INTRODUCTION
Biosensors are analytical devices consisting of a biological sensing element connected to a physicochemical transducer. These elements generate digital electronic signals proportional to the interaction of biomolecules, thus allowing label-free detection of analytes. Due to the specificity of biomolecular interactions, a biosensor can be used to analyze complex substrates including samples such as blood, serum, plasma, urine, milk, and culture media often with minimum preparative treatment.
Different types of biosensors have been developed including amperometric, potentiometric, piezoelectric, calorimetric, and optical biosensors. These have been applied mostly in food and water analysis and pharmaceutical processes due to their demands for sensitivity, specificity, speed, and accuracy of analyte measurements. Among them, optical biosensors correlating changes in concentration, mass, or number of molecules to direct changes in characteristics of light have preferentially evolved in these past years. Optical detection using surface plasmon resonance (SPR) biosensors has been increasingly popular due to its speed of detection, high specificity, high sensitivity, and possibility of real-time analysis1.
SPR-BASED BIOSENSORS
SPR appeared as a revolutionary technology > 20 years ago, when their first commercial instrumentation, the Biacore, was launched on the market by the Swedish company Pharmacia Biosensors AB2. Since then, many researchers adopted the SPR in various analytical fields such as the food industry, pharmaceutical approaches, doping analysis, proteomics and genomics, and environmental monitoring. Beyond that, clinical and biomedical analyses have also been explored with promising results1,3.
In a typical SPR experiment, one interacting molecule, referred as the ligand, is bound to the biosensor surface while the other, called analyte, is delivered to the surface in a continuous flow through a complex microfluidic system. The biosensor consists of a glass piece coated with a layer of gold, creating a platform for a range of specialized surfaces designed to optimize the binding of a variety of molecules including inorganic compounds, proteins, lipids, nucleic acids, carbohydrates, and even whole cells. The gold layer in the biosensor allows the generation of the SPR events that essentially detect changes in mass in the aqueous layer close to the biosensor surface by measuring changes in the refractive index of an incident polarized light beam (Fig. 1). The data obtained provide real-time quantitative information about binding specificity, active concentration of a molecule in a sample, kinetics, and affinity of binding models, among others. The complex theory behind SPR has been extensively detailed before4; thus, it will not be further discussed here.
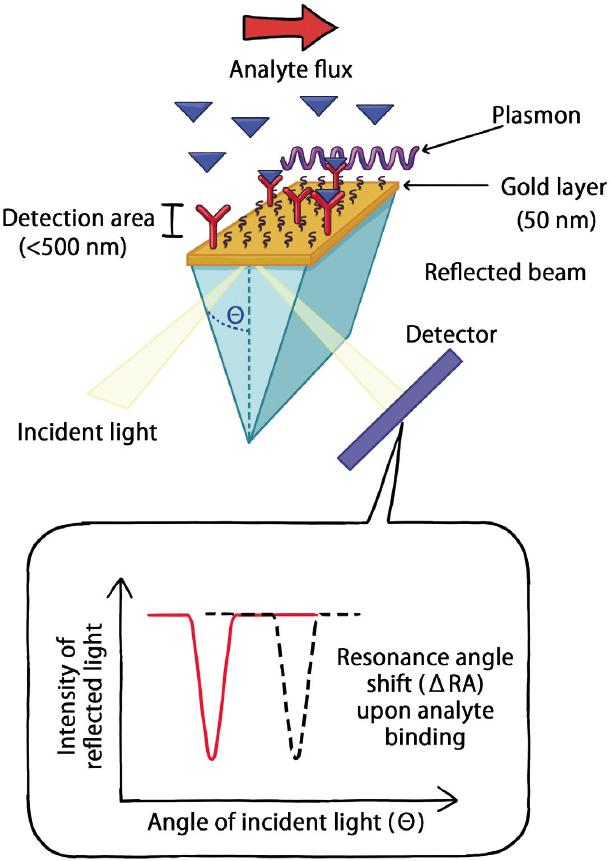
Figure 1 Fundamentals of surface plasmon resonance (SPR) sensor. SPR is a physical phenomenon that occurs when polarized light strikes an electrically conducting surface (made of gold in most cases) at the interface between two media, a high-refractive-index glass or crystal prism and a low-refractive-index buffer solution. This generates intermittent waves of electrons (charge density waves), also known as plasmons, that reduce the intensity of reflected light at a specific angle known as the resonance angle (RA), in proportion to the mass deposited on the conducting surface. These arrays, referred to as SPR sensors, are used to detect a refractive index change of the polarized light beam within a detection area (< 500 nm) as a change of the RA, when the interactions between ligand molecules (attached to the sensor) and the analytes (flowing in solution) occur.
