INTRODUCTION
Sepsis, characterized by a dysregulated host response to infection leading to life-threatening organ dysfunction, poses a significant burden on global health. However, data on its global incidence and mortality remain limited and the mortality rates linked to septic shock remain extremely high1,2. Over the past three decades, sepsis trials have been conducted in an effort to reduce mortality rates. Recent studies based on data from high-income countries indicate an annual occurrence of 19.4 million sepsis cases and 5.3 million sepsis-related deaths in adults admitted to hospitals2.
Accordingly, adjunctive therapies have been developed to reduce mortality. Over the past 40 years, numerous medications, often referred to as "magic bullets," have been investigated with the goal of reducing mortality3. None of these studies succeeded in achieving this outcome4. Criticism arose regarding the study design and the heterogeneity of sepsis populations included in these studies4,5. Considering these challenges, blood purification emerged as a potential adjunctive therapy for sepsis in the mid-1980s, initially in the form of continuous arterio-venous hemofiltration and subsequently in continuous veno-venous hemofiltration (CVVH)6,7. At present, convection and high-volume hemofiltration is the primary techniques being analysed8,9. However, despite numerous randomized controlled trials (RCTs), no significant improvement in outcomes has been observed10,11. In this context, adsorption has emerged as a promising option for blood purification in sepsis12.
NEW CLASSIFICATION FOR ADSORPTION DURING BLOOD PURIFICATION
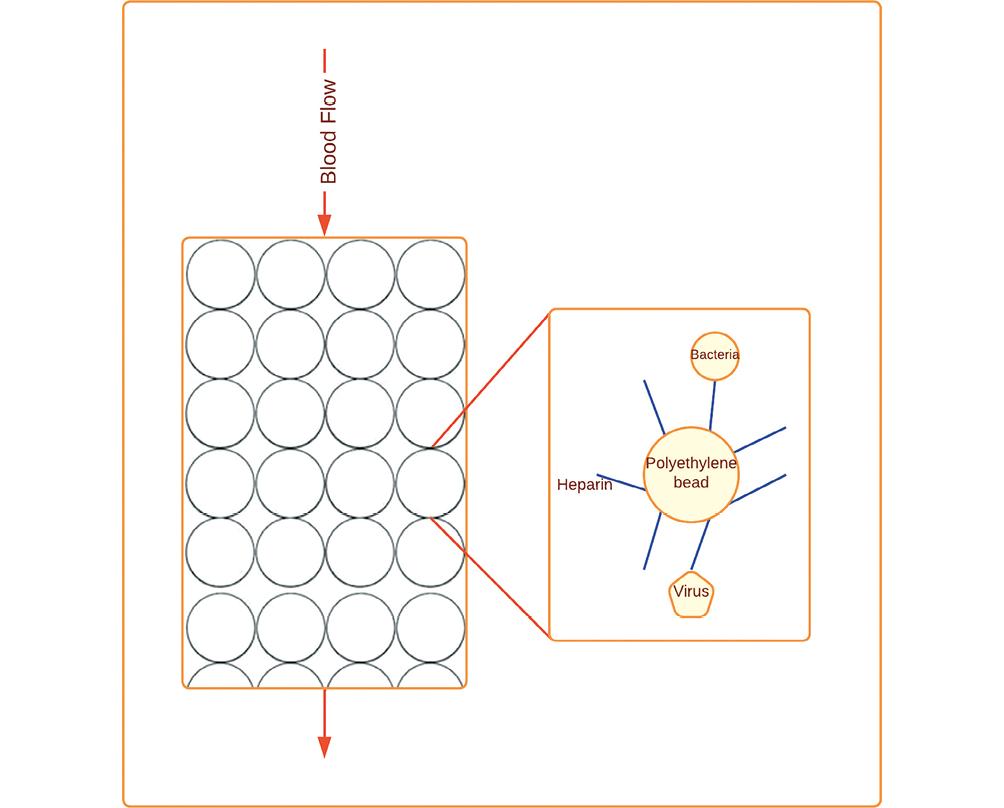
A new classification for adsorption during blood purification has been proposed, encompassing various techniques and sorbents13. In the category of hemofilters with adsorptive membranes, there are specific options for endotoxin adsorption such as AN69 oXiris® (Baxter International, USA), as well as non-specific choices including AN69-ST (Surface Treated), polymythylmetacryllate (PMMA), and SepteXTM (Baxter International, USA). Another category involves sorbents with adsorptive capacity for mediators, where hemoperfusion with polymyxin B stands out as the specific option for endotoxin adsorption. Non-specific alternatives in this category include CytoSorb® (Cytosorbents Corporation, USA), the Jafron Cartridge, and a new polymyxin B-immobilized resin column known as disposable endotoxin adsorber (KCEA). Finally, the classification includes sorbents with adsorptive capacity for bacteria and viruses. While non-specific choices such as Seraph® 100 (Seraph® 100 Microbind® Affinity Blood Filter, ExThera Medical Corporation, USA), GarnetTM Filter (BOATM Biomedical, USA), and Hemopurifier® (Aethlon Medical, USA) are available, there are currently no identified specific sorbents for this purpose. Thus, this classification framework offers a comprehensive overview of different adsorption techniques and sorbents utilized in blood purification, categorizing them based on their specificity for endotoxin, mediators, bacteria, and viruses13 (Table 1).
