic 3 and 4 with the chiral auxiliar (+)-(R)-α-methylbenzylamine [10] [(+)-(R)-α-MBA] to obtain the diastereomerically pure 4-hydroxybutyramides 5 and 6. Of relevance is the efficient chromatography separation of amides 5 and 6 and their mild acidic transformation to the enantiomerically pure lactones 3 and 4 with recovery of the chiral auxiliary. An advantage of the present methodology is that the absolute configuration of both enantiomers of 3 and 4 is established by the X-ray structures of the diastereomers (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6.
Results and Discussion
The preparation of β-phenyl-γ-butyrolactone (3) is shown in Scheme 1. Accordingly, reaction of styrene oxide (7) with the sodium salt of diethyl malonate in ethanol [11a,b] afforded the α-carboethoxy-γ-lactones 8 (26%) and 9 (13%) and the remnant was unidentified materials. The subsequent hydrolysis and decarboxylation of 8 was achieved with alumina/H2O [12] affording 3 in 67% yield. In order to increase the yield of the desired lactone 3, styrene oxide 7 was treated with ethyl cyanoacetate instead of diethyl malonate [11c,d] to obtain the corresponding α-cyano-γ-butyrolactones 10 (48%) and 11 (5%). The subsequent decyanation of 10 with alumina/H2O afforded 3 in 91% yield. Decarboalkoxylation of β-keto esters and decyanation of α-cyano-γ-lactones have been carried out with alumina/H2O under reflux in different solvents [12]. However, it is remarkable that in this work the decarboethoxylation of 8 and decyanation of 10 proceeded in solid phase at 100 °C in good yields.
The preparation of 4 is also shown in Scheme 1. Thus, the p-chlorostyrene (12) was oxidized with dimethyldioxirane (DMD) generated in situ from oxone® and acetone in a buffer of bicarbonates (pH 7) [13], to afford the p-chlorostyrene oxide (13) in 74% yield, which was reacted with the sodium salt of diethyl malonate in ethanol to give the α-carboethoxy-γ-lactones 14 and 15 in 24 and 7% yield, respectively. The solid phase decarboethoxylation of 14 with alumina/H2O afforded 4 in 64% yield. When compound 13 was treated with the sodium salt of ethyl cyanoacetate an inseparable mixture of the α-cyanolactones 16 and 17 was obtained in a 1.3:1 ratio (44% yield) as judged by 1H NMR.
As the yield of the desired lactones 3 and 4 was poor using the above methodologies specially for compound 4, we used as an alternative the Reformatsky reaction on acetoxyacetophenones 20 and 21 [11b,14]. Thus, the α-acetoxylation of the readily available 18 and 19 with 30% aq H2O2, Ac2O, BF3∙OEt2, PhI [15] afforded 20 (70%) and 21 (80%), respectively. Treatment of 20 with Zn and ethyl bromoacetate followed by hydrolysis and cyclization with AcOH and 38% aq HCl gave the α,β-unsaturated γ-butyrolactone 22 in 70% yield [11b,14]. Catalytic hydrogenation of 22 with H2-Pd/C in EtOAc afforded 3 in 96% yield [14]. Similarly, treatment of 21 gave 23 in 40% yield, whose reduction with NaBH4 in MeOH afforded 4 in 95% yield [11b,14].
In addition, lactone 3 was also prepared through reduction of phenylsuccinic anhydride (24) with NaBH4 in THF [16] affording 3 in 44% yield together with α-phenyl-γ-butyrolactone (25) in 52% yield (Scheme 2).

As lactone 4 has been used as a key intermediate for the total synthesis of 2, but in the process to obtain 4 a partial racemization at the benzylic carbon occurs [9], we used as an alternative methodology the resolution of 4 [9b,17c] with the readily available (+)-(R)-α-MBA. This methodology was also applied to the lactone 3 [17a-c] with good results. Thus, reaction of (±)-3 and (±)-4 with (+)-(R)-α-MBA gave a mixture of diastereoisomeric (2'R,3S)- and (2'R,3R)-5 and (2'R,3S)- and (2'R,3R)-6, respectively [18] (Scheme 3). These compounds were easily separated by flash column chromatography [19] eluting with CH2Cl2/EtOAc/ acetone (210:90:1) to give separately (2'R,3S)-5 (41%), (2'R,3R)-5 (41%), (2'R,3S)-6 (40%) and (2'R,3R)-6 (40%). The high yield separation (>99%) of each pair (2'R,3S)-5, (2'R,3R)-5 and (2'R,3S)-6, (2'R,3R)-6 was determined on the basis of significant peaks in their respective 1H NMR spectra.
