Introduction
Among its objectives, the World Health Organization seeks to reduce mortality in children younger than five years by two thirds, which currently amounts to 8.8 million per year; 37 % occurs within the first month of life and 43 % is associated with infectious diseases, including pneumonia, which is responsible for 14 %,1 with 1.9 million deaths and 156 million episodes per year worldwide.2-4
Acute respiratory infections (ARI) are one of the main causes of morbidity and mortality, both in neonates and infants.5 Etiological agents include bacteria and viruses, such as human type 2 orthopneumovirus (hOPV), human orthorubulavirus (hORUV) and emerging viruses such as human metapneumovirus (hMPV).6 The World Health Organization estimates that approximately 25 % of hospitalizations in children are due to acute respiratory infections. hOPV7 is responsible for 80 % of this type of infections, followed by hMPV. These agents circulate in the pediatric population throughout the year; however, from December to February there is an increase in the incidence of respiratory pathologies of infectious origin in children younger than five years. Premature newborns and infants with asthma, congenital heart disease or bronchopulmonary dysplasia are more susceptible to these infections, the clinical presentation of which is usually more severe in comparison with that observed in children without comorbidities.8
In Mexico, acute lower respiratory tract infections are the leading cause of childhood morbidity due to infectious diseases, and are reported among the top 10 causes of death in children between one and four years of age.9,10 The diagnosis is established according to the clinical practice guidelines, which describe that these infections usually occur with cough, wheezing, rales, shortness of breath and, in some cases, respiratory failure that causes death.11
Despite the impact of these infections in the world, in Mexico there is no epidemiological surveillance network that provides information on the circulation patterns of these viruses, the incidence of viral coinfections, or their impact on clinical manifestations.12 Viral coinfections are associated with an increase in childhood morbidity and mortality and cause atypical clinical presentations, which makes correct diagnosis and etiologic agent identification difficult, hence the contradictory results regarding the incidence and severity of ARI when viral infections coexist.12-14
Due to the above, establishing the incidence of viral coinfections in pediatric patients with ARIs was proposed, for which molecular diagnosis by reverse transcription followed by endpoint polymerase chain reaction (RT-PCR), previously standardized in the laboratory, was implemented, which allowed the identification and genotyping of 200 viral isolates, as well as determining the incidence of viral infections or coinfections, virus circulation patterns and their geographical distribution. Cumulative incidences were as follows: hMPV, 0.215; hMPV-hORUV, 0.015 and hMPV-hOPV, 0.23; hOPV, 0.42; hMPV-hORUV-hOPV, 0.035; hORUV-hMPV, 0.005; hORUV, 0.01. With these data, generating a circulation pattern outline for the different viruses, as well as for viral co-infections caused by hOPV, hMPV and hORUV, was possible.
Method
After informed consent letter was obtained, between August 30, 2004 and February 13, 2014, 200 pharyngeal and nasopharyngeal exudate samples were obtained from pediatric patients (0 to 14 years of age) with acute respiratory infection. The project was reviewed and approved by the Research and Ethics Commission of the Faculty of Medicine, National Autonomous University of Mexico (project approval 089/2014, registry code FMED/CI/SPLR/134/2014).
From the viral isolates obtained from the clinical samples, identification and determination of the viral group and subgroup was carried out using oligonucleotides designed in the laboratory (Table 1). For amplification of the viral gene of interest, a thermocycler (iCycler, Bio-Rad, California, USA) was used with the parameters observed in Figure 1, where X represents the specific alignment temperature of each oligonucleotide. Phase 2 is repeated in 35 cycles.
Table 1 Oligonucleotide sequences for genotyping of viral isolates obtained from clinical samples of children with acute respiratory infection
| hOPV (human orthopneumovirus) |
| Ff5ATGAACAGTTTAACATTACCAAGTGA3 |
| Fr5CCACGATTTTTATTGGATGCTG3 |
| Subgroup A |
| Gf5CCCAACATACCTCACT3 |
| Gr5GAGGAGGTTGAGTGGAAG3 |
| Subgroup B |
| MLf5CATGCCAAACACAAGAATCAAC3 |
| MLr5ATTCATCATCTCTGCCAATCAC3 |
| hMPV (human metapneumovirus) |
| Nf5CAACAGCAGTGACACCCTC3 |
| Nr3ACTCATACCGTTTCGTAA5 |
| Lf5GCCATAGCCCAAACCATA3 |
| Lr5CCCTGTAACGACTAGACT3 |
| hORUV (type 2 human orthorubulavirus) |
| HNf5GACGCCTAAATATGGACCTCTC3 |
| HNr5CACGTCTGGTCTTCCATCTTT3 |
hOPV. F gene 1127-1320 region amplification (Ff = sense, Fr = antisense).