Table 1. Classification techniques for adsorption during blood purification, categorized by their specificity for endotoxin, mediators, bacteria, and viruses
| Type of device | Mechanisms | Results | Challenges |
|---|---|---|---|
| Hemofiltration with a specific sorbent for endotoxins | |||
| Hemofiltration with a specific adsorptive membrane for Endotoxins (AN69 oXiris) | Has dual functionality as a toxin remover
and cytokine adsorber. The filter is made from a synthetic polymer material called AN69. AN69 has high biocompatibility and excellent adsorption propities. |
In a comparative study, a higher
percentage of patients in the oXiris filter group (77.8%) showed
decreased endotoxin levels compared to the standard filter group
(16.7%). AN69 oXiris demonstrated the ability to reduce lactate, norepinephrine, TNF-α, IL-6, IL-8, and IFNγ compared to standard filters. The study did not assess mortality and clinical outcomes. In another observational study on septic shock patients, those treated with AN69 oXiris had improved survival compared to standard therapy: 28-day mortality rate in the AN69 oXiris group (47.3%) compared to the control group (73.3%). In a pediatric study, eleven patients diagnosed with vasoplegic shock and AKI received AN69 oXiris therapy with mixed results and a survival rate of 37.5%. |
Assay choice is crucial: EAA providing
more reliable measurements of endotoxin levels than LAL.
Treatment focusing on patients with optimal EAA levels (0.6-0.89) is essential for maximizing therapeutic benefits. Blind treatment Gram-negative sepsis has limited success: a more tailored approach based on specific pathogens is needed. Lack of clear guidelines for AN69 oXiris filter replacement frequency. Findings raise questions about the potential impact of AN69 oXiris therapy in pediatric patients. |
| Hemofiltration with a Non-Specific Adsorption Membrane for Cytokines (AN69 -ST, PMMA, ...) | |||
| AN69 -ST | Designed with the goal of enhanced
biocompatibility and reduced immune system activation compared to
the original AN69 polymer. The surface treatment process aims to minimize the adsorption of blood components. Aims to decrease the activation of inflammatory pathways. |
Studies are relatively limited, except for
specific targets like HMGB-1. The AN69-ST membrane showed rapid decrease in circulation and did not exhibit saturation when repeatedly exposed to HMGB-1 during hemofiltration. |
Lack of clear indications for the removal
of pro- or anti-inflammatory mediators. The optimal cut-off point for the elimination of mediators remains unknown, resulting in the presence of mediators that surpass the defined threshold and remain unremoved. The timing of initiating non-specific adsorption therapy has yet to be determined. The lack of large-scale RCTs makes it difficult to assess the overall efficacy of these techniques. |
| PMMA | Porous structure with larger and longer pores and a highly specific surface area dedicated to trapping high molecular weight substances. | In comparative study between PMMA-CVVHDF
treatment and standard polyacrylonitrile hemofilters in septic shock
patients with AKI: improved urine output and hemodynamic stability.
