INTRODUCTION
Acute pulmonary embolism (PE) is one of the manifestations of venous thromboembolism and is the third most frequent acute cardiovascular event, accounting for approximately 5-10% of deaths in hospitalized patients1,2. However, the mortality rates change between groups according to clinical and radiological findings; in intermediate-risk PE, the expected mortality rate was 3-8% whereas in high-risk PE, as much as 25-52%3. The PE severity index (PESI) determines early mortality risk in patients and is calculated as part of the risk stratification process according to European Society of Cardiology (ESC) PE Guidelines4. This risk score is solely based on clinical parameters; however, various studies have shown that sensitivity can be enhanced using biomarkers such as high-sensitivity cardiac troponin I and N- terminal pro- brain natriuretic peptide5-7.
Inflammation promotes thrombosis and plays a key role in the pathophysiology of PE8. C-reactive protein (CRP) is an acute phase reactant that is produced by the liver and induced by several cytokines. A previous research showed that high CRP levels correlate with sepsis and mortality in critically ill patients9. Moreover, CRP has been investigated for the diagnosis, follow-up and prognosis of PE6,10. Serum albumin is another protein produced by the liver; it is a negative acute phase reactant and negatively correlates with inflammatory processes11. It also plays an anti-inflammatory, antioxidant, anticoagulant, and antiplatelet aggregation role12. The CRP/albumin ratio (CAR) has been defined as an inflammation-based prognostic marker, and the relationship between CRP and albumin levels is thought to indicate the prognosis of critical diseases and malignancies13,14 and various cardiovascular disorders15,16. According to the best of our knowledge, there has been no study in the medical literature in English that looked at the association and the prognostic value of the CRP/albumin ratio in patients with PE. Therefore, we aimed to evaluate the prognostic role of CAR in patients with acute PE.
METHODS
Study design
In this study, a total of 256 patients with acute PE who were hospitalized from 1 March 2016 to 30 December 2020 were reviewed retrospectively. Acute PE was diagnosed with Computed Tomography (CT) angiography or pulmonary angiography. Exclusion criteria were: < 18 years of age, previous diagnosis of autoimmune disease, active infection (pulmonary, urinary tract, etc.), malignancy, acute transient ischemic attack (TIA)/stroke, albuminuria, and chronic liver disease. Albumin replacement therapy in the past 6 months, and lack of serum CRP or albumin levels in laboratory results were also exclusion criteria. The study protocol was approved by the institutional ethics committee and was conducted in accordance with the Declaration of Helsinki.
Data collection
Demographic and clinical data were recorded by reviewing the electronic database. PESI was calculated based on the initial clinical parameters. Patients were classified according to their final scores: ≤ 65, class I (very low risk); 66–85, class II (low risk); 86–105, class III (intermediate risk); 106-125, class IV (high risk); > 125, class V (very high risk). In-hospital mortality and 6-month mortality were assessed. PE-related parameters such as treatment with thrombolytic agents, positive inotrope therapy or mechanic ventilation requirement were noted.
Blood samples were obtained on admission. The albumin and CRP levels were obtained using Roche Diagnostics Cobas 8000 c502 analyzer (Indianapolis, USA). CAR was obtained by dividing the CRP level (mg/L) by the albumin (g/dL) level.
Statistical analyses
All statistical tests were conducted using the Statistical Package for the Social Sciences 19.0 for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to analyze normality of the data. Continuous data are expressed as mean ± SD, and categorical data are expressed as percentages. Chi-square test was used to assess differences in categorical variables between groups. Student's t-test or Mann-Whitney U test was used to compare unpaired samples as needed. Variables having linear correlation were evaluated using Pearson's correlation test and nonlinear variables were evaluated using Spearman's correlation test. Binary logistic regression analysis was used to identify independent variables of death. For the best cut-off value of the CRP/albumin ratio and PESI, receiver operating characteristic (ROC) curves were obtained and the optimal values with the greatest total sensitivity and specificity in the prediction of death were selected. Significance was assumed at a 2-sided p < 0.05.
