Introduction
Tobacco smoking has deleterious effects on kidney function1. The use of tobacco among living kidney donors has been associated with a decreased survival of the recipient and renal graft2. This negative impact on the renal graft function persists in the long term after transplant3. Simultaneously, smoking is associated with an increased short-term risk of developing chronic kidney disease (CKD) in living kidney donors4. Despite these findings, few studies have evaluated the impact of smoking on the donor's kidney function. The aim of this study is to assess the short- and mid-term impact of smoking on kidney donors after nephrectomy.
Methods
This is a retrospective observational study of prospectively collected data from 308 patients who underwent living donor nephrectomy (LDN) at a tertiary referral hospital in Mexico City from 2008 to 2017. This study was approved by the Institutional Review Board. We compared baseline characteristics as well as functional outcomes following LDN according to history of tobacco smoking. Non-smoking donors were defined as donors with no medical history of smoking whereas smoking donors were those former or current smokers. The cumulative smoking was calculated by multiplying the total number of smoking years by the number of cigarettes per day divided by 20, known as pack-years. Estimated glomerular filtration rate (eGFR) was calculated with the modification of diet in renal disease equation in 6 time periods: pre-operative, 1 week, 1 month, 6 months, 12 months and 24 months after surgery. CKD was defined as eGFR < 60 mL/min/1.73 m2.
The local Institutional Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán reviewed and approved this study.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 for IBM. For descriptive statistics, we used central tendency measures such as mean or median. Standard deviation or interquartile range and range were used as dispersion descriptive measures. Bivariate analysis was performed using paired samples test by t-test while non-parametric variables were compared with Mann-Whitney U-test. Proportions were compared using Chi-square test. Kaplan-Meier analysis was performed to determine CKD outcome based on smoking history and cumulative smoking using pack-year formula (0, 0.1-10, 10.01-20, 20-30, and > 30 pack-years). Binary logistic regression analysis was performed to identify independent risk factors associated CKD at 24 months of follow-up. Receiver operating characteristic curve analysis was performed to identify a pack-year cutoff point associated with CKD. Any p value equal or lower than 0.05 or 5% was considered as statistically significant for a two-tied distribution.
Results
The median follow-up was 36 months. Demographic characteristics of the kidney donors are presented in table 1. The mean age was 39 ± 16 (18-65) years and 58.1% were female. Mean body mass index (BMI) was 25.5 ± 2.99 (15.6-34) kg/m2. Median pre-operative serum creatinine was 0.78 ± 0.27 (0.43-1.56) mg/dL with an eGFR of 94 ± 29 mL/min/1.73 m2. Left laparoscopic nephrectomy was performed in 94.2%. Past or current use of tobacco was reported by 106 (34.4%) donors. Most of the smoking donors were male (57.5% vs. 33.7%, p ≤ 0.01). Pre-operative and 24-month post-operative renal function was compared between groups (Table 2). Compared with non-smokers, smokers had lower pre-operative eGFR (90 ± 26.3 mL/min/m2 vs. 96 ± 27 mL/min/1.73m2, p = 0.02), as well as lower 1st-week post-operative eGFR (72.5 ± 35 vs. 83 ± 38 mL/min/1.73 m2, p = 0.01), 1st month (66 ± 27.5 vs. 73 ± 23.2 mL/min/1.73 m2, p ≤ 0.01), 6th month (66.7 ± 26.3 vs. 72 ± 20 mL/min/1.73m2, p = 0.01), and 12th month (66 ± 25.3 vs. 74 ± 22 mL/min/1.73m2, p = 0.01). There was no difference between groups at the 24th month of follow-up (p = 0.40). The pack-years cutoff point associated to CKD was 3.2 with an AUC of 0.67 (Fig. 1). A total of 38 (12.3%) patients developed CKD of whom 22/106 were smokers (20.7%) and 16/202 non-smokers (7.9%). Kaplan-Meier analysis showed that non-smokers had a greater disease-free period than smokers (p = 0.001) (Fig. 2). In addition, we classified patients in groups according to their cumulative smoking using the pack-year formula (< 0.1 [1.16%], 0.1-10 [86.04%], 10.01-20 [8.13%], 20-30 [1.16%], and > 30 [3.48%] pack-years), which did not show a significant difference between heavy smokers and CKD (p = 0.763). The univariate and multivariate analyses showed that age ≥ 40 years before nephrectomy (OR 6.59; 95% CI 2.71-16.02; p ≤ 0.01) and tobacco smoking (OR 3.35; 1.58-7.12; p ≤ 0.01) were independent risk factors to develop CKD in kidney donors after nephrectomy (Table 3).
