INTRODUCTION
HIV infection continues to be a serious health hazard, particularly in developing countries. In Mexico, in 2016, there were 200,000-240,000 people living with HIV, 11,000-14,000 new HIV infections, and 3300-5300 AIDS-related deaths1. A high percentage of HIV/AIDS patients requires intensive care2, although their mortality rates have improved over the past decades2-6. In addition, the causes of admission into intensive care units (ICUs) have changed since the introduction of highly active antiretroviral therapy (ARVT)7,8. In high-income countries, opportunistic respiratory infections such as Pneumocystis jiroveci pneumonia (PJP) no longer represent the primary reason for ICU admission in patients with HIV infection. However, in developing countries, a high percentage of individuals are unaware that they are carriers of HIV and have not received ARVT or prophylaxis9,10. These patients represent the greatest number of admissions to respiratory ICUs (RICUs) due to severe acute hypoxemic respiratory failure (SAHRF) secondary to PJP. Therefore, tools that allow the prediction of mortality are needed to assist in clinical decision-making, optimizing the limited health resources available, and communicating realistic expectations regarding prognosis to patients and their families. The objectives of this study were to describe the clinical characteristics and outcomes of a prospective cohort of patients with SAHRF, HIV infection, and PJP requiring RICU admission and determine the variables associated with mortality to develop a predictive scoring system for inhospital mortality.
METHODS
Design and patients
We conducted an observational study of a prospective cohort admitted to the RICU at the National Institute of Respiratory Diseases in Mexico City, Mexico. This is a 241-bed third-level teaching and research reference center for patients with HIV infection and respiratory diseases with a 15-bed RICU. All patients who were admitted to the RICU between January 2013 and January 2018 with a diagnosis of HIV infection (confirmed by serological tests and viral load) and SAHRF and PJP (confirmed by aspirate, BAL, and/or biopsy) that required invasive mechanical ventilation were included in the analysis. We excluded patients with evidence of coinfection due to bacteria or fungi on admission (documented by microbiological study of aspirate, BAL, and/or biopsy). If a patient was admitted to the RICU on more than one occasion, only the data from the first admission were considered. Due to the study design, the ethics committee did not request informed consent from the patients or their kin. The study was registered and approved by the institutions biomedical and ethics research committee and adhered to the TRIPOD guidelines on prediction model development and validation studies11.
Variables and definitions
The patients clinical data were obtained from the electronic database and clinical records. The following data were collected by two researchers: demographic information; risk factors for HIV infection; previous diagnosis of HIV infection; treatment with ARV drugs and anti-infective prophylaxis; time between admission to the emergency room and transfer to the RICU; acute kidney injury defined according to the classification of Mehta et al.12; hemodynamic and respiratory parameters on RICU admission; markers of infection; anthropometric data comprising weight, height, and body mass index (BMI: weight/height2); and the sequential organ failure assessment (SOFA) score obtained in the first 24 h of admission to RICU13. PJP was diagnosed by tracheal aspiration or bronchoalveolar lavage, which was performed when analysis of the tracheal sample did not allow for identification of the microorganism. Samples were examined after applying the Gomori methenamine silver and Giemsa stains; moreover, all samples were evaluated for viruses, bacteria, and fungi. ARDS was defined according to the recent Berlin definition14. The primary endpoint was inhospital mortality, defined as death before discharge, regardless of the length of stay.
Statistical analysis
Data analysis was performed using the statistical software SPSS version 21.0 (IBM Statistics, Armonk, New York). Data were summarized as counts or percentages for categorical variables and the median with interquartile range (25th-75th percentiles) for continuous variables. Group comparisons were made by applying Chi-squared test for categorical variables and the WilcoxonMannWhitney test for continuous variables. All tests of significance were two-tailed, and p < 0.05 was considered to be statistically significant.
Missing data and multiple imputation
The amount of missing data was determined, and the candidate variables were excluded if more than 25% of the total data for that variable were unavailable. Before the imputations, a test was performed to determine the randomness of the lost data to avoid bias. The imputation of the missing candidate variables was performed using the linear regression method for continuous variables and logistic regression for the nominal variables. After five imputations, the average values of the observed data were compared with those of the imputed data; as there were no significant differences, the imputed database was used for the statistical analysis15,16.