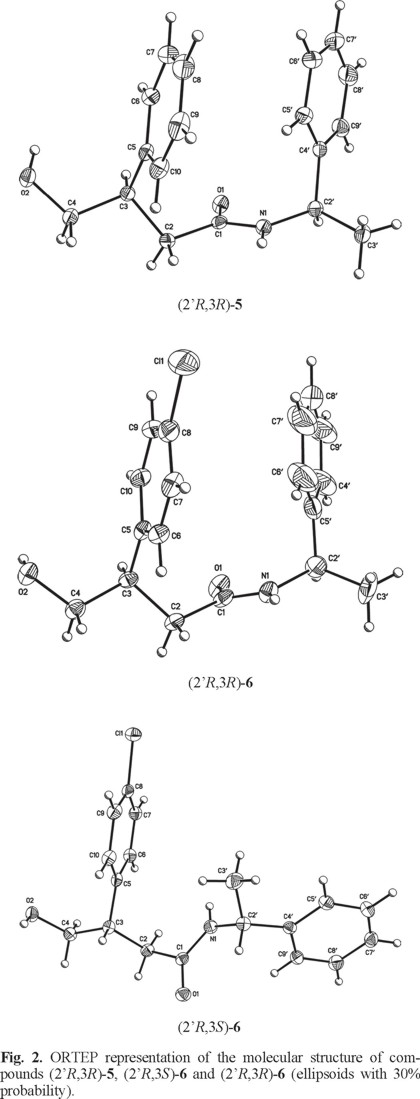
With the methodology developed in this work, the absolute configuration of both enantiomers of 3 and 4 could be establish through the X-ray crystallographic analysis of diastereoisomeric (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6. Fig. 2 shows the X-ray diffraction structures revealing the absolute configuration at C-3 in (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6. The pure diastereoisomers (2'R,3S)-5, (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6 were hydrolyzed and cyclized in acidic medium to give (+)-(S)-3 (78%) [α]20 = +48.3° (c = 1.0, CHCl) {lit. [17c], [α]20= + 46.5° (c = 0.5, CHCl)}, (-)-(R)-3 (73%) [α]20 = -48.9° (c = 1.0, CHCl), D 3D 3D 3 (+)-(S)-4 [9b,20] (75%) [α]20 = +47.9° (c = 1.0, CHCl) {lit. [17c], [α]20 = + 52.0° (c = 0.4, CHCl)} and (-)-(R)-4 (80%) [α]20 = D 3D 3D -48.6° (c = 1.0, CHCl3), respectively. The chemical nature of the stereogenic center at C-3 together with values and signals of optical rotation of each pair of enantiomers confirm that the hydrolysis proceed without significant racemization.
Conclusion
The β-phenyl- and -β-(4-chlorophenyl)-γ-butyrolactones 3 and 4 were resolved with (+)-(R)-α-MBA based on the efficient and simple silica gel flash column chromatography separation and subsequent mild acidic hydrolysis of the corresponding diastereoisomeric 4-hydroxybutyramides (2'R,3S)-5, (2'R,3R)-5 and (2'R,3S)-6, (2'R,3R)-6. The absolute configuration of enantiomers of 3 and 4 was indirectly determined using the X-ray crystallographic structures of (2'R,3R)-5, (2'R,3S)-6 and (2'R,3R)-6. Due to 3 and 4 are key intermediates for β-phenylGABA (1) and baclofen (2), respectively, this methodology constitutes a convenient approach for the enantiomeric synthesis of these important compounds.
