Introduction
Coronavirus disease 2019 (COVID-19) is mainly a respiratory disorder due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1. However, more recent evidence now considers it a multisystemic infectious disease, and the digestive tract is one of the systems that could be affected2. This disease is characterized by fever, dry cough, fatigue, and lymphopenia, in some cases leading to severe acute respiratory syndrome (SARS), organ dysfunction, and death3. Fever is the most common symptom of COVID-19, which occurs in 83-98% of patients followed by cough in 46-82% of patients. Overall, 80% of those infected in Hubei Province, China, were described as mild cases, 13.8% were severe cases warranting hospitalization, and 6.1% needed intensive care unit (ICU) care. COVID-19 may progress to severe bilateral pneumonia and acute respiratory distress syndrome requiring prolonged mechanical ventilation4.
Many extrapulmonary manifestations of COVID-19 have been described, suggesting that the hematologic, cardiovascular, renal, gastrointestinal (GI), hepatobiliary, endocrinologic, neurologic, ophthalmologic, reproductive, and dermatologic systems can all be affected5,6.
SARS-CoV-2 belongs to the beta coronavirus genus and enters cells through the angiotensin-converting enzyme 2 (ACE2) receptor7. A virus surface spike protein mediates SARS-CoV-2 binds to its receptor ACE2 through its receptor-binding domain (RBD) and is proteolytically activated by human proteases8. ACE2 is highly expressed in many cells from various human organs9; particularly lungs, vascular endothelial cells, kidneys, gastrointestinal tract, liver, and arterial smooth muscle cells. Thus, all of these organs might be susceptible to SARS-CoV-2 infection6.
More attention has been paid to the GI manifestations of SARS-CoV-2 since SARS-CoV-2 RNA was first detected in the stool of the first reported COVID-19 case in the United States, who also presented with diarrhea, nausea, and vomiting10,11. GI manifestations are common in patients with COVID-19. A study from China has reported that 50.5% of COVID-19 patients have at least one GI manifestation12; among GI manifestations of COVID-19, diarrhea seems to be the most commonly reported in children and adults13.
Methods
This is a narrative review. We searched PubMed, EMBASE, MEDLINE, and Web of Science up to January 2021 to identify all studies documenting the presence of gastrointestinal symptoms in patients with a confirmed diagnosis of COVID-19. The following search terms alone or matched with the Boolean operators “AND” or “OR” were used: “COVID-19,” “SARS-CoV-2,” “coronavirus,” “pandemic,” “diarrhea,” “gastrointestinal symptom(s),” “stool,” “feces,” “pancreas,” “pancreatitis,” “liver injury,” “hepatitis,” “biliary tract,” and “gastrointestinal bleeding.” No temporal, study design, or language restrictions were applied. We focused on full-text articles, but abstracts were considered if relevant (Fig. 1).
Overview of SARS-CoV-2 viral infection
SARS-CoV-2 binds its envelope homotrimeric spike protein to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2) at the host cell membrane to enter the host target cell14. The union of the virus with its ACE2 cellular receptor triggers internalization of the complex into the cell, leading to the downregulation of the ACE215 and favoring the interruption of angiotensin II (AngII) metabolism; causing an increase in its plasma concentration. AngII is responsible for a prothrombotic effect, endothelial and platelet activation, vasoconstriction, and inflammation16.
Specific ACE2 expression in extrapulmonary tissues and its relation to gastrointestinal symptoms
SARS-CoV-2 contagiousness and pathogenicity mostly depend on the interactions, including virus attachment, receptor recognition, protease cleaving, and membrane fusion17. Cell entry is a key component of cross-species transmission, mainly for the beta coronaviruses. All coronaviruses encode a surface spike protein, which binds to the host cell receptor and mediates viral entry18. The primary host receptor that mediates the mechanism as the SARS-CoV-2 enters the cell is the ACE2 receptor, which regulates the virus’s cross-species and human-to-human transmissions18-20. The broad distribution of ACE2 receptors in the venous and arterial endothelial cells, arterial smooth muscle cells, biliary tract, cardiovascular, renal, and GI tissues predisposes to the involvement of multiple organs, which may also explain the various extrapulmonary symptoms3.