Logistic regression modeling
The binary logistic regression analysis included the candidate variables selected as independent covariates and inhospital mortality as the dependent variable. The candidate variables were first tested for inclusion in univariate models; those that were significant based on p < 0.20 were entered into multivariable models, and a series of progressive backward eliminations was used to identify the variables for the final model17. To ensure that the scoring system was suitable for use at the bedside, the number of variables was reduced to ensure simplicity and clinical relevance. The results are presented as adjusted odds ratios with 95% confidence intervals (CIs) and regression coefficients (β values).
Conversion to the clinical prediction rule
The logistic regression model was converted into a clinical prediction score by transforming the estimates of the individual parameters of the model into weights (risk units) according to the method used in the Framingham Heart Study18. A risk unit was represented by the regression coefficient associated with a BMI decrease of 1.5 units (kg/m2). The points were assigned to all other variables, where their regression coefficients were divided by the BMI coefficient. The individual risk of the patient was estimated by calculating the sum of the scores from the risk factors. A new logistic regression analysis was performed using the predictive score as the only independent variable and the inhospital mortality as the dependent variable. Low-, intermediate-, and high-risk strata were defined based on the estimated probability of mortality. We present the predicted inhospital mortality risks for the different scores together with the sensitivity, specificity, and inhospital mortality observed in the sample of complete derivation. The score was evaluated using the models diagnosis19. The overall predictive performance of the score was evaluated using the NagelkerkeR2 for binary exposure20, which refers to the proportion of variation explained by the model. Discrimination, which refers to the ability of the scoring system to distinguish patients who die from those who are alive, was evaluated using the area below the receiver operating characteristic (ROC) curve21. Finally, the calibration was evaluated as recommended in the literature through calibration and intersection graphs of α and slope β of the regression of the binary result (inhospital mortality) in the predicted values, complemented by the HosmerLemeshow goodness-of-fit statistic22.
Internal validation
The model was validated internally using bootstrapping23. One thousand random samples with replacement were obtained from the original cohort. The model was developed again in each bootstrap sample to yield new parameter values for each bootstrap model. Finally, the mean, standard error, and CIs of the coefficients were obtained.
Sample size and power
A power analysis indicated that a logistic regression model with 202 patients and 54 events would have sufficient power to include four predictive variables24.
RESULTS
Demographic and clinical characteristics of patients
During the study period, 1415 admissions to the RICU were registered, of which 1235 were non-HIV-related and were excluded. The diagnosis of HIV infection was confirmed in 219 patients, 17 of whom were excluded because they did not meet the inclusion criteria. Therefore, the final analyzed sample comprised 202 patients (Fig. 1).

Figure 1 Flow diagram of HIV/AIDS patients enrolled in the study at RICU from January 2013 to January 2018.
The general characteristics of surviving and non-surviving patients are shown in Table 1. The age and sex distributions were similar in the two groups, while the BMIs were different; non-survivors included a higher percentage of underweight patients (BMI <18.5 kg/m2) (37% vs. 12.8%, p < 0.0001), while survivors had a higher percentage of overweight patients (BMI >25 kg/m2) (27% vs. 9.2%, p < 0.001). Practically, all the patients were unaware of their HIV infection status on admission to the RICU; fewer than 4% were receiving ARV treatment and antimicrobial prophylaxis on admission. Patients in both the groups were severely immunosuppressed, with CD4+ cell counts of 23 (39) (median, interquartile range) and 19 (59) (median, interquartile range) among survivors and non-survivors, respectively. The viral loads were not different between the two groups (5.36 [1.32] vs. 5.59 [1.13] log10 copies/ml). The main reason for admission in both the groups was severe hypoxemic respiratory failure (SHRF) secondary to PJP, requiring invasive ventilation. The number of days of mechanical ventilation, RICU stay, and inhospital stay was not different between the two groups. RICU and inhospital mortality rates were 25.2% and 26.7%, respectively.