Superior efficacy of PMMA in removing several cytokines, reducing systemic inflammation, and improving hemodynamic stability and renal function in critically ill patients. |
|
| Hemofiltration with a Specific Sorbent for Endotoxins (Polymyxin B) | Polymyxin B, incorporated into a hemofilter, effectively binds and eliminates endotoxins due to its high affinity for them. | The EUPHRATES involving critically ill
adults with septic shock and high endotoxin activity (EAA levels
≥ 0.60) concluded that polymyxin B hemoperfusion, in addition
to conventional medical therapy, did not reduce 28-day mortality
compared to the placebo treatment. Trial revealed a subgroup of patients with extremely high endotoxin activity that exceeded the cartridge's capacity suggesting potential benefit in patients with non-extreme endotoxin activity. |
Restrict the patient population to those
with endotoxin levels between 0.6 and 0.9. Appropriate assay for endotoxin detection is crucial, with the EAA demonstrating superiority over the LAL assay. Benefits of therapy in patients with endotoxin levels above 0.6 and sterile cultures, suggesting a role for gut translocation in endotoxin release: rationale to focus on this specific patient group. |
| Hemofiltration with a Non-Specific Sorbent for Mediators | |||
| CytoSorb | Made of biocompatible porous polymer beads designed to adsorb various hydrophobic, lipophilic, and hydrophilic substances. | In study utilizing standardized human in
vivo model of systemic inflammation and immunological tolerance
induced by administering bacterial lipopolysaccharide revealed that
CytoSorb hemoperfusion effectively reduces circulating cytokine
concentrations during systemic inflammation in humans without
compromising long-term immune function. CytoSorb therapy may hold potential in conditions characterized by excessive cytokine release, providing a valuable approach for managing such conditions. |
Non-specific removal mechanism raises
concerns about its selectivity and targeted action. The optimal timing for administering the therapy remains uncertain, emphasizing the need for establishing appropriate treatment windows. The frequency of cartridge changes lacks clear guidelines. Identifying the appropriate patient population for treatment is a challenge, with suggested indicators such as IL-6 levels above 5,000 p g/ml or procalcitonin levels above 3 ng/ml. The optimal number of cartridges needed for effective treatment is yet to be determined. The absence of mortality data from RCTs presents a gap in understanding the therapy's impact on patient outcomes, necessitating further mechanistic studies. |
| Jafron Cartridge | Similar to CytoSorb, consisting of a specially designed cartridge containing adsorptive materials with high affinity for cytokines and capable of capturing and removing them from the bloodstream. | An in vitro study
comparing CytoSorb and Jafron HA 380 performances in removing
interleukins-6 and 10, tumor necrosis factor-α, and monocyte
chemoattractant protein-1 from blood. Both devices demonstrated the ability to remove cytokines from blood in a benchtop model. However, CytoSorb exhibited significantly greater efficiency, accomplishing the majority of cytokine removal within the first 120 minutes. |
Limited literature and research available
on the use of the Jafron Cartridge, particularly in sepsis and its
mechanisms of action. Key questions include: - Specificity of the removal - Timing and duration of treatment - Determination of patient population - Number of cartridges to be used |
| New polymyxin B device (KCEA) | Functions by adsorbing and neutralizing endotoxins, aiming to mitigate their harmful effects i n septic conditions. | KCEA hemoadsorption effectively detoxified circulatory endotoxin and inflammatory mediators leading to a reduction in mortality rate observed in septic beagles. | Further research and clinical studies are needed to validate the efficacy and safety of the KCEA device in human sepsis cases and explore its potential as a therapeutic intervention in a clinical setting. |
| Hemofiltration with a Non-Specific Sorbent for Bacteria and Viruses | |||
| Seraph-100 | Novel sorbent with the capacity to capture both bacteria and viruses, including SARS-CoV-2. | RCT examining use of Seraph-100 in
COVID-19 patients showed a lower mortality rate in patients treated
with Seraph-100 than in the control group (32.1% vs.