Experimental
General Experimental Procedures
Flash column chromatography [19] was carried out using silica gel Merck grade 60 (230-400 mesh) eluting with the specified solvent mixture. Analytical thin layer chromatography was performed on silica gel 60 F254 coated aluminum sheets (0.25 mm thickness) with a fluorescent indicator. Visualization was accomplished with UV light (254 nm). All commercially available reagents were used as received. All of the solvents employed were distilled prior to use or were spectral or HPLC grade. Melting points were determined on a Büchi B-504 apparatus and are uncorrected. IR spectra were recorded on a Perkin Elmer 2000 FT-IR spectrophotometer. Optical rotation measurements were performed on a Perkin-Elmer 341 polarimeter. The 1H and 13C NMR spectra were obtained on a JEOL Eclipse+ 400 spectrometer working at 400 and 100 MHz, respectively, using DMSO-d6 or CDCl3 as the solvent. Data are reported as follows: chemical shifts in ppm from TMS, multiplicity (s singlet, d doublet, t triplet, q quartet, m multiplet, br broad), coupling constants (Hz), integration and assignment. Low-resolution mass spectra were recorded at an ionizing voltage of 70 eV on a Hewlett Packard 5989-A spectrometer. High-resolution (HR) mass spectra were measured on a JEOL JMSSX 102A mass spectrometer at Instituto de Química UNAM-México. Microanalytical determinations were performed on a Perkin-Elmer 2400 series PCII apparatus.
Preparation of 2-(4-chlorophenyl)oxirane (13)
To a solution of 12 (400 mg, 2.89 mmol) in acetone (15 mL) was added a solution of NaHCO3 (840 mg, 10 mmol) in H2O (5 mL). The resulting thick mixture was treated dropwise with a solution of oxone monopersulfate complex (2.22 g, 3.6 mmol) and disodium EDTA (7 mg) in water (7 mL) and the resulting reaction mixture was stirred at room temperature for 2 h. The acetone was evaporated under reduced pressure and the residue was dissolved in EtOAc (30 mL), washed with brine (2 × 20 mL), dried over anhydrous Na2SO4 and concentrated. The crude product was purified by flash column chromatography on silica gel (EtOAchexane 1:8) to afford 13 (330 mg, 74%).
General procedure for the decarboethoxylation of 8 and 14 and decyanation of 10
To a solution of appropriate γ-lactone 8 (500 mg, 2.13 mmol), 14 (500 mg, 1.86 mmol) or 10 (100 mg, 0.53 mmol) in THF (25 mL for 8 and 14, and 9 mL for 10) and water (1.4 mL for 8 and 14, and 0.3 mL for 10) was added activated neutral alumina (3.5 g for 8 and 14, and 1.0 g for 10) (Brockmann 1, 150 mesh, 58 Å, d = 3.970 from Aldrich Co.). The mixture was stirred for 5 min and the THF was evaporated. The impregnated alumina was heated at 100 °C for 26 h for 8 and 14, and 5 h for 10. The reaction mixture was cooled to room temperature, ethyl acetate was added and the alumina was filtered and washed with ethyl acetate (3 × 20 mL). The resulting solution was evaporated affording 3 (232 mg, 67% from 8 and 78 mg, 91% from 10) and 4 (247 mg, 64% from 14).
General procedure for the preparation of 4-hydroxylamides 5 and 6
A solution of 3 (200 mg, 1.23 mmol) or 4 (300 mg, 1.53 mmol) in (R)-(+)-α-MBA (1.26 mL for 3 and 1.56 mL for 4; 8 equiv/mol) was heated at 55 °C for 9 h. The mixture was cooled to room temperature and diluted with ethyl acetate (30 mL), washed with aqueous 10% HCl (3 x 15 mL), brine (3 × 15 mL), dried over anhydrous Na2SO4, and concentrated. The (R)-(+)-αMBA was recovered from aqueous phase with saturated NaHCO3 aqueous solution. The residue was separated by flash column chromatography on silica gel (CH2Cl2-EtOAc-acetone 210:90:1) to give, in sequence, pure (2'R,3S)-5 and (2'R,3R)-5 from 3 and (2'R,3S)-6 and (2'R,3R)-6 from 4.