RESULTS
A total of 186 patients were enrolled. Overall, 54 patients were in the intermediate risk, 34 patients in high risk, and 98 patients were in the very high-risk group according to PESI score. We formed two groups according to 6-month survival data of the included patients: 137 patients (86 female, 51 male) formed the survivor group; and 49 patients (33 female, 16 male) who died within 6 months formed the non-survivor group. Both groups were similar in terms of age, gender, HT, DM, CAD, congestive heart failure, COPD, and heart rate at admission (Table 1). However, the numbers of patients having systolic blood pressure lower than 100 mmHg, ventilation rate higher than 30 times/min, and body temperature < 36°C were significantly higher in the non-survivor group (51.09% vs. 73.46%, p = 0.002; 9.48% vs. 53.06%, p < 0.0001; 1.45% vs. 8.16%, p = 0.042, respectively). Concerning the biochemical parameters, glomerular filtration rate (81.31 ± 25.48 vs. 61.55 ± 24.58, p < 0.0001), plasma albumin level (3.7 ± 0.5 vs. 3.2 ± 0.6, p < 0.001), left ventricular ejection fraction (56.4 ± 4.5 vs. 53.0 ± 6.2, p < 0.001) were significantly higher; uric acid level (5.80 ± 1.91 vs. 7.06 ± 3.03, p < 0.001), PESI score (113.74 ± 44.05 vs. 175.10 ± 64.42, p < 0.0001), high-sensitivity troponin I (hs-TpI) (3 [0-51] vs. 34 [22-263], p < 0.001), CRP (5.1 [2-10] vs. 15.8 [4.8-24.6], p < 0.001), and CRP/Albumin ratio (1.57 ± 6.07 vs. 5.97 ± 6.83, p < 0.001) were significantly lower in the survivor group compared to non-survivors. Furthermore, right ventricle (RV) dimensions were significantly higher in the non-survivor group (34.8 ± 5.6 vs. 39.4 ± 5., p < 0.001). All demographical, clinical, and biochemical characteristics of the two groups are presented in detail in table 1.
Table 1 Demographic, biochemical, and clinical data of acute pulmonary embolism patients
| Clinical characteristics | Survivor (n = 137) | Non-survivor (n = 49) | p |
|---|---|---|---|
| Age (years) | 63.2 ± 15.0 | 67.5 ± 17.3 | 0.157 |
| Male, n (%) | 51 (37.22) | 16 (32.65) | 0.375 |
| Heart rate > 110, beats/min, n (%) | 136 (99.27) | 49 (100) | 0.737 |
| Respiratory rate > 30, times/min, n (%) | 13 (9.48) | 26 (53.06) | < 0.0001 |
| Systolic arterial pressure < 100, mmHg, n (%) | 67 (51.09) | 36 (73.46) | 0.002 |
| Saturation O2 < 90, n (%) | 108 (78.83) | 42 (85.87) | 0.400 |
| Right ventricle/Left ventricle (Computerized Tomography), n (%) | 83 (60.58) | 38 (77.55) | 0.016 |
| Deep venous thrombosis, n (%) | 26 (18.97) | 14 (28.57) | 0.083 |
| Hospital stay (days) | 5(3-7) | 1 (1-6) | < 0.001 |
| Intensive care unit admission, n (%) | 87 (63.50) | 40 (81.63) | 0.013 |
| Mental status, n (%) | 2 (1.45) | 20 (40.81) | < 0.0001 |