SPR APPROACHES FOR BIOMEDICAL AND CLINICAL APPLICATIONS
SPR analyses possess a great potential for clinical and biomedical applications due to their inherent advantages when dealing with biomolecules. Optical SPR-based studies can be carried out with colored or even opaque samples, and there is no need to label molecules of interest with fluorescent or radioactive tags, thus avoiding the possibility that labels may compromise molecular activity or association features. In this way, analytes derived from clinical samples, human cells, or tissues can be studied in their native state to provide results that reflect more accurately their activity in vivo3,5.
In recent years, there has been a significant increase in research articles reporting the development of SPR techniques for biomolecular analysis; accordingly, several studies report the detection of different peptides, proteins (including antibodies), hormones, microRNA, and DNA. Consequently, multiple SPR-related reports emerged showing the analysis of clinical samples from patients suffering specific diseases, which is usually the ultimate objective of biomedical researchers looking for new diagnostic tools.
SPR approaches have been applied to clinical tests for monitoring antibodies, proteins, enzymes, drugs, small molecules, peptides, nucleic acids, and even bacterial components or viruses. Amazingly, while the molecules detected were usually at concentrations of nanomolar scales or higher, several studies reported picomolar detection or even lower concentrations. This technique has been applied in a number of biofluids including plasma, serum, whole blood, urine, stools, saliva, cervicovaginal secretion, endometrial tissue, synovial, cerebrospinal, amniotic, and ascites fluids1,3,6-9. The analytes detected by SPR in these samples represent an everyday growing list of molecules with great potential for clinical-related applications, as exemplified in the next sections and detailed in Table 1.
Table 1 Clinically relevant analytes determined by SPR analyses.
| Disease or condition | Analytes measured by SPR | Sample |
|---|---|---|
| Antibodies | ||
| Antiphospholipid syndrome | Anti-cardiolipin, anti-β2 glycoprotein I | Serum |
| Chagas disease | Anti-Trypanosoma cruzi | Serum |
| Dengue | Anti-dengue virus IgM antibodies | Serum |
| Diabetes | Insulin and proinsulin autoantibodies | Serum |
| EpsteinBarr virus infection | Anti-VCA, anti-EBNA, and anti-EA, all viral antigens | Serum |
| Hepatitis A and B | Anti-hepatitis A or B antigens | Serum |
| Leukemia | Anti-asparaginase, κ and λ immunoglobulin light chain | Serum |
| Lyme disease | Anti-Borrelia spp. | Serum |
| Neonatal thrombocytopenia | Anti-HPA-1a alloantibodies | Serum |
| Peanut allergies | IgE | Serum |
| Red cell aplasia | Anti-erythropoiesis-stimulating agent IgG4 antibodies | Serum |
| Rheumatoid arthritis | Anti-glucose 6-phosphate isomerase, anti-citrullinated protein antibodies | Synovial fluid and serum |
| Syphilis | Anti-Treponema pallidum | Serum |
| Systemic lupus erythematosus | Anti-dsDNA autoantibodies | Serum |
| Typhoid fever | Anti-Salmonella enterica serotype typhi | Serum |
| Other proteins | ||
| Alzheimers disease | Tau protein | Serum |
| Bladder cancer | Podoplanin | Serum, urine |
| Cancer | Galectin-1 | Serum |
| Cardiopulmonary bypass surgery | IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ | Serum |
| Diabetes | Insulin | Serum |
| Head and neck squamous cell carcinoma | p53, p38αMAP kinase | Serum |
| Heart disease | C-reactive protein, BNP, MG, and cTnI | Serum |
| Hematopoiesis | CXCL12 | Urine |
| Hepatocellular carcinoma | Lipocalin-2 | Serum |
| Lung cancer | Rac | Serum |
| Osteoarthritis | TNF-α, MMP-3 | Synovial fluid |
| Pancreatic cancer | ALCAM | Serum |
| Preeclampsia | Albumin | Urine |