(S)-4-Hydroxy-3-phenyl-N-[(R)-1-phenylethyl]butanamide [(+)-(2'R,3S)-5]. Obtained from 3 as a white solid (143 mg, 41%): mp 73-74°C; [α]20 D +106.0° (c 1.0, EtOH); IR (KBr) νmax 3328, 3246, 3030, 2973, 1638, 1554, 1450 cm-1; 1H NMR (CDCl3, 400 MHz) δ7.29-7.14 (10H, m, H-6 to H-10 and H-5' to H-9'), 6.28 (1H, d, J = 6.9 Hz, NH), 4.96 (1H, q, J = 6.9 Hz, H-2'), 3.70 (1H, dd, J = 11.0, 5.9 Hz, H-4A), 3.64 (1H, dd, J = 11.0, 7.4 Hz, H-4B), 3.52 (1H, br s, OH), 3.23 (1H, q, J = 6.9 Hz, H-3), 2.66 (1H, dd, J = 14.3, 7.4 Hz, H-2A), 2.43 (1H, dd, J = 14.3, 7.0 Hz, H-2B), 1.25 (3H, d, J = 7.0 Hz, C-3' Me); 13C NMR (CDCl3, 100 MHz) δ 171.3 (C-1), 143.0 (C-4'), 141.6 (C-5), 128.6 and 128.4 (C-7, C-9 and C-6', C-8'), 127.6 (C-6, C-10), 127.0 and 126.8 (C-7'and C8), 126.0 (C-5', C-9'), 66.8 (C-4), 48.7 (C-2'), 44.9 (C-3), 40.2 (C-2), 21.4 (C-3'); EIMS m/z (rel. int.): 283 [M]+ (6), 265 (12), 162 (18), 132 (23), 120 (35), 105 (100), 77 (35); HRMS (FAB) m/z 284.1645 (calc. for C18H22O2N ([M+H]+), requires 284.1651); Anal. C 75.73, H 7.30, N 4.28, calcd for C18H21O2N, C 76.33, H 7.42, N 4.95.
(R)-4-Hydroxy-3-phenyl-N-[(R)-1-phenylethyl]butanamide [(+)-(2'R,3R)-5]. Obtained from 3 as colorless crystals (142 mg, 41%): mp 117-118°C (EtOAc-CH2Cl2); [α]D 20 +33.1° (c = 1.0, EtOH); IR (KBr) νmax 3234, 3031, 2966, 1645, 1565, 1451 cm-1; 1H NMR (DMSO-d6, 400 MHz,) δ7.29-7.15 (8H, m, H-6 to H-10 and H-6' to H-8'), 7.03 (2H, dd, J = 7.7, 1.5 Hz, H-5', H-9'), 6.16 (1H, d, J = 8.1 Hz, NH), 4.99 (1H, q, J = 7.0 Hz, H-2'), 3.75 (1H, dt, J = 11.0, 5.5 Hz, H-4A), 3.69 (1H, dt, J = 10.6, 6.6 Hz, H4B), 3.34 (1H, t, J = 5.9 Hz, OH), 3.25 (1H, q, J = 6.9 Hz, H-3), 2.71 (1H, dd, J = 14.3, 7.0 Hz, H-2A), 2.45 (1H, dd, J = 14.3, 7.0 Hz, H-2B), 1.37 (3H, d, J = 7.0 Hz, C-3' Me); 13C NMR (DMSO-d6, 100 MHz) δ171.2 (C-1), 142.8 (C-4'), 141.5 (C-5), 128.6 and 128.4 (C-7, C-9 and C-6', C-8'), 127.6 (C-6, C-10), 127.0 and 126.8 (C-7' and C-8), 126.0 (C-5', C-9'), 66.8 (C-4), 48.6 (C2'), 44.9 (C-3), 40.3 (C-2), 21.5 (C-3'); EIMS m/z (rel. int.) 283 [M+, 6], 265 (12), 162 (18), 132 (23), 120 (35), 105 (100), 77 (35); HRMS (FAB) m/z 284.1652 (calc. for C18H22O2N ([M+H]+), requires 284.1651); Anal. C 75.96, H 7.38, N 5.19, calcd for C18H21O2N, C 76.33, H 7.42, N 4.95.