| Patients receiving thrombolytic, n (%) | 35 (25.54) | 23 (46.93) | 0.005 |
| Body temperature (ºC) < 36, n (%) | 2 (1.45) | 4 (8.16) | 0.042 |
| Body mass index (kg/m2) | 28.89 ± 5.61 | 27.67 ± 4.78 | 0.672 |
| Comorbidity | |||
| Hypertension, n (%) | 73 (53.28) | 22 (44.89) | 0.200 |
| Diabetes Mellitus, n (%) | 33 (24.08) | 7 (14.28) | 0.107 |
| Coronary artery disease, n (%) | 21 (15.32) | 5 (10.20) | 0.264 |
| Congestive heart failure, n (%) | 2 (1.45) | 2 (4.08) | 0.284 |
| Malignity, n (%) | 5 (3.64) | 13 (26.53) | < 0.0001 |
| Chronic obstructive pulmonary disease, n (%) | 20 (14.59) | 3 (6.12) | 0.093 |
| Cerebrovascular Incident, n (%) | 6 (4.37) | 3 (6.12) | 0.011 |
| Laboratory findings | |||
| Hemoglobin (g/dL) | 12.2 ± 1.9 | 12.3 ± 2.0 | 0.816 |
| Platelets | 231.3 ± 69.5 | 243.2 ± 90.4 | 0.408 |
| Leukocytes (103 /µL) | 10.1 ± 3.2 | 11.7 ± 3.9 | 0.319 |
| Neutrophiles | 9.0 ± 4.4 | 10.1 ± 4.2 | 0.480 |
| Laboratory findings | |||
| Lymphocytes | 2.5 ± 1.1 | 3.0 ± 1.4 | 0.057 |
| Red cell distribution width | 14.1 ± 1.7 | 14.8 ± 3.1 | 0.091 |
| Platelet distribution width | 14.7 ± 5.1 | 13.4 ± 3.0 | 0.175 |
| Serum creatinine (mg/dL) | 1.01 ± 0.89 | 1.12 ± 0.41 | 0.385 |
| Glomerular filtration rate (CPK-EPI) | 81.31 ± 25.48 | 61.55 ± 24.58 | < 0.0001 |
| Sodium (mmol/L) | 138.7 ± 3.9 | 136.6 ± 6.3 | 0.017 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.5 ± 0.6 | 0.059 |
| Glucose (mg/dL) | 153.0 ± 71.9 | 171.9 ± 57.8 | 0.171 |
| Albumin | 3.7 ± 0.5 | 3.2 ± 0.6 | < 0.001 |
| Uric acid | 5.80 ± 1.91 | 7.06 ± 3.03 | < 0.001 |
| Pulmonary embolism severity index | 113.74 ± 44.05 | 175.10 ± 64.42 | < 0.0001 |
| C-reactive protein (mg/dL) | 5.1 (2-10) | 15.8 (4.8-24.6) | < 0.001 |
| High-sensitivity Troponin I (Normal range < 14 pg/mL) | 3 (0-51) | 34 (22-263) | < 0.001 |
| D-dimer (ng/mL) | 5 (2-8) | 7 (5-8) | 0.237 |
| CRP/Albumin | 1.57 ± 6.07 | 5.97 ± 6.83 | < 0.0001 |
| Left ventricular ejection fraction | 56.4 ± 4.5 | 53.0 ± 6.2 | < 0.001 |
| Left ventricular dimension (LVD) | 44.6 ± 4.6 | 44.0 ± 4.6 | 0.523 |
| Right ventricular dimension (RVD) | 34.8 ± 5.6 | 39.4 ± 5.1 | < 0.001 |
| RVD/LVD | 0.8 ± 0.4 | 0.9 ± 0.1 | 0.454 |
| Pulmonary artery systolic pressure | 46.6 ± 10.9 | 54.3 ± 9.7 | < 0.001 |
In the correlation analysis, we observed moderately positive correlations between CRP/albumin ratio, troponin and PESI score (r = 0.584, p < 0.0001; r = 521, p < 0.0001, respectively) (Fig. 1). Regression analysis revealed that only CRP/albumin ratio and PESI score were independent risk factors associated with 6-month mortality of acute PE patients (Table 2).
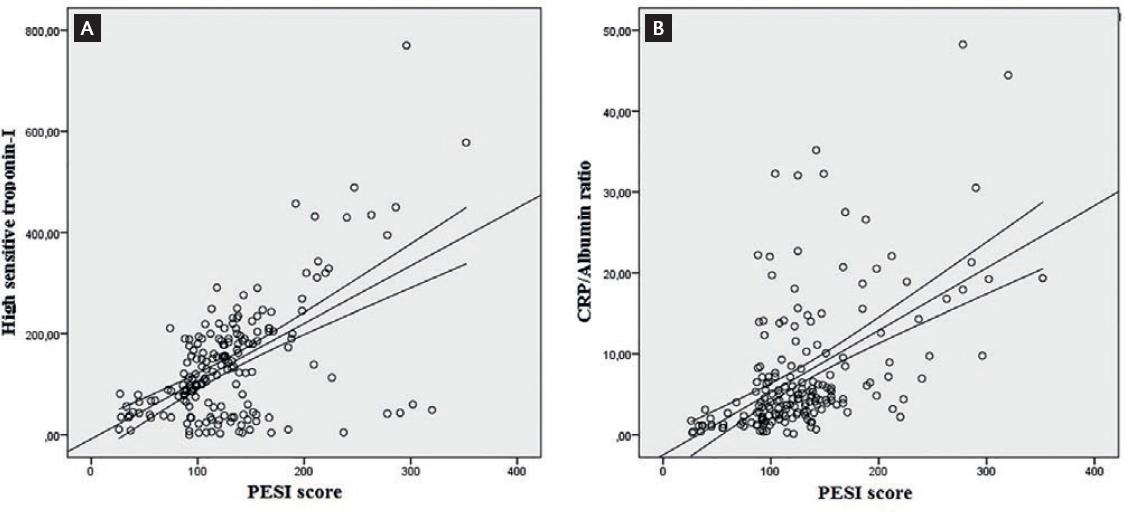
Figure 1 Graphs show the correlation between PESI score and (A) high-sensitivity troponin-I level. (B) CRP/albumin ratio.