Subgroup A. G gene 275-524 region amplification. (Gf = sense, Gr = antisense). Subgroup B. ML gene 102-204 region amplification. (MLf = sense, MLr = antisense).
hMPV. N gene 148-747 region amplification (Nf = sense, Nr = antisense) and L gene 8920-9367 region amplification (Lf = sense, Lr = antisense).
hORUV. HN gene 525-826 region amplification (HNf = sense, HNr = antisense).

Figure 1 Viral genes amplification. Cycle 1. cDNA denaturation for each of the viruses. Cycle 2. Amplification of the selected region flanked by each pair of oligonucleotides. X represents the alignment temperature of each pair of oligonucleotides selected for each viral gene sequence. The last phase of this cycle corresponds to extension time; this cycle is repeated 35 times (35x). Cycle 3. Corresponds to an extra cycle of the oligonucleotide extension phase on its tempered strand. The last phase corresponds to amplification product maintenance. hOPV = human orthopneumovirus, hMPV = human metapneumovirus, hORUV = type 2 human orthorubulavirus.
The SPSS version 19 program was used for data statistical analysis. The samples were considered valid when all the information was available: clinical presentation data, patient history and diagnosis, state of origin of the sample and date of collection, identification of viral infection or coinfection. Samples in which any of these data was not available were considered as lost elements.
Results
Viral typing and viral coinfection cumulative incidence
Viral isolates were genotyped by endpoint RT-PCR; out of 200 clinical samples, 186 were positive for one or more of the three studied viruses and 14 were negative. In the studied pediatric population, hOPV and hMPV cumulative incidences were 0.42 and 0.215 (84 and 43 positive samples, respectively). Regarding cumulative incidence of viral coinfections, it is important to highlight that subgroup B hMPV-hOPV coinfection was the one with the highest incidence, with 24 positive cases. If coinfection by two or more viruses or viral subgroups (hOPV, hMPV and hORUV) is considered rather than the subtype or the amplified gene, hMPV-hOPV coinfection was the one with the highest incidence, with 47 positive cases (23 %), in contrast with hOPV-hORUV and hMPV-hORUV coinfections, which corresponded to two positive cases each (1 %), and hOPV-hORUV-hMPV, with five positive cases (2.5 %) (Table 2).
Table 2 Typing and cumulative incidence of viral infections and coinfections with hOPV, hMPV and hORUV in 200 clinical samples of children with acute respiratory infection
| hOPV | hMPV | hORUV | hOPV-hMPV | hOPV-hORUV | hMPV-hORUV | hOPV-hMPV-hORUV | Unidentified |
|---|---|---|---|---|---|---|---|
| 84* | 43* | 2* | 46* | 1* | 3* | 7* | 14* |
| 0.42** | 0.215** | 0.01** | 0.23** | 0.005** | 0.0015** | 0.0035** |
*Positive samples typed by reverse transcription followed by endpoint polymerase chain reaction (RT-PCR).
**Cumulative incidence of viral infections and coinfections in children with acute respiratory infection. hOPV = human orthopneumovirus, hMPV = human metapneumovirus, hORUV = type 2 human orthorubulavirus.
hOPV-positive clinical samples by amplification of the fragment corresponding to the F gene were subtyped in subgroups A and B. For subgroup A, a fragment of the gene that codes for glycoprotein G, and for subgroup B, a fragment of the gene that codes for proteins M and L. Our results showed that, of the 40 hOPV-positive samples, 48 % corresponded to subgroup B, while 27 % corresponded to subgroup A and 3 % to co-infections by both subgroups. In 22 % of hOPV-positive samples, identifying the viral subgroup was not possible, which could be due to probable mutations in subgroup A or B amplified regions (Fig. 2).