(S)-3-(4-Chlorophenyl)-4-hydroxy-N-[(R)-1-phenylethyl]butanamide [(+)-(2'R,3S)-6]. Obtained from 4 as colorless crystals (195 mg, 40%): mp 153-154°C (EtOAc-CH2Cl2); [α]D 20 +96.4° (c = 1.0, EtOH); IR (KBr) νmax 3397, 3329, 3090, 2982, 1633, 1541, 1446 cm-1; 1H NMR (DMSO-d6, 400 MHz) δ8.18 (1H, d, J = 7.7 Hz, NH), 7.37-7.15 (9H, H-6, H-7, H-9, H-10 and H-5' to H-9'), 4.81 (1H, m, H-2'), 4.73 (1H, m, OH), 3.50 (2H, m, H-4A and H-4b), 3.17 (1H, m, H-3), 2.57 (1H, dd, J = 14.3, 6.2 Hz, H-2A), 2.39 (1H, dd, J = 14.6, 9.1 Hz, H-2B), 1.17 (3H, d, J = 7.0 Hz, C-3' Me); 13C NMR (DMSO-d6, 100 MHz) δ169.8 (C-1), 144.6 (C-4'), 141.8 (C-5), 130.6 (C-8), 129.8 (C-6',C-8'), 128.1 and 127.8 (C-6, C-10 and C-7, C-9), 126.5 (C-7'), 125.9 (C-5', C-9'), 65.1 (C-4), 47.6 (C-2'), 44.1 (C-3), 38.2 (C-2), 22.3 (C-3'); EIMS m/z (rel. int.) 252 [M+- 65, 7], 224 (3), 125 (27), 111 (55), 97 (100), 83 (98), 69 (79), 57 (100), 55 (90); HRMS (FAB) m/z 318.1258 (calc. for C18H21O2NCl ([M+H]+), requires 318.1261); Anal. C 67.93, H 6.38, N 4.53, calcd for C18H20O2NCl, C 68.04, H 6.30, N 4.41.
(R)-3-(4-Chlorophenyl)-4-hydroxy-N-[(R)-1-phenylethyl]butanamide [(+)-(2'R,3R)-6]. Obtained from 4 as colorless crystals (193 mg, 40%): mp 112-113°C (CHCl3); [α]20 D +18.7° (c = 1.0, EtOH); IR (KBr) νmax 3257, 3068, 2965, 1634, 1558, 1442 cm-1; 1H NMR (DMSO-d6, 400 MHz) δ8.18 (1H, d, J = 8.1 Hz, NH), 7.30 (2H, d, J = 6.6 Hz, H-7 and H-9), 7.22 (2H, d, J = 6.6 Hz, H-6 and H-10), 7.16-7.12 (m, 3H, H-6' to H-8'), 6.86 (2H, m, H-5', H-9'), 4.80 (1H, q, J = 7.3 Hz, H-2'), 4.78 (1H, t, J = 5.5 Hz, OH), 3.51 (2H, m, H-4A and H-4b), 3.15 (1H, m, H-3), 2.57 (1H, dd, J = 14.2, 5.5 Hz, H-2A), 2.43 (1H, dd, J = 13.9, 9.9 Hz, H-2B), 1.27 (3H, d, J = 7.0 Hz, C-3' Me); 13C NMR (DMSO-d6, 100 MHz) δ169.9 (C-1), 144.4 (C-4'), 141.4 (C-5), 130.8 (C-8), 130.0 (C-6, C-10), 127.9 (C-7,C-9), 127.8 (C-6', C-8'), 126.2 (C-7'), 125.5 (C-5', C-9'), 65.3 (C-4), 47.3 (C-2'), 44.6 (C-3), 38.4 (C-2), 22.3 (C-3'); EIMS m/z (rel. int.) 252 [M+-65, 3], 224 (1), 125 (15), 111 (35), 97 (73), 83 (80), 69 (73), 57 (100), 55 (100); HRMS (FAB) m/z 318.1256 (calc. for C18H21O2NCl ([M+H]+), requires 318.1261); Anal. C 68.04, H 6.42, N 4.53, calcd for C18H20O2NCl, C 68.04, H 6.30, N 4.41.