Table 1 Living donor characteristics
| n | 308 |
| Age (years) | 39±16 (18-65) |
| Gender | |
| Male | 129 (41.9%) |
| Female | 179 (58.1%) |
| Laterality | |
| Left | 290 (94.2%) |
| Right | 18 (5.8%) |
| BMI (kg/m2) | 25.5±2.99 (15.6-34) |
| BSA (m2) | 1.7±0.24 (1.32-2.25) |
| Hypertension | |
| Yes | 2 (0.6%) |
| No | 306 (99.4%) |
| Pre-operative creatinine (mg/dL) | 0.78 ± 0.27 (0.43-1.56) |
| Pre-operative MDRD eGFR (mL/min/1.73m2) | 94 ± 29 |
| Smoking history | |
| Current smoking | 54 (17.4%) |
| Cigarettes per day | 4 ± 8 |
| Past smoking | 52 (17%) |
| No smoking history | 202 (65.6%) |
| Pack-years in smoking living donors | 2±4.73 |
BMI: body mass index; BSA: body surface area; MDRD: modification of diet in renal disease; eGFR: estimated glomerular filtration rate.
Table 2 Smoking history and perioperative renal function of living kidney donors
| Variable | Non-smoking donor | Smoking donor | p value |
|---|---|---|---|
| n (%) | 202 (65.6%) | 106 (34.4%) | |
| Age (years) | 40 ± 15 | 38.5 ± 17.8 | 0.99 |
| Male (%) | 68 (33.7%) | 61 (57.5%) | <0.01 |
| Pre-operative Creatinine (mg/dL) | 0.73 ± 0.27 | 0.86 ± 0.26 | <0.01 |
| Creatinine 1 week PO (mg/dL) | 0.96 ± 0.36 | 1.15 ± 0.35 | <0.01 |
| Creatinine 1 month PO (mg/dL) | 1.05 ± 0.35 | 1.24 ± 0.33 | <0.01 |
| Creatinine 6 months PO (mg/dL) | 1.05 ± 0.3 | 1.24 ± 0.28 | <0.01 |
| Creatinine 12 months PO (mg/dL) | 1.03 ± 0.31 | 1.21 ± 0.26 | <0.01 |
| Creatinine 24 months PO (mg/dL) | 1.05 ± 0.31 | 1.16 ± 0.23 | 0.01 |
| Pre-operative eGFR MDRD mL/min/1.73 m2 | 96 ± 27 | 90 ± 26.3 | 0.02 |
| eGFR 1-week PO MDRD mL/min/1.73 m2 | 83 ± 38 | 72.5 ± 35 | 0.01 |
| eGFR 1-month PO MDRD mL/min/1.73 m2 | 73 ± 23.2 | 66 ± 27.5 | <0.01 |
| eGFR 6-months PO MDRD mL/min/1.73 m2 | 72 ± 20 | 66.7 ±26.3 | 0.01 |
| eGFR 12- months PO MDRD mL/min/1.73 m2 | 74 ± 22 | 66 ± 25.3 | 0.01 |
| eGFR 24- months PO MDRD mL/min/1.73 m2 | 72 ± 19.5 | 72 ± 24.8 | 0.40 |
PO: post-operation; MDRD: modification of diet in renal disease; eGFR: estimated glomerular filtration rate.

Figure 1 Receiver operating characteristic curve analysis showing the association of smoking history and development of chronic kidney disease in the overall population with an AUC of 0.67.