Figure 2 Typing of human orthopneumovirus viral isolates in subgroups A and B; it was carried out by RT-PCR (reverse transcription followed by polymerase chain reaction) at endpoint, and amplification products were observed on Gelred®-stained agarose gel. The amplification products were processed and analyzed with the Quantity One Bio-Rad program of ChemiDoc Hood II.
Circulation patterns and their distribution in time
In Mexico, there are no respiratory viruses circulation patterns, incidence of coinfections and geographic distribution available. In addition to the implications on the severity of clinical presentation, it is important to define the incidence of circulation patterns based on seasonality. For this reason, date of collection, chronology of circulation and place of origin of all 136 samples that had this information were determined. The cumulative incidence results showed that the largest number of positive cases of circulating or co-circulating viruses occurred from January to March (Fig. 3), which is consistent with the seasonality of these viruses; however, identifying viruses circulating or co-circulating between April and September, a period that corresponds to the rainy season in Mexico, was possible, although cumulative incidence was actually lower, which is consistent with reports from other countries.
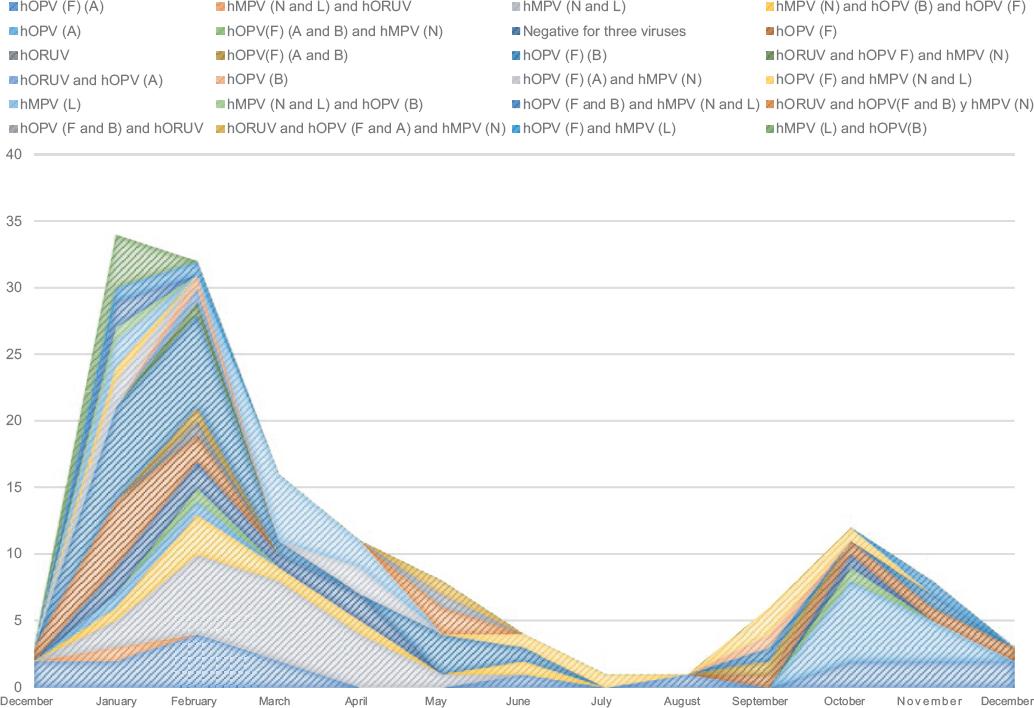
Figure 3 Cumulative incidence and its relationship with weather seasonality. The cumulative incidence of the cases typed as positive for each viral infection and coinfection was determined and was related to the date of sample collection in order to establish circulation patterns and their weather seasonality. hOPV =human orthopneumovirus, hMPV = human metapneumovirus, hORUV = type 2 human orthorubulavirus.
Viral coinfection geographic maps according to cumulative incidence
The determination of cumulative incidence allowed to draw geographic maps related to the circulation patterns of the study population and to determine the flow of respiratory viruses in different states of Mexico (Fig. 4). Similarly, the geographic circulation pattern was established in hOPV-positive viral isolates, subtyped in A or B (Fig. 5).