Table 1 Demographic and clinical characteristics of patients
| Characteristics | Survivors (n = 148) | Non-survivors (n = 54) | p value |
|---|---|---|---|
| Age (years) | 33 (27; 41) | 34 (29; 41) | NS |
| Male (%) | 89 | 92 | NS |
| BMI (%) | 23.7 (20.1; 26.3) | 20.2 (17; 23.4) | 0.001 |
| Normal (18.5-24.9) | 54 | 51.8 | NS |
| Underweight (<18.5) | 12.8 | 37 | 0.0001 |
| Overweight (25-29.9) | 27 | 9.2 | 0.007 |
| Obesity 1 (30-34.9) | 4.7 | 1.8 | NS |
| Obesity 2 (35-39.9) | 1.3 | 0 | NS |
| Education | |||
| Primary (%) | 36.6 | 31.3 | NS |
| Secondary school (%) | 28.6 | 31.3 | NS |
| High school (%) | 14 | 9.8 | NS |
| University (%) | 20.6 | 27.4 | NS |
| Sexual preference | |||
| Heterosexual (%) | 39.3 | 49 | NS |
| Homosexual (%) | 56 | 41 | NS |
| Bisexual (%) | 2.6 | 3.9 | NS |
| Transgender (%) | 0.6 | 5.8 | NS |
| Condom use (%) | 17.3 | 7.8 | NS |
| Days since HIV infection diagnosis | 2.0 (0; 30) | 7 (0; 159) | 0.005 |
| Use of ARV before admission to RICU (%) | 16 | 29 | NS |
| Use of ARV at the time of admission to RICU (%) | 3.3 | 3.9 | NS |
| Antimicrobial prophylaxis* (%) | 3.3 | 3.9 | NS |
| CD4+ count (cells µl-1) | 23 (11; 50) | 19 (8; 65) | NS |
| HIV viral load, log10 (copies ml-1) | 5.36 (4.63; 5.95) | 5.59 (4.87; 5.93) | NS |
| SOFA score | 4 (3; 8) | 8 (7; 11) | 0.001 |
| Albumin | 2.1 (1.87; 2.5) | 1.7 (1.17; 2.13) | 0.001 |
| DHL | 409 (322; 518) | 427 (276; 575) | NS |
| Norepinephrine days | 5 (3; 9) | 10 (6; 16) | 0.001 |
| Time admission-RICU (days) | 1 (0; 2) | 3 (1; 8) | 0.001 |
| Length of ventilation (days) | 8 (5; 13) | 11 (8; 18) | NS |
| RICU stay (days) | 9 (6; 14) | 13 (8; 21) | NS |
| Hospital stay (days) | 20 (14; 29) | 19 (10; 30) | NS |
| RICU mortality | | 25.3 | |
| Hospital mortality | | 26.7 |
*Trimethoprim-Sulfamethoxazole. Median (25th and 75th percentiles), NS: Non-significant (p>0.05). BMI: Body mass index (weight in kilograms divided by the square of height in meters), RICU: Respiratory intensive care units, SOFA: Sequential organ failure assessment, HIV: Human immunodeficiency virus, ARV: Antiretroviral.
Model development and performance
A binomial logistic regression was performed to ascertain the effects of underweight, albumin, norepinephrine administration days, and time to RICU admission on the rate of inhospital mortality (Table 2). The linearity of the continuous variables with respect to the logit of the dependent variable was assessed using the Box-Tidwell procedure25. A Bonferroni correction was applied using all terms in the model, resulting in statistical significance being accepted when p<0.00625. Based on this assessment, all continuous independent variables were found to be linearly related to the logit of the dependent variable. The logistic regression model was statistically significant, χ2 (4) = 54.8, p < 0.0005. The model explained 33.0% (Nagelkerke R2) of the variance in mortality and correctly classified 78.0% of cases. The area under the ROC curve was 0.809 (95% CI, 0.721-0.890), which is an excellent level of discrimination according to Hosmer et al.26.
Table 2 Multivariate model of predictors of inhospital mortality in patients with human immunodeficiency virus infection and Pneumocystis jiroveci pneumonia
| Characteristic | β | SE | OR (95% CI) | Bootstrap bias (95% CI) |
|---|---|---|---|---|
| Underweight (BMI <18.5) | 1.197 | 0.431 | 3.31 (1.42-7.70) | 0.019 (0.299-2.208) |
| Albumin <1.5 g/dl | 1.066 | 0.447 | 2.90 (1.21-6.97) | 0.061 (0.109-2.146) |
| Norepinephrine (days) | 0.108 | 0.029 | 1.11 (1.05-1.18) | 0.004 (0.067-0.158) |
| Time to admission to RICU (days) | 0.194 | 0.056 | 1.21 (1.08-1.35) | 0.014 (.088-0.370) |
| Constant | −2.983 | 0.406 | −0.102 (−3.96-2.34) |
BMI: Body mass index, RICU: Respiratory intensive care units, SE: Standard error, CI: Confidence interval, OR: Odds ratio.