The ACE2 cell surface receptor in the liver was more highly expressed in cholangiocytes (near to 60%) than hepatocytes (less than 3%). The level of ACE2 expression in cholangiocytes was similar to that in type 2 alveolar cells of the lungs; thus, the liver and biliary tract could be infected for SARS-CoV-2, causing dysfunction21. In the liver, immunohistochemistry stains for ACE2 were negative on T and B lymphocytes, Kupffer cells, as well as the sinusoidal endothelium22.
Zhang et al. revealed that ACE2 mRNA and protein are importantly expressed in small intestinal enterocytes but not on intestinal immune cells or goblet cells. ACE2 may mediate the invasion, amplification, and activation of GI inflammation in the GI tract, leading to GI symptoms susceptibility3. Furthermore, SARS-CoV-2 RNA can be detected in the stool of patients; thus, the virus could replicate in the enterocytes of the small intestine3,23. Among 20% of COVID-19 patients, viral RNA has been detected in the stool even after the nasopharyngeal swabs become negative24-26. Viral RNA can be detected in the stool of more than 50% of the COVID-19 patients with GI symptoms27. Chen et al. studied 42 laboratory-confirmed patients with COVID-19; 67% of these patients tested positive for SARS-CoV-2 RNA in stool samples; 64% of them remained positive during 7 (6-10) days for viral RNA in the stool after the pharyngeal swabs became negative28. Moreover, Wu et al. have shown that SARS-CoV-2 viral RNA may be present in fecal samples for nearly 5 weeks after the respiratory samples tested negative for the virus29.
Positivity for SARS-CoV-2 RNA in stool specimens was not always related to GI symptoms or the severity of the disease since only 19% of patients in the case series of Chen Y had any GI clinical manifestation28.
The high expression of ACE2 protein, as a functional receptor for SARS-CoV-2 in the enterocytes, and the finding of the virus in the stool of patients indicate that fecal-oral transmission may also exist23-29.
Frequency of gastrointestinal manifestations
There are two main phases in SARS-CoV-2 infection. The early or viral phase occurs shortly after infection. It is characterized by low inflammatory activity and a high viral load, with scarcely any symptoms, but also associated with GI symptoms30. Then, in the progressive or late infection phase, patients develop the most severe symptoms such as respiratory distress and fever31.
GI symptoms can be frequent in COVID-19 and, in some cases, be the first manifestation even before respiratory symptoms and fever10,32 (Table 1). The exact incidence of GI symptoms is a matter of debate33. Furthermore, wide variation regards the frequency of GI symptoms exists, but taking in count, cohort studies with at least 99 patients or more included, the frequency of any GI symptom ranges from as low as 2.0%34 to as high as 50.5%12. Most of the available data come from China, where a large cohort, including 1099 patients reported a frequency of any GI symptom of 5.0%4. Another large cohort study conducted in China by Luo et al. found that of 1141 confirmed COVID-19 cases, 183 (16%) presented with GI symptoms35.
Table 1 Frequency of the most common gastrointestinal manifestations reported in COVID-19 patients
| GI manifestation | Estimated frequency (%) | Source of information | n | Relevant comments |
|---|---|---|---|---|
| Any GI symptom | 2.0 | [34] | 99 | – Wide variability across the studies. |
| 5.0 | [4] | 1099 | – Most relevant information from large cohort studies comes from China. | |
| 11.5 | [42] | 4434 | ||
| 16 | [35] | 1141 | ||
| 17.6 | [36] | 4243 | ||
| 22.2 | [49] | 293 | ||
| 25 | [38] | 892 | ||
| 50.5 | [12] | 204 | ||
| Diarrhea | 2.0 | [34] | 99 | – Seems to be the most common GI manifestation from COVID-19 |