64.2%). This difference in mortality rate remained significant even after adjusting for confounding factors. Among the 86 treated patients in the overall cohort, only 3 experienced serious adverse events out of 177 total treatments. While the study did not consistently demonstrate significant clinical benefits in all endpoints and comparisons, it suggests that using broad-spectrum, pathogen-agnostic blood purification, like Seraph-100, can be safely employed as a response to emerging pathogen threats while awaiting targeted therapies and vaccines. |
Estimated number and mass of SARS-CoV-2
virions in infected individuals during peak infection exceed the
capacity of a single Seraph-100 filter. Rapid production of SARS-CoV-2 during the resident extracellular time surpasses the filter's capacity, raising doubts about the practicality and efficiency of the therapy. The timing of therapy and stages of SARS-CoV-2 infection are crucial. Future randomized controlled trials focusing on patients in early stages of infection (stage I and IIA) with high viral loads would provide better insights into the effects of Seraph-100 therapy. |
| Garnet Hemofilter | The inner surface of polysulfone fibers is
coated with an engineered protein possessing pathogen-binding
capabilities known as MBL. MBL is a component of the human innate immune system that recognizes and binds to carbohydrate patterns on the surface of pathogens including bacteria, viruses, fungi, and parasites. Incorporates genetically engineered recombinant MBL fused with the Fc portion of a human immunoglobulin (FcMBL). FcMBL allows for direct binding of pathogens and PAMP. Specifically targets mannose, which is universally present on the surface of all pathogens, effectively capturing and removing a wide range of pathogens from the blood during hemofiltration. |
The effectiveness is still under
evaluation, and its limitations have not been fully determined.
A prospective single-arm, multi-center human study is currently underway to assess the safety and feasibility of hemodialysis with the Garnet device in chronic hemodialysis patients with bloodstream infections. The results are not yet available. |
|
| Hemopurifier | Specialized lectin affinity plasmapheresis
device designed to target viruses, including enveloped viruses like
coronaviruses and filoviruses. Uses a lectin protein derived from the common snowdrop, which has a strong affinity for GPs found on the surface of viruses. Also bind soluble GPs shed from virus-infected cells, further enhancing its effectiveness. |
Has shown promise in the treatment of
critically ill patients, including those with severe Ebola virus
disease. In combination with dialysis, it has demonstrated a significant reduction in hepatitis C virus viral l oad (57% in 1 week). |
A clinical trial is expected to begin
recruiting patients to evaluate the therapy's efficacy in
COVID-19 treatment. However, there is a concern regarding the timeliness of patient recruitment, which may impact the availability of sufficient data for making conclusive statements. The early stage of the trial calls for cautious interpretation of the results. |
EAA: endotoxin activity assay; LAL: limulus amoebocyte lysate; HMGB-1: high-mobility group-1 protein; PMMA: polymythylmetacryllate; CVVHDF: continuous veno-venous hemodiafiltration; AKI: acute kidney injury; MBL: mannose-binding lectin; PAMP: pathogen-associated molecular patterns; GP: glycoproteins; TNF: tumor necrosis factor; IL: interleukin.
HEMOFILTRATION WITH A SPECIFIC ADSORPTIVE MEMBRANE FOR ENDOTOXINS (AN69 OXIRIS®)
AN69 oXiris® is a type of hemofilter that combines two key functionalities: it acts as both a hemofilter for removing toxins and as an adsorber for removing cytokines from the blood. The AN69 oXiris® filter is made from a unique synthetic polymer material called AN69, which has high biocompatibility and excellent adsorption properties14 (Fig. 1).