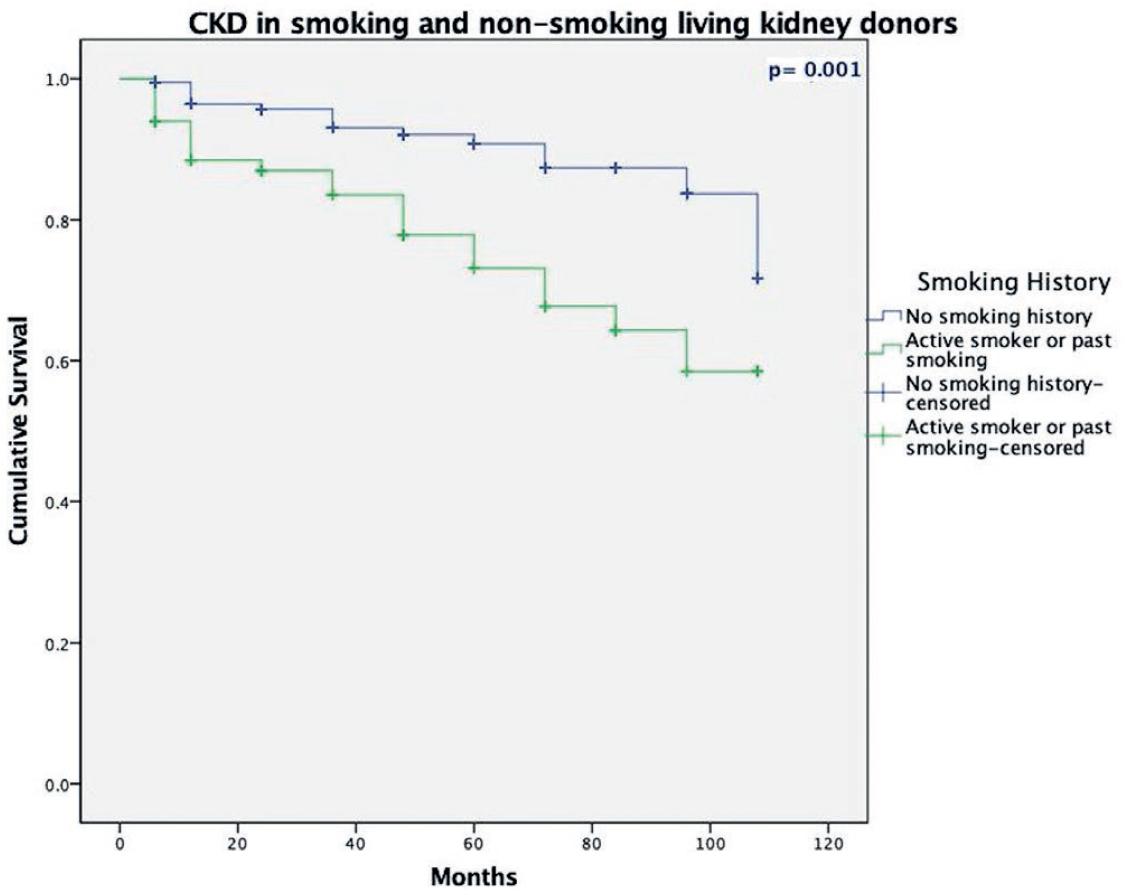
Figure 2 Kaplan-Meier survival plots of chronic kidney disease in smoking living donors and non-smoking living donors.
Table 3 Logistic regression analysis of prognostic factors in relation to development of chronic kidney disease at 24 months
| Independent risk factors for chronic kidney disease at 24 months | |||
|---|---|---|---|
| Variable | OR | CI | p value |
| Univariate | |||
| Age at donation ≥ 40 years | 6.3 | 2.25-12.4 | <0.01 |
| Gender (male) | 2.116 | 1.06-4.22 | 0.03 |
| Laterality (right) | 1.39 | 0.38-5 | 0.61 |
| Obesity (BMI >30 kg/m2) | 1.38 | 0.38-5 | 0.62 |
| Smoking history | 3.133 | 1.56-6.3 | 0.01 |
| Multivariate | |||
| Age at donation ≥ 40 years | 6.59 | 2.71-16.02 | <0.01 |
| Gender (male) | 1.94 | 0.92-4.13 | 0.08 |
| Smoking history | 3.35 | 1.58-7.12 | 0.02 |
BMI: body mass index; OR: odds ratio; CI: confidence interval.