Table 2 Binary logistics regression analysis on the risk factors associated with mortality in patients with acute pulmonary embolism
| Variable | Beta | 95% CI | p |
|---|---|---|---|
| CRP/albumin ratio | −0.80 | 0.872-1.078 | 0.006 |
| Troponin | 0.002 | 0.994-1.004 | 0.926 |
| Left ventricular ejection fraction | 0.58 | 0.982-1.144 | 0.134 |
| PESI score | −0.14 | 0.975-1.118 | 0.027 |
ROC curves for accuracy of PESI score and CRP/albumin ratio for predicting 30- day, 3-month and 6-month mortality in acute PE patients are shown in figure 2. For 30-day mortality, area under the curve (AUC) for PESI score was 0.640 (95% CI: 0.543-0.737). A cut-off value of 125.5 for PESI score was associated with 57.6% sensitivity and 58.2% specificity in prediction of 30-day mortality. For 3-month mortality, AUC for PESI score was 0.815 (95% CI: 0.740-0.889). A cut-off value of 133.5 for PESI score was associated with 69.8 % sensitivity and 71.3 % specificity in prediction of 3-month mortality. AUC for PESI score was 0.813 (95% CI: 0.741-0.885). A cut-off value of 132.5 for PESI score was associated with 71.4% sensitivity and 72.2% specificity in prediction of 6-month mortality.
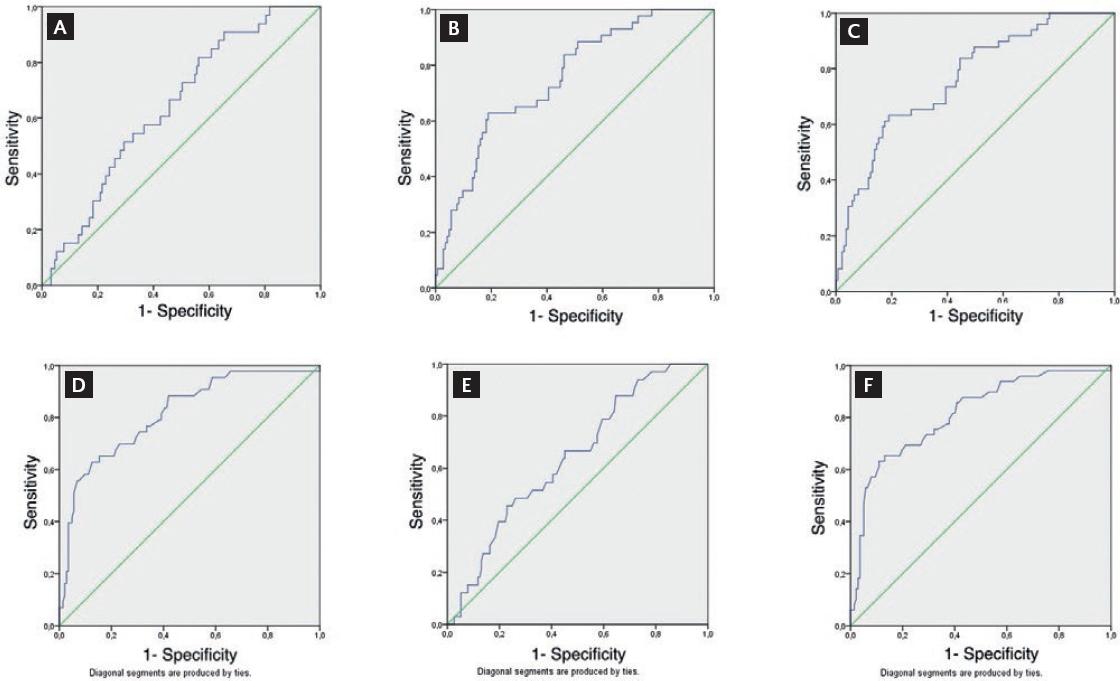
Figure 2 ROC curves of the performance of CAR for diagnosing (A) 30-day, (B) 90-day, and (C) 6-month mortality. ROC curve analysis of the performance of PESI scores for diagnosing (D) 30-day, (E) 90-day, and (F) 6-month mortality.