General procedure for the hydrolysis of 4-hydroxyamides 5 and 6
To a solution of the appropriate 4-hydroxylamides 5 (200 mg, 0.71 mmol) and 6 (200 mg, 0.63 mmol) in EtOH-H2O 1:1 (0.4 mL) was added a solution of aqueous 36% HCl (0.15 mL). The resulting white suspension was heated to 120°C for 6 h in a sealed tube. The reaction mixture was cooled to room temperature, the solvent was evaporated under reduced pressure and the residue dissolved in CH2Cl2 (30 mL). This solution was washed with brine (2 x 20 mL), dried over anhydrous Na2SO4 and concentrated. The crude product was purified by flash column chromatography on silica gel (1:8 EtOAc-hexane) to afford (+)-(S)-3 (89.6 mg, 78% from (2'R,3S)-5) and (-)-(R)-3 (84.0 mg, 73% from (2'R,3R)-5), and (+)-(S)-4 (92.4 mg, 75% from (2'R,3S)-6) and (-)-(R)-4 (98.6 mg, 80% from (2'R,3R)-6).
Single crystal X-ray analyses
Compounds (2'R,3R)-5 and (2'R,3R)-6 were crystallized from AcOEt/CH2Cl2, while (2'R,3S)-6 from CHCl3. X-ray data were collected on a Bruker Smart 6000 CCD diffractometer. A total of 1321 frames were collected at a scan width of 0.3° and an exposure time of 10s/frame, using Mo Kαradiation (λ0.7073 Å). The frames were processed with the SAINT software package, provided by diffractometer manufacturer, by using a narrow-frame integration algorithm, and the structure was solved and refined by using the SHELXS-97 program [20] included in the WINGX VI.6 crystallographic software package [21]. The non-hydrogen atoms were treated anisotropically, and the hydrogen atoms, included in the structure factor calculation, were refined isotropically. Crystallographic data for the structures reported in this paper are in deposit at the Cambridge Crystallographic Data Center. Table 1 summarizes the relevant data of the X-ray procedures.

Acknowledgements
We are pleased to acknowledge the financial support from CONACYT (Mexico) grant 132048 and SEP-PROMEP (Mexico) grant UAEHGO-PTC-244. JGG thanks fellowship No. 256339 from CONACYT.
References
1. (a) Collins, A. N.; Sheldrake, G. N.; Crosby J., in: Chirality in Industry, Eds., John Wiley and Sons, Chichester, 1992; [ Links ] (b) Collins, A. N.; Sheldrake, G. N., Crosby, J., in: Chirality in Industry II, Eds., John Wiley and Sons: Chichester 1997. [ Links ]
2. (a) Ikunaka, M. Chem. Eur. J. 2003, 9, 378-388; [ Links ] (b) Wandrey, C.; Liese, A.; Kihumbu, D. Org. Proc. Res. Dev. 2000, 4, 286-290. [ Links ]
3. (a) Allan, R. D.; Bates, M. C.; Drew, C. A.; Duke, R. K.; Hambley, T. W.; Johnston, G. A. R.; Mewett, K. N.; Spence, I. Tetrahedron 1990, 46, 2511-2524; [ Links ] (b) Ong, J.; Kerr, D. I. S.; Doolette, D. J.; Duke, R. K.; Mewett, K. N.; Allen, R. D.; Johnston, G. A. R. Eur. J. Pharmacol. 1993, 233, 169-172. [ Links ]