| 4.5 | [48] | 132 | ||
| 6.5 | [49] | 293 | ||
| 7.4 | [40] | 4805 | ||
| 7.8 | [42] | 4434 | ||
| 10.4 | [39] | NA | ||
| 19.8 | [38] | 892 | ||
| 31.9 | [56] | |||
| Nausea/vomiting | 1.0 | [34] | 99 | – Unspecific clinical manifestation |
| 1.5 | [48] | 132 | ||
| 2.4 | [49] | 293 | ||
| 3.6 | [42] | 4434 | ||
| 4.6 | [40] | 4805 | ||
| Hypoxia | 2.4 | [42] | 4434 | – Unspecific clinical manifestation |
| 12.1 | [48] | 132 | ||
| 15.2 | [49] | 293 | ||
| Abdominal pain | 0.7 | [49] | 293 | – Related to a severe clinical course of COVID-19 |
| 0.8 | [42] | 4434 | ||
| Liver injury | – Extremely variability though the different
available studies according to if the studied population was
constituted by an ambulatory, hospitalized, or intensive care
patients. – Related to a worse prognosis, need for intensive care, and higher mortality rate. – Controversy if it is SARS-CoV-2 direct injury or drug-induced liver injury, or due to acute systemic inflammatory response. |
|||
| ALT (elevation) | 14.6 | [40] | 4805 | |
| AST (elevation) | 20 | [44] | 1827 | |
| ALT (elevation) | 61.6 | [45] | 100 | |
| AST (elevation) | 83.4 | [48] | 132 | |
| AP (elevation) | 22.7 | [46] | 80 | |
| TBIL (elevation) | 16.1 | [49] | 293 | |
| LFT (altered) | 62.4 | [50] | 74 | |
| Liver enzymes (increased) | 24.2 | [54] | 1611 | |
| ALT (elevation) | 13.8 | |||
| AST (elevation) | 18.8 | |||
| TBIL (elevation) | 6.3 | |||
| Albumin (decreased) | 27.5 | |||
| Pre-albumin (decreased) | 38.8 | |||
| ALT (elevation) | 2.1 | |||
| AST (elevation) | 2.5 | |||
| ALT (elevation) | 29.7 | |||
| AST (elevation) | 25.7 | |||
| AP (elevation) | 6.7 | |||
| TBIL (elevation) | 16.2 | |||
| GGT (elevation) | 27 | |||
| LFT (altered) | 45.2 |
ALT: alanine aminotransferase; AP: alkaline phosphatase; AST: aspartate aminotransferase; COVID-19: coronavirus disease 2019; GGT: gamma-glutamyl transferase;
GI: gastrointestinal; LFT: liver function test; SARS-CoV-2: severe respiratory acute syndrome coronavirus-2; TBIL: total bilirubin.
In a systematic review with meta-analysis by Cheung et al. including 60 studies comprising 4243 patients, the pooled prevalence of all GI symptoms was 17.6% (95% confidence interval [CI], 12.3-24.5); 11.8% of patients with non-severe COVID-19 had GI symptoms (95% CI, 4.1-29.1), and 17.1% of patients with severe COVID-19 had GI symptoms (95% CI, 6.9-36.7). Likewise, in this study, the pooled prevalence of stool samples that were positive for virus RNA was 48.1% (95% CI, 38.3-57.9); of these samples, 70.3% of those collected after the loss of virus from respiratory specimens tested positive for the virus (95% CI, 49.6-85.1)36.
The most common GI reported symptoms in several studies are diarrhea, anorexia, nausea, vomiting, and abdominal pain or discomfort36,37. Of all these GI symptoms, only the presence of abdominal pain seems to be associated with a more severe course of the disease (odds ratio [OR] 7·10; 95% CI: 1·93-26·07)37.
In a large cohort from New York, which included 892 patients, 25% of patients had any GI symptom, the most common was diarrhea in 19.8%38. Furthermore, D’Amico et al. performed a pooled analysis of the available studies until March 2020, founding an overall diarrhea rate of 10.4%39.
In a systematic review with a meta-analysis conducted from November 2019 to March 2020 by Parasa et al., including a total of 4805 patients extracted from 23 published and six preprint studies, the pooled rate reported 7.4% (95% CI, 4.3%-12.2%) of cases presenting diarrhea and 4.6% (95% CI, 2.6%-8.0%) of cases with nausea or vomiting. Fecal tests positive for SARS-CoV-2 were reported in eight studies, and viral RNA shedding was detected in the stool of 40.5% (95% CI, 27.4%-55.1%) of patients40.