| Prostate cancer | PSA | Serum |
| Rheumatoid arthritis | Cathepsin G | Endometrial tissue |
| Tuberculosis | CFP-10 | Urine |
| Other molecules | ||
| Alzheimers disease | Amyloid-β | Cerebrospinal fluid |
| Cancer | Exosomes | Ascites fluid |
| Cancer treatment | Methotrexate | Serum |
| Celiac disease | Gluten peptides | Urine |
| Enterohemorrhagic Escherichia coli infection | PCR products of Escherichia coli O157:H7 | Stool |
| Fertility monitoring | Estriol 3-sulfate 16α-glucuronide | Serum |
| Pancreatic cancer | miR-21 and miR-10b | Plasma |
| Sport doping | Human growth hormone | Serum |
| β-thalassemia | Genomic DNA | Blood |
Molecules used in clinical approaches, detected in human samples by SPR methods. The table was adapted from Mariani and Minunni3.PSA: prostate-specific antigen, SPR: surface plasmon resonance, IL: interleukin, Ig: immunoglobulin, TNF: tumor necrosis factor, IFN: interferon, BNP: brain natriuretic peptide, MG: myoglobin, cTnI: cardiac troponin I, PCR: polymerase chain reaction.
Protein Biomarkers
A protein biomarker is used in clinical diagnosis to monitor the status of several associated diseases, displaying a potential utility for targeted therapy and to evaluate the therapeutic responses. The implementation of proteomic approaches together with the growing number of clinical biomarkers of proteic nature is accelerating the development of reliable methods for their detection in complex clinical samples such as SPR techniques. Examples of these clinically relevant protein biomarkers include molecules related with specific diseases1,3,6-9, as explained below.
Cancer Biomarkers
An important aspect of all types of cancer management should include the monitoring of protein biomarkers related with these diseases, preferentially in easily collected physiological fluids over surgically obtained biopsies; examples of these proteins are the prostate-specific antigen, podoplanin, lipocalin 2, galectin 1, and CD166 (ALCAM), among others. Besides providing practical information to guide clinicians decisions, cancer biomarkers are also linked to specific alterations in molecular pathways controlling cancer pathogenesis, thus evidencing their potential for deciding about therapeutic strategies8,9.
Cardiac Disease Biomarkers
Cardiac biomarkers such as C-reactive protein, brain natriuretic peptide, myoglobin, and cardiac troponin I are proteins released into the bloodstream on damage of the heart or associated tissues. These biomarkers help to diagnose acute coronary syndrome and cardiac tissue ischemia, both conditions associated with insufficient blood flow. Cardiac biomarker proteins can also be used to estimate an individuals risk to develop these conditions or to help monitor and deal with a patient suspected to suffer from these conditions3.
Antibodies
Although SPR has been extensively applied for the characterization of monoclonal antibodies, both used in therapeutics or biomedical research, its utility in diagnosis by measuring antibody levels in circulation is a neglected application. However, there are reports of SPR playing a crucial role mainly in the analysis of autoantibodies in human serum for real-time monitoring of autoimmune disorders including for example anti-dsDNA autoantibodies in lupus erythematosus and anti-citrullinated protein antibodies in rheumatoid arthritis3.
Another field where antibody detection by SPR sensors could be important is the biopharmaceutical industry. Here, the immunogenicity of biological drugs is always a concern since they are primarily proteins (monoclonal antibodies, cytokines, growth factors, hormones, enzymes, or fusion proteins) and peptides that, when administered, often could induce a drug-specific immune response characterized by the presence of anti-drug antibodies (ADAs)10.