For 30-day mortality, AUC for CAR score was 0.643 (95% CI: 0.550-0.737). A cut-off value of 5.07 for CAR was associated with 57.6% sensitivity and 57.5% specificity in prediction of 30-day mortality. For 3-month mortality, AUC for CAR was 0.751 (95% CI: 0.672-0.830). A cut-off value of 5.35 for CAR was associated with 65.1% sensitivity and 65.0% specificity in prediction of 3-month mortality. The AUC for CRP/albumin ratio was 0.763 (95% CI: 0.687-0.838). A cut-off value of 5.33 for CRP/albumin ratio was associated with 65.3% sensitivity and 65.6% specificity in predicting 6-month mortality (Figure 2).
DISCUSSION
The aim of this study was to assess whether serum inflammatory and nutritional markers can estimate mortality in patients with acute PE. CRP (inflammatory marker), albumin (nutritional marker), and their ratio CRP/albumin were analyzed. Results showed that CRP/albumin ratios were associated with 6-month mortality in acute PE. Furthermore, a positive correlation between PESI and CAR was detected.
Present ESC guidelines recommend measuring biomarkers in patients with PE at moderate risk4. Results of our study supported the prognostic accuracy of the PESI risk score for all-cause 6-month mortality in patients with PE. However, from a clinical perspective, our data also suggest that combinations of PESI risk score and biomarkers (single or combined) provide a better estimation of complications.
Acute phase proteins are a class of proteins whose serum concentrations increase (positive acute phase proteins) or decrease (negative acute phase proteins) in response to inflammation9. CRP is the first defined positive acute phase protein and is a sensitive marker of systemic inflammation17,18. On the other hand, serum albumin is a negative acute phase protein, such as transferrin and transthyretin19. Numerous clinical studies have established that hypoalbuminemia is a powerful prognostic marker in the general population as well as in many diseases12. Low serum albumin is independently and inversely correlated and a strong prognostic marker in different cardiac conditions such as coronary artery disease, heart failure, atrial fibrillation, stroke and venous thromboembolism20-22. Furthermore, its prognostic value persists after correcting malnutrition and inflammation12.
Cardiovascular disease and venous thromboembolism (VTE) are closely linked conditions that have common risk factors and may have common pathophysiological mechanisms22-24. CAR has recently been explored as a potential index to predict significant cardiovascular outcomes in a series of clinical entities9,14. CAR contains both CRP and albumin, and therefore, has the advantage of reflecting not only proinflammatory status but also nutritional status. Prognostic nutritional index, which contains albumin as the nutritional component, has already been found to be a prognostic parameter at short- and long-term follow-up of PE patients25 that correlates with our findings, emphasizing the role of combined inflammatory and nutritional status on prognosis. Recent studies evaluating CAR in patients with CVD had been encouraging. Accordingly, we showed that although CAR has lower sensitivity than PESI risk score in estimating mortality risk in PE, it has similar specificity. CAR has the potential to predict mortality in patients with PE, but its clinical applicability has yet to be proven with an internal validation cohort. Furthermore, using CAR, it is possible to modify our therapy to identify high-risk patients and to deal with possible high-risk-related adverse events in patients with PE. It can be speculated that the higher specificity of CAR is attributed to the advantage of containing two important inflammatory biomarkers.
Our study has some limitations. First of all, this is a retrospective study, and it has all the related down sides such as selection bias, lack of control of all variables and patient fall-out. Secondly, it includes a relatively small number of patients, which may explain lower sensitivity and specificity for validated PESI score. Furthermore, all data are based on a single measurement and may not reflect the relationship of CRP/albumin ratio and PE for changes over time, as follow-up measurements are not available. Definitely, larger and prospectively designed studies are needed to demonstrate the relationship between CRP/albumin ratio and PE. Biomarkers such as hs-CRP, BNP, and pro-BNP may increase the predictive power.
In conclusion, the results of our study showed decreased albumin levels and increased CRP levels in patients with PE, although both were still within the normal range. These results reinforce the role of inflammation in PE. The CRP/albumin ratio, an inexpensive and easily measurable laboratory variable, may be a useful prognostic marker of PE, especially when other causes that alter serum levels are excluded from the study.











 nueva página del texto (beta)
nueva página del texto (beta)