In a recent systematic review by Almeida et al., the prevalence of GI symptoms ranged from 6.8% to 61.3%, including diarrhea (8.14% to 33.7%), nausea/vomiting (1.53%-26.4%), anorexia (12.1%-40.0%), and abdominal pain (0%-14.5%). The presence of viral RNA in stools was rarely tested but positive in 0%-48.1%41. Likewise, Merola et al. identified 33 studies that were included in a meta-analysis. Out of 4434 COVID-19 patients, GI manifestations’ pooled prevalence was 11.51% (95% CI: 8.16-15.35). Similar to other studies, they found that the most frequent GI symptom was diarrhea (7.78% of cases; 95% CI: 5.05-11.04), followed by nausea/vomiting (3.57%; 95% CI: 1.87 to 5.80), hypoxia (2.39%; 95%CI: 0.55-5.46), and abdominal pain (0.78%; 95% CI: 0.26-1.57). Positivity for COVID-19 in stool samples was observed in 41.5% (95% CI: 17.70-67.65) of cases42.
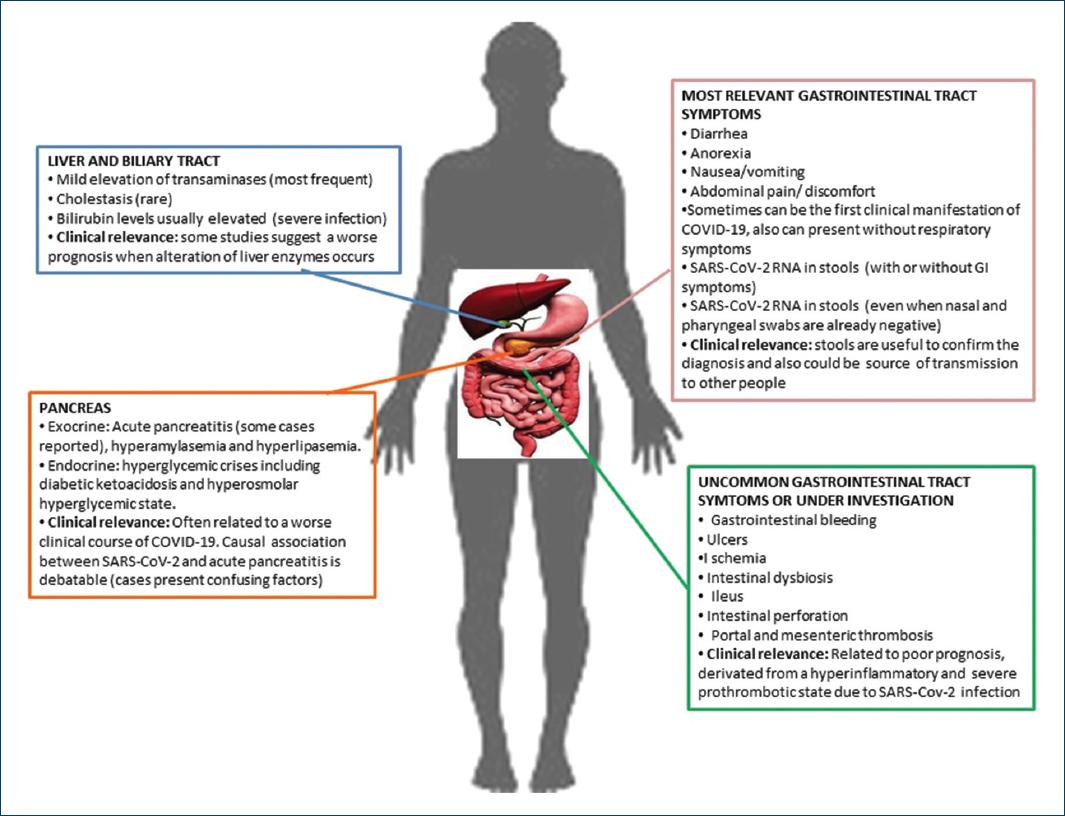
GI symptoms are of particular significance in COVID-19 patients because, in contrast to other coronaviruses, they appear early and may worsen during the disease, whereas in some cases may be solitary. In patients presenting solely with GI symptoms, there is usually a delay in disease diagnosis and time to first respiratory symptoms, rendering them a source of viral dissemination33. In these times, the presence of diarrhea should generate awareness of a possible SARS-CoV-2 infection31 (Fig. 2).
Adequate rehydration and potassium monitoring are recommended in all COVID-19 patients with diarrhea. There is not enough evidence about the safety and efficacy of antidiarrheal drugs in this context31.