4. (a) Olpe, H.-R.; Demieville, H.; Baltzer, V.; Bencze, W. L.; Koella, W. P.; Wolf, P.; Haas, H. L. Eur. J. Pharmacol. 1978, 52, 133-136; [ Links ] (b) Dambrova, M.; Zvejniece, L.; Liepinsh, E.; Cirule, H.; Zharkova, O.; Veinberg, G.; Kalvinsh, I. Eur. J. Pharmacol. 2008, 583, 128-134. [ Links ]
5. Sytinsky, K. A.; Soldatenkov, A. T. Prog. Neurobiol. 1978, 10, 89-133. [ Links ]
6. Mann, A.; Boulanger, T.; Brandau, B.; Durant, F.; Evrard, G.; Heaulme, M.; Desaulles, E.; Wermuth, C. G. J. Med. Chem. 1991, 34, 1307-1313. [ Links ]
7. (a) Kerberle, H.; Faigle, J. W.; Wilhelm, M. Swiss Patent 499,046 (CA 1968 69, 106273f); [ Links ] (b) Wall, G. M.; Baker, J. K. J. Med. Chem. 1989, 32, 1340-1348; [ Links ] (c) Pifferi, G.; Nizzola, R.; Cristoni, A. Il Farmaco 1994, 49, 453-455 (CA 1995, 122, 204918); [ Links ] (d) Ibuka, T.; Schoenfelder, A.; Bildstein, P.; Mann, A. Synth. Commun. 1995, 25, 1777-1782; [ Links ] (e) Carpes, M. J. S.; Correia, C. R. D. Tetrahedron Lett. 2002, 43, 741-744; [ Links ] (f) Chen, Z.; Chen, Z.; Jiang, Y.; Hu, W. Tetrahedron 2005, 61, 1579-1586; [ Links ] (g) Coehlo, F.; de Azevedo, M. B. M.; Boschiero, R.; Resende, P. Synth. Commun. 1997, 27, 2455-2465; [ Links ] (h) Chang, M.-Y.; Sun, P.-P.; Chen S.-T.; Chang N.-C. Tetrahedron Lett. 2003, 44, 52715273. [ Links ]
8. (a) Ordóñez, M.; Cativiela, C. Tetrahedron: Asymmetry 2007, 18, 3-99; [ Links ] (b) Evans, D. A.; Mito, S.; Seidel, D. J. Am. Chem. Soc. 2007, 129, 11583-11592; [ Links ] (c) Fujimori, I.; Mita, T.; Maki, K.; Shiro, M.; Sato, A.; Furusho, S.; Kanai, M.; Shibasaki, M. Tetrahedron 2007, 63, 58205831; [ Links ] (d) Wang, Y.; Li, P.; Liang, X.; Zhang, T. Y.; Ye, J. Chem. Commun. 2008, 1232-1234; [ Links ] (e) Deng, J.; Duan, Z.-C.; Huang, J.-D.; Hu, X.-P.; Wang, D.-Y.; Yu, S.-B.; Xu, X.-F.; Zheng, Z. Org. Lett. 2007, 9, 4825-4828; [ Links ] (f) Ji, L.; Ma, Y.; Li, J.; Zhang, L.; Zhang, L. Tetrahedron Lett. 2009, 50, 6166-6168; [ Links ] (g) Maltsev, O. V.; Kucherenko, A. S.; Beletskaya, I. P.; Tartakovsky, V. A.; Zlotin, S. G. Eur. J. Org. Chem. 2010, 2927-2933; [ Links ] (h) Khatik, G. L.; Khurana, R.; Kumar, V.; Nair, V. A. Synthesis 2011, 19, 3123-3132; [ Links ] (i) Jensen, K. L.; Poulsen, P. H.; Donslund, B. S.; Morana, F.; Jørgensen, K. A. Org. Lett. 2012, 14, 1516-1519; [ Links ] (j) Yang, X.-F.; Ding, Ch.-H.; Li, X.-H.; Huang, J.-Q.; Hou, X.-L.; Dai, L.-X.; Wang, P.-J. J. Org. Chem. 2012, 77, 8980-8985; [ Links ] (k) Brodzka, A.; Koszelewski, D.; Cwiklak, M.; Ostaszewski, R. Tetrahedron: Asymmetry 2013, 24, 427-433. [ Links ]