Mortality score performance
The score for each of the predictive variables is shown in Table 3. The adjusted logistic regression model for the association between the mortality risk in hospital (p) and the score is given as follows:
Table 3 Scores assigned to the different levels of the mortality predictor variables
| Predictor | Points |
|---|---|
| BMI (g/m2) | |
| 18.5-25 | 0 |
| 18.5-17.5 | 2 |
| 17.4-16.5 | 5 |
| <16.5 | 7 |
| Albumin (g/dl) | |
| 4.5-3.5 | 0 |
| 3.4-2.5 | 1 |
| 2.4-1.5 | 3 |
| 1.4-0.5 | 5 |
| Time to RICU admission (days) | |
| 0-3 | 0 |
| 4-7 | 1 |
| 8-11 | 2 |
| 12-15 | 3 |
| >16 | 4 |
| Days with norepinephrine | |
| 1-5 | 1 |
| 6-10 | 2 |
| 11-15 | 4 |
| 16-20 | 6 |
| >20 | 8 |
BMI: Body mass index, RICU: Respiratory intensive care units.
Logit(p) = −5.436+0.481(score points)
With a predicted mortality risk estimated by the following equation
Predicted inhospital mortality
= Exp (−5.436+0.481 [score points]/1+exp (−5.436+0.481 [score points])
The overall performance of the score (NagelkerkeR2) was 0.467; the discrimination (area under the ROC curve) was 0.869 (95% CI: 0.821-0.917) (Fig. 2) and calibration intercept (α) and slope (β) were 0.03 and 0.99, respectively. The sensitivity was 47.2%, the specificity was 84.6%, the positive predictive value was 89.2%, and the negative predictive value was 82.6%.

Figure 2 Receiving operating characteristic curve for the derived score. Area under the curve = 0.869 (95% confidence interval: 0.821-0.917).
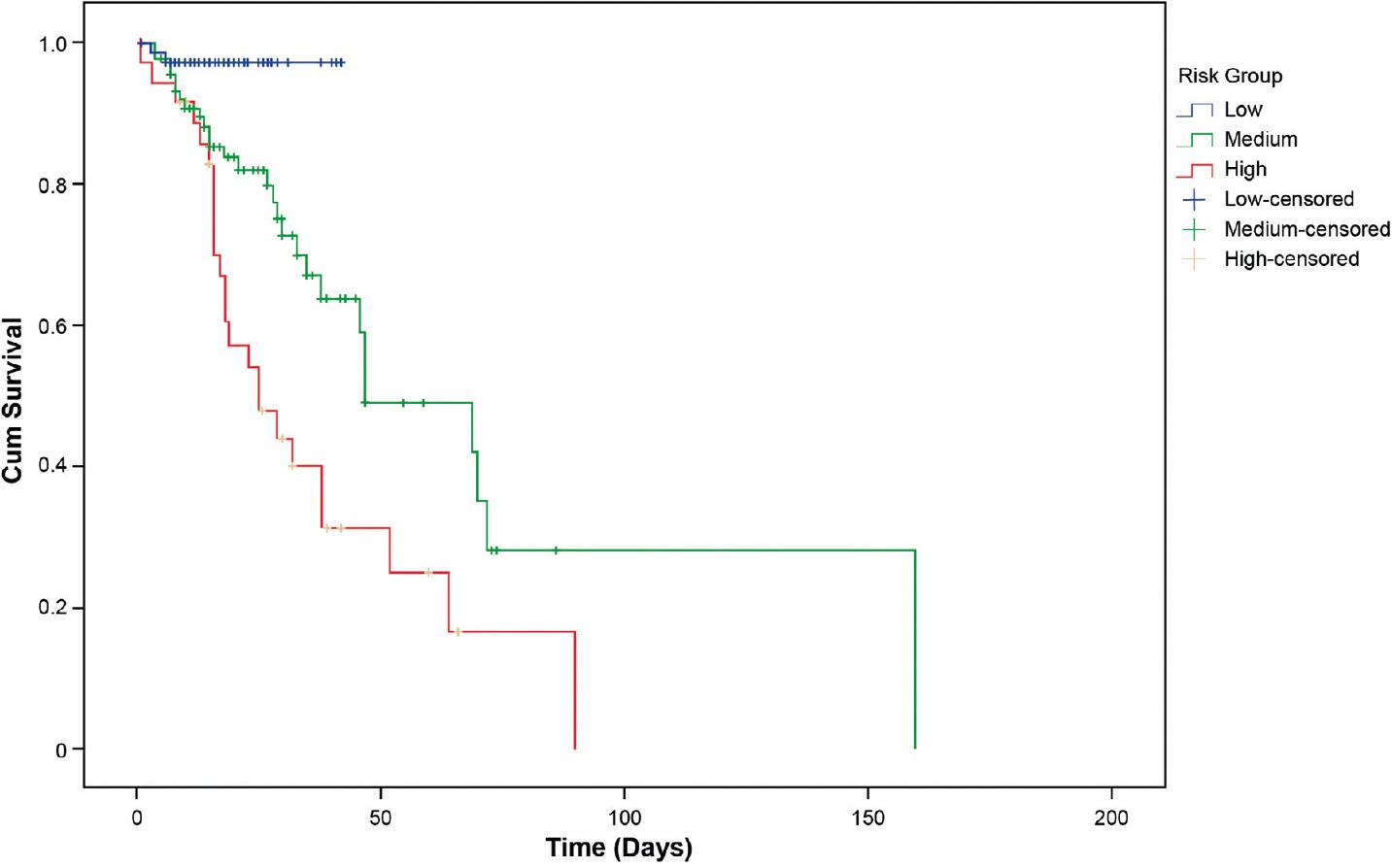
Based on the obtained scores, the population was initially divided into quartiles to obtain risk groups. Since there was no significant difference between Groups 2 and 3, these were merged into an intermediate risk group. Finally, the three risk groups were integrated: low risk with a score of 0-6 and a probability of mortality of 2.6%, intermediate risk with a score of 7-12 and a probability of mortality of 36.1%, and high risk with a score of ≥13 and a probability of mortality of 63.3%. Log-rank pairwise comparisons were performed to determine which intervention groups had different survival distributions. A Bonferroni correction was made with statistical significance accepted at p < 0.0167. There was a statistically significant difference in survival distributions for the risk groups: low versus intermediate (p = 0.003), low versus high (p < 0.0005), and intermediate versus high (p = 0.003) (Fig. 3).
DISCUSSION