Intestinal dysbiosis and COVID-19
Significantly few studies have addressed the link between COVID-19 and intestinal dysbiosis. Zuo et al. performed shotgun metagenomic sequencing analyses of fecal samples from 15 patients with COVID-19. These patients had significant alterations in fecal microbiomes compared with controls, characterized by enrichment of opportunistic pathogens and depletion of beneficial commensals. The baseline abundance of Coprobacillus, Clostridium ramosum, and Clostridium hathewayi correlated with COVID-19 severity; there was an inverse correlation between the abundance of Faecalibacterium prausnitzii (an anti-inflammatory bacterium) and disease severity. During hospitalization, Bacteroides dorei, Bacteroides thetaiotaomicron, Bacteroides massiliensis, and Bacteroides ovatus, which downregulate the expression of angiotensin-converting enzyme 2 (ACE2) in the murine gut, correlated inversely with SARS-CoV-2 load in fecal samples from patients43. From these results, we must remember that strategies to alter the intestinal microbiota might reduce COVID-19 severity, but further investigation is required in this field.
Liver and biliary tract involvement
Several observational studies have reported liver function test abnormalities in COVID-19 patients40,44-51 (Table 1).
Liver injury in COVID-19 patients is mainly characterized by a mild elevation of aspartate transaminase (AST) and alanine transaminase (ALT) with a rare incidence of cholestasis52,53. However, bilirubin levels are usually more than double in those with severe infection when compared to those with milder disease24, the incidence of elevated ALT and AST is widely variable, ranging from 2.5%-50.0% to 2.5%-61.1%, respectively53. In a study by Parasa et al., the pooled rate for AST levels outside reference ranges was 20% (95% CI, 15.3%-25.6%) of patients, and the pooled rate for ALT levels outside reference ranges was 14.6% (95% CI, 12.8%-16.6%) of patients40. Hundt et al. conducted a study including 1827 COVID-19 patients admitted to hospital, where abnormal liver tests were commonly seen, both at admission. At admission, most patients with abnormal liver tests had minimal elevation 1-2× the upper limit of normal (ULN). Abnormal liver tests are mainly related to the severe course of COVID-19. Medications used to treat COVID-19 (lopinavir/ritonavir, hydroxychloroquine, remdesivir, and tocilizumab) are also associated with liver transaminases rising >5× ULN during hospitalization44.
In the study by Lenti et al., liver function tests were altered in 62.4% of COVID-19 patients and improved during follow-up. Altered liver function tests were associated with worse outcomes in those who developed SARS. In patients with altered liver function tests, PaO2/FiO2 < 200 was associated with more significant mortality and needed intensive care45. In the study by Li et al., liver function test abnormalities were seen most commonly in severe COVID-19 cases compared with mild cases48. Higher AST levels and lower levels of albumin and pre-albumin were significantly associated with mortality46. Furthermore, the elevation of ALT and AST correlated with the development of coagulopathy50. In severe COVID-19 pediatric patients, a high percentage (50%) of liver function tests abnormalities were also reported51.
Recently, our group published the data of a prospective Latin American cohort of 1611 patients with COVID-19. On admission, abnormal liver function tests were present in 45.2% (95% CI 42.7-47.7) patients. Overall, 15.1% of patients died. Patients with abnormal liver tests on admission had higher mortality compared to those with normal liver biochemistries. After excluding patients with a history of chronic liver disease, abnormal liver tests on admission were independently associated with death [OR 1.5 (95% CI 1.1-2.0); p= 0.01] and severe COVID-19 (2.6 [2.0-3.3], p < .0001), both adjusted by age, gender, diabetes, pneumonia, and body mass index > 3054.
Abnormal liver function tests have consistently shown to be more prevalent in severe COVID-1955. Elevated liver enzymes are more common in those with a severe disease course (40-60%) than those who are asymptomatic or have a mild disease (18-25%)24.
In a cohort study, factors at admission predicting the requirement for invasive mechanical ventilation during hospitalization for COVID-19 were AST ≥ 250 IU/L and D-dimer ≥ 3500 ng/mL56.