ADAs have been detected in clinical studies resulting in significant alterations in toxicology, pharmacokinetics, and efficacy of biotechnology-derived pharmaceuticals. Consequently, regulatory authorities are now requesting immunogenicity studies that include the detection of ADAs, before the approval of any biological drug10. On this scenario, the use of SPR devices for ADAs detection represents a valuable approach due to the intrinsic and previously discussed advantages of this technology.
Hormones Measurement
The analysis of both lipidic and small peptide hormones is an important area in clinical diagnostics and, currently, in anti-doping regulations. Endocrine diseases, where these mediators are typically involved, usually require measuring hormone levels by direct or indirect methods. Accordingly, SPR has demonstrated the ability to provide sensitive solutions in this clinical area by determining, for example, estriol metabolites and human growth hormone, among other hormones3,7.
Nucleic Acid Analyses
SPR assays related with nucleic acids could be subdivided into two main classes: analyses for the detection of chromosome abnormalities including point mutations and single-nucleotide polymorphisms such as those occurring in β-thalassemia, and assays for the quantification of genetic material as biomarkers such as microRNAs3.
Pathogens Detection
Beyond the identification of pathogens through specific DNA sequences targeting, other approaches based on the detection of a number of cell pathogen components have been reported. Particularly, several bacterial and parasite components, including Salmonella spp. and Schistosoma mansoni antigens, or even whole of H1N1 influenza virus particles, have been subject to SPR analyses6.
Whole-cell SPR Analyses
The detection depth of conventional SPR sensors reaches no > 500 nm beyond the gold layer; therefore, they are useful for the detection of changes near the plasma membrane of live cells with a high sensitivity. In this way, different SPR techniques have been developed to perform real-time evaluation of exogenous stimuli-induced responses in living cells. These approaches might reflect the reorganization of proteins as a consequence of intracellular signal transduction processes.
Examples of whole-cell analyses by SPR devices include the detection of real-time adhesion and morphological changes in living cells due to the action of toxins or enzymes5. A similar approach is the SPR measurement of the apoptosis rate of cancer cells in response to different oncological drugs, which can be applied in a clinical setting to evaluate the individual therapeutic potential of different treatments including pharmacodynamic interactions5. In addition, SPR sensors could reveal real-time alterations in intracellular signaling pathways of abnormal cells such as cancer cells, making them able to detect malignant tumors5.
Finally, the recently developed SPR imaging systems and the long-range SPR sensors for living cells may allow the visualization and deep through analyses of single cell reactions, potentially expanding the application of SPR whole-cell sensing for clinical diagnosis in the near future.
CONCLUSIONS
To be implemented as a routine analytical method, SPR sensing will need to replace existing technologies. In this way, several clinical results obtained with SPR devices have been compared to those from ELISA assays, chemiluminescence approaches, polymerase chain reaction, or liquid chromatographymass spectrometry, generally demonstrating similar quality8.
Since ELISA employs the same experimental design as SPR sensing, regarding the detection of analytes using direct, indirect, or sandwich assays, it is frequently the method against which SPR systems are compared. The studies show that ELISA and SPR deliver the same dynamic range of sensitivity, although the advantages of SPR reside in the label-free and rapid detection of analytes plus the possibility of reusing the same sensor many times, which could decrease the cost of serial measurements. The SPR method is also preferred for the detection of molecules with low binding affinities, which can otherwise be washed away in ELISA assays.
Although the detection of antibody and general protein markers dominates the current applications of SPR approaches that undoubtedly support medical practice, the increasingly recognized usefulness of genetic testing, microRNA detection, and special molecules analyses in clinical practice could represent an interesting niche for SPR applications.
In the upcoming years, novel integrated, simpler, and even portable SPR systems, capable of detecting biomolecules with high sensitivity ideally in undiluted biofluids, will be surely available to clinicians for daily use, allowing for a more accurate decision-making in the management of a given patient. For now, the promising results of this technology for the analysis of human samples in detecting biomarkers and helping with diagnostic or prognostic approaches in several diseases position SPR techniques as a powerful tool in clinical management with a great potential in precision medicine.











 nueva página del texto (beta)
nueva página del texto (beta)