Discussion
Smoking causes acute and chronic toxic effects on the kidneys. The acute effects include intense sympathetic stimulation, an increase in blood pressure, tachycardia5, and an increase in the concentration of circulating catecholamines5,6. In addition, smoking causes an increase in renovascular resistance (11%) and a decrease in the GFR (−15%) and filtration fraction (−18%)6. In our study, we found that kidney donors who were smokers had a lower pre-operative eGFR compared to non-smokers, suggesting that before nephrectomy, smokers have some kind of kidney damage. On the other hand, the chronic effects of smoking on the kidney are still unknown5,7. One of the theories suggests a decrease in renal plasma flow and elevation of endothelin, which induces functional kidney abnormalities secondary to its vasoconstrictive effect7. Similarly, smoking has been associated with thickening of the renal arterioles8. The prevalence of smoking among living kidney donors has not been studied widely and ranges from 14.5% to 34%9-12. Our study showed a greater prevalence of smoking in living kidney donors (34.4%) than in the Mexican general population (17.9%)13. This difference increases the need for attending physicians to take action and implement better strategies to promote smoking cessation.
Recently, the negative impact of smoking on kidney recipients and grafts has been reported. Smoking is a risk factor for developing cardiovascular events in the 1st year after transplant due to a higher risk of fibrous thickening of the vascular intima14,15. On the other hand, it has been demonstrated that donor smoking history is closely related to reduced survival of the recipient (HR 1.06 95% CI 1.01-1.12) and kidney graft (HR 1.05 95% CI 1.01-1.09)2,16. Moreover, recipients with a personal history of smoking have worse self and graft survivals (HR 1.6 95% CI 1.02-2.53 and HR 1.7 95% CI 1.14-2.7, respectively)17. Finally, a decrease in the recipient's GFR has been associated with grafts provided by smokers3.
Long-term follow-up of living kidney donors is essential to reduce the risk of renal function impairment secondary to the development of comorbidities, including hypertension and obesity18. Monitoring should include smoking cessation, since several studies have associated an increased number of complications in living donors who smoke, such as higher perioperative complication rate (OR 1.4, 95% CI 1.02-1.94)20, higher rate of surgical wound infections (OR 4.78, 95% CI 2.30-9.94)21, and elevated serum creatinine levels in the 1st year after donation (57% in smokers vs. 40% in non-smokers, p < 0.001)3 and higher mortality (HR 5.3, 95% CI 2.6-10.8)19. These results were similar to those in our study, where we found that smokers more often developed CKD compared with non-smokers in the Kaplan-Meier analysis, which correlates with the increase in creatinine after donation. In addition, Bahous et al. reported a significant association between pack-years and lower GFR 1 year after nephrectomy, which is similar to our findings22. Although in the pack-years sub-analysis, there was no significant difference between a higher calculated pack-years and CKD (p = 0.763), this can be the result of a lower proportion of heavy smokers in the sample.
Strategies to stop smoking after donation were discussed at the Amsterdam International Forum on the care of the live kidney donor. In this consensus, experts acknowledged the controversies and lack of solid evidence regarding the role of active smoking on morbidity or mortality of living kidney donors. Conversely, there was observational evidence for the benefit of quitting smoking before donation23,24. Despite the high risk of complications associated with tobacco, smoking cessation is still not recommended by all donation centers, which points to the need to implement new clinical guidelines for smokers who are candidates to donor nephrectomy. The recommendation issued at the Amsterdam Forum was as follows: all potential donors should stop smoking for at least 4 weeks before donation while they receive health promotion advice from anesthesiologists or other health professionals, focusing on smoking cessation in the context of other risk factors24. In an overview regarding smoking policies for organ donation, 20% of transplant programs do not include a smoking policy while only 7% exclude smokers. Instead, active smokers are accepted in 71% of programs, of which 36% require the donor to stop smoking before surgery25. There are few studies evaluating smoking behavior of kidney donors. Kelles et al. reported that 47% of donors had a history of smoking before nephrectomy, with 29% quitting smoking 12 months later (p = 0.001); however, their levels of nicotine dependence remained similar to those who continued smoking26.
One of the limitations of our study was its retrospective nature, which limited information obtained from clinical records, precluding a thorough identification of current smokers versus former smokers. In addition, the sample size could be reduced in comparison to larger series. Besides, a 24-month renal function assessment was performed only in 181 patients (58.7%), which could restrict the long-term results. However, we believe our findings, along with the previous investigations from other authors, are strong enough to recommend smoking cessation to all potential living donors.
In conclusion, living kidney donors with a tobacco smoking history had an increased risk of developing CKD following nephrectomy. Smoking-cessation strategies should be implemented before and after donor nephrectomy.











 nueva página del texto (beta)
nueva página del texto (beta)