In addition, it is important to mention that preexisting CLD is a condition with low reported prevalence (2.5%) in COVID-19 patients57. Nevertheless, this coexistence has been associated with a worse outcome57,58. The patients with cirrhosis are at increased risk of death from COVID-19, particularly those with more advanced cirrhosis and alcohol-related liver disease. A study found that the mortality rate was 32% in cirrhosis patients compared to 8% in those without cirrhosis (p < 0.001). Acute hepatic decompensation occurred in 46% of patients with cirrhosis, of whom 21% had no respiratory symptoms. Near 50% of those with decompensation had acute-on-chronic liver failure59.
Current data are limited, and it is difficult to ascertain whether the liver injury could be due to direct viral infection, though there is no evidence of active replication of the SARS-CoV-2 in hepatocytes24; or drug-induced liver injury; or may be due to complications such as ischemic hepatitis driven for hypoxemia52,53. The elevated transaminases are often accompanied by high creatine kinase and lactate dehydrogenase, suggesting viral myositis60. In addition, the activation of the immune system and “cytokine storm” may contribute to an immune-mediated liver injury process in COVID-1961.
Pancreas
The possible association between SARS-CoV-2-related infection and pancreatic disorders remains uncertain and not well defined47,62. Whether SARS-CoV-2 plays a role in acute pancreatitis (AP), etiology remains controversial since the main evidence is based on some reports of clinical cases63.
Hadi et al. described two female clinical cases of AP due to SARS-CoV-2 (other causes of AP were exhaustively excluded). The main finding in both cases was the rapidly increasing value of amylase during the disease64. Gadiparthi et al. also reported a case of severe AP63. Kataria et al. also reported a case of AP related to COVID-19 in a woman without other risk factors for AP65. A systematic review conducted on May 15, 2020, including six case reports and two retrospective cohorts, found 11 COVID-19 patients with AP. Most cases were considered SARS-CoV-2 induced, but the authors were able to identify other possible causes in most of them that could explain the development of AP66.
In a case series of 52 patients admitted for COVID-19, Wang et al. found evidence of pancreatic injury, defined as elevated amylase and lipase, in up to 17% of these patients67. Similarly, in a descriptive study including 71 patients, McNabb-Baltar et al. found that 9 (12.1%) developed hyperlipidemia, with 2 (2.8%) greater than 3 times the upper limit stock. However, nobody developed acute pancreatitis, and the presence of hyperlipidemia was not associated with poor outcomes or symptoms62. The endocrine pancreas seems altered too. In a South Korean report of two patients, COVID-19 infection was implicated in severe acute hyperglycemic crises68.
GI bleeding as an atypical manifestation of COVID-19
GI bleeding as the first symptom of COVID-19 is a scarce report. It seems to affect mainly older men, and it was more frequently reported in severe COVID-19 cases; most of them required a blood transfusion, and the source of bleeding was not detected or was related to GI ulcers affected upper, middle, and lower GI tract69-75.
Guotao et al. described the case of an 83-year-old man with hematochezia as the initial symptom of SARS-CoV-2 infection, in whom no source of bleeding was identified by colonoscopy or abdominal computed tomography (CT) scan69. Amarapurkar et al. also reported the case of a 63-year-old man who presented with acute abdomen and was diagnosed as hemorrhagic enteritis without any predisposing conditions but positive for SARS-CoV-270. Another interesting case was reported by Carvalho et al., a 71-year-old woman with hemorrhagic colitis due to SARS-CoV-2. The main findings, in this case, were the endoscopic evaluation to 40 cm from the anal verge, which revealed patchy areas of focal erythema without ulceration in the descending colon, sigmoid colon, and rectum; and the histological examination of the colon and rectal biopsies showing a slight expansion of the lamina propria with edema with normal cellularity and intact crypts71. Gulen et al. also reported a case of a 53-year-old man with GI bleeding with COVID-19 and the absence of other major risk factors for GI bleeding72.
Gadiparthi et al. reported three cases of GI bleeding in patients with severe SARS-CoV-2. In these cases, the source of bleeding was mainly attributed to GI ulceration. In this case series, two out of three patients had a higher Glasgow-Blatchford bleeding score of 7 and 11 on admission, which translates to high-risk GIB with a need for intervention >50% two patients died due to respiratory failure although GIB resolved73.