In this study, we described the clinical characteristics and outcome in a cohort of HIV/AIDS patients with P. jiroveci pneumonia and severe acute hypoxemic respiratory failure and developed a predictive scoring system to estimate the risk of mortality in these patients. Our results show that three factors that are easily obtainable on the patients admission to the ICU (BMI, serum albumin, and time interval between admission to the emergency room and admission to the RICU) predict inhospital mortality. The duration of vasopressor support was also significantly predictive of mortality. Having a tool to predict the probability of survival is important for patients, families, and hospital personnel before selecting the appropriate treatment given the ethical and economic implications that these decisions entail. As such, the scoring system developed in our unit may guide these decisions.
Several markers of malnutrition have been associated with excess mortality in severely ill patients, including low BMI or body weight27,28. A low BMI has also been associated with a prolonged hospital stay, an increased risk of nosocomial infection, and a greater number of days of mechanical ventilation29,30. In our population of patients with HIV infection and SAHRF, a low BMI (<18.5 kg/m2) showed a significant independent association with inhospital mortality, which is consistent with previous results obtained in critically ill HIV-infected and non-HIV-infected patients27-31. These data highlight nutritional status as an important factor for survival in critically ill HIV-infected patients. HIV patients with high viral loads have been shown to have a hypermetabolic state mediated by elevated levels of inflammatory cytokines with increased basal energy expenditure and accelerated proteolysis32. The median viral loads of our patients, survivors and non-survivors, were 5.36 and 5.59 log10 copies/ml, respectively. In this context, early detection of HIV infection is important, as studies show that delaying ARV initiation increases the risk of opportunistic infections that contribute significantly to HIV-associated wasting syndrome28,33. Therefore, it is of concern that, in 94% of our patients, the diagnosis of AIDS and PJP was made at the time of admission, which was reflected in high viral loads and low CD4 cell counts. Early detection requires strengthening and restructuring primary care medical services in the country, a task for which the coordinated participation of the government and private investment is indispensable.