9. (a) Mazzini, C.; Lebreton, J.; Alphand, V.; Furstoss, R. Tetrahedron Lett. 1997, 38, 1195-1196; [ Links ] (b) Schoenfelder, A.; Mann, A.; Le Coz, S. Synlett 1993, 63-64; [ Links ] (c) Hayashi, M.; Ogasawara, K. Heterocycles 2003, 59, 785-791; [ Links ] (d) Capitta, F.; Frongia, A.; Ollivier, J.; Piras, P. P.; Secci, F. Synlett 2011, 89-93; [ Links ] (e) Oliveira, C. C.; Angnes, R. A.; Correia, C. R. D. J. Org. Chem. 2013, 78, 4373-4385; [ Links ] (f) Xu, S.; Wang, Z.; Zhang, X.; Zhang X.; Ding, K. Angew. Chem. Int. Ed. 2008, 47, 2840-2843. [ Links ]
10. Juaristi, E.; Escalante, J.; León-Romo, J. L.; Reyes, A. Tetrahedron: Asymmetry 1998, 9, 715-740. [ Links ]
11. (a) DePuy, C. H.; Breitbeil, F. W.; Eilers, K. L. J. Org. Chem. 1964, 29, 2810; [ Links ] (b) Sato, M.; Kosasayama, A.; Uchimaru, F. Chem. Pharm. Bull. 1981, 29, 2885-2892; [ Links ] (c) Zuidema, G. D.; Cook, P. L.; Van Zyl, G. J. Am. Chem. Soc. 1953, 75, 294-296; [ Links ] (d) Orszulik, S. T.; Porter, M. J. Chem. Research (S) 1998, 258-259. [ Links ]
12. (a) Greene, A. E.; Cruz, A.; Crabbé, P. Tetrahedron Lett. 1976, 17, 2707-2708. [ Links ] (b) Morales-Ríos, M. S.; Suárez-Castillo, O. R.; García-Martínez, C.; Joseph-Nathan, P. Synthesis 1998, 17551759. [ Links ]
13. Suárez-Castillo, O. R.; Sánchez-Zavala, M.; Meléndez-Rodríguez, M.; Castelán-Duarte, L. E.; Morales-Ríos, M. S.; Joseph-Nathan, P. Tetrahedron 2006, 62, 3040-3051. [ Links ]
14. Linville, R. G.; Elderfield, R.C. J. Org. Chem. 1941, 6, 270272. [ Links ]
15. Sheng, J.; Li, X.; Tang, M.; Gao, B.; Huang, G. Synthesis 2007, 1165-1168. [ Links ]
16. Bailey, D. M.; Johnson, R. B. J. Org. Chem. 1970, 35, 3574-3576. [ Links ]
17. (a) Arcadi, A.; Bernocchi, E.; Cacchi, S.; Marinelli, F. Tetrahedron 1991, 47, 1525-1540; [ Links ] (b) Satoh, T.; Kamide, Y.; Sugiyama, S. Tetrahedron 2004, 60, 11805-11812; [ Links ] (c) Lawston I. W.; Inch, T. D. J. Chem. Soc., Perkin Trans. I 1983, 2629-2635. [ Links ]
18. (a) Helmchen, G.; Nill, G.; Flockerzi, D.; Schühle, W.; Youssef, M. S. K. Angew. Chem. Int. Ed. Engl. 1979, 18, 62-63; [ Links ] (b) Helmchen, G.; Nill, G.; Flockerzi, D.; Youssef, M. S. K. Angew. Chem. Int. Ed. Engl. 1979, 18, 63-65; [ Links ] (c) Helmchen, G.; Nill, G. Angew. Chem. Int. Ed. Engl. 1979, 18, 65-66. [ Links ]
19. Still, W. C.; Kahn, M.; Mitra, A. J. Org. Chem. 1987, 43, 2923-2925. [ Links ]
20. Sheldrick, G. M. Programs for Crystal Structure Analysis. In Institüt für Anorgnische Chemie der Universität, University of Göttingen: Göttingen, Germany 1988. [ Links ]
21. Farrugia, L. J. J. Appl. Crystallogr. 1999, 32, 837-838. [ Links ]