Discussion
In this project, 200 clinical samples from pediatric patients with acute respiratory infection were analyzed. Our research is a prospective, observational, cross-sectional pilot study, which ran from August 30, 2004 to February 13, 2014. Each sample was obtained with written authorization and after information was provided to the parents or legal guardians. Cumulative incidence, which is a measure of the dynamic index associated with the appearance of new cases over time and in the selected population, was determined. hOPV, hMPV and hORUV infection and coinfection cumulative incidence was defined based on 200 viral isolates; with these data, circulation patterns and geographic distribution with regard to seasonality were drawn. Cumulative incidence data for infections associated with a single virus showed that the most frequent was hOPV, with an incidence of 0.42, 48 % corresponded to subtype B, and 38 %, to A. This result is consistent with reports of subgroup B prevalence in different Chinese populations during the 2009-2010, 2012-2013 and 2006-2014 outbreaks,15,16 out of which BA9 and GB5 had the highest circulation.16,17
Drawing the geographical distribution was also possible: Mexico City, Chihuahua, San Luis Potosí, Jalisco and Guerrero showed the highest number of positive cases for subgroup B hOPV. Subgroups A and B cumulative incidence and seasonality in Chihuahua and San Luis Potosí may be related to the winter period and cold fronts; in Jalisco and Guerrero, the increase in the number of cases is associated with the rainy season, which is produced by the humid currents of the trade winds from both hemispheres in the so-called intertropical convergence zone. This seasonality pattern was repeated in the other two viruses; however, hMPV geographical distribution was broader: samples were identified in Mexico City, Chihuahua, San Luis Potosí, Querétaro, Jalisco, Michoacán and Guerrero. The circulation patterns of these viruses during the winter season and the rainy season may be associated with the effect of weather on innate immune defense mechanisms in the respiratory system, such as decreased mucociliary movement and impaired ability of the upper respiratory tract to adequately heat cold air, which favors this type of infection. During the rainy season, environmental humidity contributes for saliva droplets to remain suspended for a longer time, which facilitates their dissemination.
Our data are consistent with the report on the predominance of type A and C rhinovirus during the rainy season and early winter.18 Furthermore, a retrospective study conducted between 1985 and 1987 in Nigerian children with respiratory infection showed that peak incidence of reported cases occurred in September, in the rainy season, which is followed by the trade wind season in West Africa, characterized by cold, dryness with dust and low humidity from November to March (Harmattan), which are environmental conditions that damage the airways and favor exposure to infections.19-21 Our results also agree with the finding that human respiratory coronaviruses (HCoV) are responsible for ARI during Harmattan and the rainy season.21-23
Our results showed that the highest cumulative incidence of viral coinfections occurred in San Luis Potosí (three cases) and Mexico City (two cases); in addition, San Luis Potosí was the only state where hORUV-positive samples were isolated and identified as the only causative agent and in coinfection with hOPV and hMPV. Our investigation allowed to detect viral coinfections also in Chihuahua, Nuevo León, Jalisco, Michoacán and Tabasco. Co-circulation of these respiratory viruses, their seasonal coincidence, and immune system conditions of infected patients favor multiple infections. In another study of our working group, it was concluded that viral coinfections modify respiratory clinical presentation, causing atypical manifestations with the appearance of signs and symptoms such as fever, abdominal pain, laryngeal stridor and hyaline rhinorrhea, which makes diagnosis and correct identification of the etiological agent difficult.12
Finally, our results suggest that pediatric age is a predisposing factor for viral infection, probably because lung maturation is achieved after eight years of age.12 In Mexico, there are no epidemiological data available, probably due to lack of information in patient records (medical-legal documents), which compile patient ailments chronological order and contribute to efficient diagnosis. Without this information, diagnosis becomes random and the factors that determine a disease or the circumstances that facilitate an accurate diagnosis are lost sight of;12 in addition, the opportunity to determine viruses circulation patterns is lost.
Recent investigations address viral coinfections and new respiratory symptoms, as well as new health strategies to confront infections and decrease mortality rates in children.12 Despite bias due to the number of analyzed samples, the reported incidence of each virus and associated coinfections provided the necessary data to generate an outline of circulation patterns and geographic distribution in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)