Hypoalbuminemia is reportedly an independent risk factor for mortality in practically all studies performed in patients with HIV infection and has also been reported as an independent predictor of non-AIDS complications and long-term mortality in patients living with HIV34. Albumin has antioxidant activity and can, therefore, protect cells from oxidative stress during sepsis35. It is also a biomarker of inflammation; albumin levels decrease during acute and chronic inflammatory processes due to the inhibition of its synthesis in the liver by cytokines36. Our results showed a strong association between low serum albumin levels and mortality, which may indicate greater chronicity or a more intense inflammatory response in patients with lower albumin levels and higher mortality.
Septic shock and the use of vasopressors are predictors of mortality in patients with PJP and HIV infection37. We found that the number of days with vasopressor support was an independent risk factor for mortality. While unknown at the time of admission, we included this factor due to its weight as a mortality predictor, and because it may serve to reevaluate expectations of survival throughout a patients course.
At the onset of the AIDS epidemic, the survival rate of patients with PJP-associated SHRF who required intubation and mechanical ventilation was no higher than 15%, making it difficult to justify their admission to an ICU4. However, their survival rates have since improved substantially38; among our patients, the survival rate was >70% on discharge from the hospital. Similar results have been reported by other researchers3,8,9. Since none of our patients were receiving ARV therapy or PJP prophylaxis at the time of admission, survival times similar to those reported in the pre-ARV era would be expected. The improvement in survival, therefore, appears to be independent of ARV therapy and most likely reflects advances in intensive care and respiratory management39. Rapid admission to the ICU, the application of lung-protective ventilation, and, in selected cases, advanced measures can be important to improve the survival of these patients.
Efforts to develop scoring systems predictive of prognosis in patients with HIV infection and PJP started at the beginning of the AIDS epidemic. Montaner et al.5 retrospectively evaluated various scoring systems to predict mortality in 56 patients with SHRF due to PJP who required intensive care between 1985 and 1991; their mortality rates were 65% overall and 72% in those who required mechanical ventilation. On multivariate analysis, only the multiple system organ failure score predicted mortality; its performance was enhanced when the serum lactate dehydrogenase (LDH) level was included in the model. The vasopressor requirement was also a significant predictor of mortality in this cohort. Speich et al.6 proposed a PJP severity scoring system based on the following parameters: LDH level, P(A-a)O2, and the percentage of neutrophils in the bronchial lavage fluid obtained in the first 24-48 h after admission. Only 4 of the 94 patients required mechanical ventilation, and the 14-day mortality rate was 7%. Despite the importance of these first studies in developing a predictive prognostic model, they have important limitations in current clinical applications due to changes in populations and treatment methods over time14.
Our developed model and scoring system for predicting mortality showed good discriminatory capacities, with an area under the ROC curve of 0.869 (i.e., if two patients in this population were randomly selected, the probability that the model correctly identified the non-survivor would be 86.9%). Calibration analysis showed an excellent match between the predicted and observed probabilities (α = 0.03 and β = 0.99). In addition, the model was validated internally and did not show a significant bias. Hence, the model and the scoring system derived from it can be used as tools to help clinicians in decision-making and communicate expectations regarding the probability of patient death with family members. In addition, the model can be useful for the quality control of ICU medical care because important deviations from the mortality predicted in different strata ought to lead to revising management protocols.
However, it is important to discuss the limitations of the study so that clinicians are fully informed of any drawbacks of this statistical tool. Although our model showed good calibration and discrimination and was internally validated, external validation remains necessary for a proper evaluation of our models generalization. In this context, our model requires recalibrating in ICUs that wish to adopt it.
In summary, we developed a model and prognostic scoring system using variables that are easy to obtain on admission to the ICU in HIV/AIDS patients with severe acute hypoxemic respiratory failure caused by PJP. The internally validated scoring system, which showed good calibration and discrimination, provides a potentially useful tool to assist clinicians to estimate the inhospital mortality risk in a specific HIV/AIDS patient population. However, more studies are required to validate the application of our score, especially in low- and middle-income countries, where PJP continues to be a significant burden for health services.











 nueva página del texto (beta)
nueva página del texto (beta)