A 77-year-old man, without previous gastrointestinal disease, was diagnosed with SARS-CoV-2 infection, which progressed rapidly, requiring mechanical ventilation. The endoscopy revealed multiple round herpetic erosions and superficial ulcers (4-6 mm in size) covered with white exudates and blood clots located on the proximal esophagus. The tissue specimens from these lesions tested positive for SARS-CoV-2 RNA, with lymphocytic infiltration typical of viral esophagitis74.
Barrett et al. described six patients who tested positive for SARS-CoV-2 and had self-limited hematochezia or melena. The range of age in these cases was 66-77 years old, most of them (4/6) were men, most of them were African-Americans (4/6), just two of them were taking anticoagulation after COVID-19 diagnosis, most of them had no history of GI diseases (one had a history of internal hemorrhoids, large and small diverticular disease, and small hiatal hernia)75.
Barberis et al. presented a rare case of a 71-year-old woman hospitalized for COVID-19 pneumonia, which presented recurrent severe intestinal bleeding; the patient underwent a colonoscopy which showed severe inflammation associated with pseudopolyps, ulcerations, and diffuse bleeding; subsequently, she developed hemorrhagic shock and a subtotal colectomy with terminal ileostomy was performed; a swab for SARS-CoV-2 was made on the abdominal fluid in which the presence of the virus was demonstrated within the abdominal cavity76; other authors have performed studies on the peritoneal fluid in COVID-19-positive patients, but without finding the virus77.
Other atypical gastrointestinal manifestations of SARS-CoV-2 infection
Paralytic ileus and intestinal perforation are also rare manifestations of SARS-CoV-2 infection. Ibrahim et al. recently published two cases; both were 33-year-old men, first hospitalized for severe COVID-19 pneumonia requiring mechanical ventilation support. He developed significant bowel dilatation and perforation of the mid-transverse colon and needed laparotomy and colonic resection. Histopathology of the resected bowel specimen showed acute inflammation, necrosis, and hemorrhage, supporting a role for COVID-19-induced microthrombosis, leading to perforation. The second patient also had severe COVID-19 pneumonia, renal failure, and acute pancreatitis. His hospital course was complicated with paralytic ileus, but he improved with conservative management78.
Ischemic GI complications, although uncommon, also have been reported in the literature. A systematic literature review performed from January 2020 to June 2020 identified 22 studies and 31 patients with the mean age of 59 ± 12.7 (age range: 28-80) years old; most of them (74.2%) were male. The significant GI imaging findings include mesenteric, arterial, or venous thromboembolism followed by small bowel ischemia. Nine patients (29%) presented with arterial compromise due to superior mesenteric thromboembolism, resulting in bowel ischemia. Besides, 6 patients (19.3%) demonstrated occlusive thrombosis of the portal and superior mesenteric veins. A 64.5% required laparotomy and bowel resection, and the mortality rate was high since 40% of patients died79.
Conclusions
The GI clinical manifestations of SARS-CoV-2 are heterogeneous, with widely variable incidence, prevalence, and frequency among the different cohorts and case series reported worldwide. The binding of the viral spike glycoprotein to ACE2 and its receptor on the target cell plays a crucial role as an entry pathway to the cells that express it, which is, in the first place, the most popular mechanism that seems to explain more reliably the occurrence of GI symptoms in COVID-19 patients.
In addition, the systemic inflammatory response and the secondary prothrombotic state developed in the most severe cases of COVID-19, which seems to be mechanisms involved in the presentation of uncommon GI clinical manifestations, such as ulceration, bleeding, ischemia, or intestinal perforation.
Concerning hepatopancreatobiliary organs, it remains to be established whether the injury to these tissues is due to a direct viral effect, or is somewhat secondary to the systemic inflammatory process, endothelial vascular damage prothrombotic phenomena. Specifically, about the liver injury seen in COVID-19 patients, another hypothesis is the occurrence of idiosyncratic drug-induced liver injury possibly related to the polypharmacy with which these patients are usually treated.
Finally, in terms of primary prevention, maybe the most important to consider is that in almost half of COVID-19 patients with GI symptoms, viral RNA can be detected in their stool for determining the diagnosis. The possibility of fecal-oral transmission of SARS-CoV-2 must be considered.
It is already known that COVID-19 is a systemic disease with the involvement of many organs, including the digestive system. This article adds to the literature a comprehensive summarized review of all reported most common GI, liver, biliary, and pancreatic manifestations of COVID-19 and included rare or atypical ones that must not be forgotten.