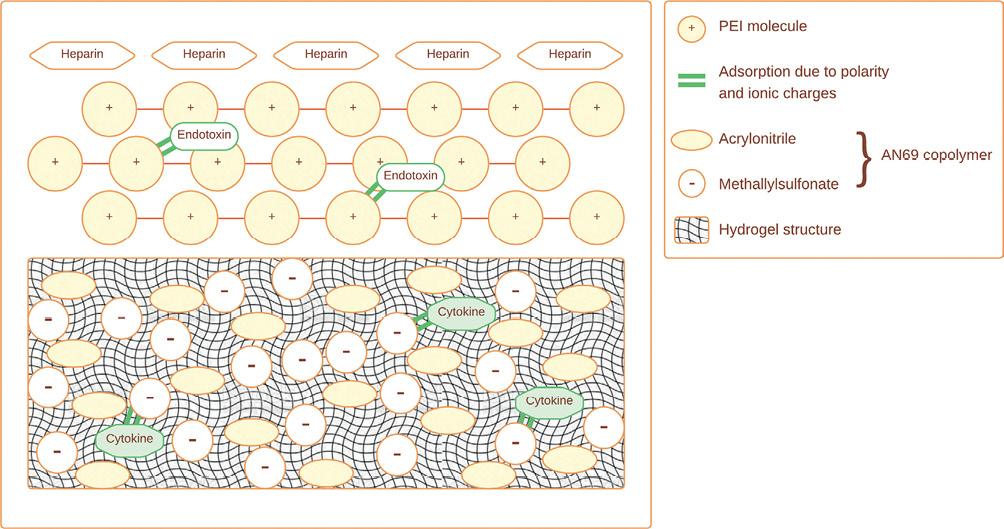
Figure 1. AN69 oXiris, hemofiltration with a Specific Adsorptive Membrane for Endotoxins. Heparin grafting reduces local thrombogenicity. Layers of polyethyleneimine improve biocompatibility and adsorb endotoxins. AN69 copolymer adsorbs cytokines. The hydrogel structure removes solute of up to 40 kDa through convection through membrane pores (modified and adapted from Monard et al., 2019)14.
A recent study conducted by Broman et al. investigated the removal of endotoxins using AN69 oXiris® in a randomized crossover double-blind study involving 16 patients. This study is the largest one to date that focuses on AN69 oXiris® and endotoxin removal. However, it is important to note that the study utilized the whole-blood limulus amebocyte lysate (LAL) assay15. Comparing the LAL assay to the endotoxin activity assay (EAA), it has been found that patients with Gram-negative infections showed higher endotoxin levels (> 50 pg/ml) when measured using the EAA compared to the LAL assay16. The use of LAL assay to detect and quantify endotoxin has been problematic, due to its variability in the prevalence of endotoxemia when comparing Gram-negative and non-Gram-negative infections16. When measured using EAA, Gram-negative infections had significantly higher mean endotoxin levels than infections with non-Gram-negative bacteria (p < 0.05, n = 10), while the LAL assay detected no difference between the same two samples16. In addition, circulating inhibitors of the limulus reaction have been described with published reports showing considerable variability in the prevalence of endotoxemia or its association with Gram-negative infection17-21. Therefore, it is strongly recommended to abandon the LAL assay in favor of the more reliable EAA assay. Regarding the results of the study done by Broman et al., endotoxin levels decreased during treatment in a higher percentage of the patients in the oXiris® filter group (7 of the 9; 77,8%) compared to the standard filter group (1 of the 6; 16.7%) (p = 0.02)14. Nonetheless, the authors state that their upgraded LAL assay was the best method to detect endotoxin in the blood22. In addition, AN69 oXiris® demonstrated the ability to reduce lactate and norepinephrine, as well as inflammatory mediators such as tumor necrosis factor-α, interleukin (IL)-6, IL-8 and IFNγ, compared to standard filters15. However, it is important to note that the study did not evaluate mortality and clinical outcomes14. Another recent observational study focusing on patients with septic shock showed improved survival in the group treated with AN69 oXiris® (n = 46) compared to standard therapy (n = 30)23. The 28-day mortality in the treatment group was lower than in the control group (47.3% vs. 73.3%; p < 0.001)23. A meta-analysis done by Wang et al. supports these results, with a significant reduction in 28-day mortality, as well as length of ICU stay in patients treated with the oXiris filter compared to other filters24. Secondary outcomes such as norepinephrine dose, IL-6 and lactate levels, and 7- and 14- day mortalities were lower in the oXiris group, whereas 90-day mortality, ICU and hospital mortality, and length of hospital stay were comparable24.
In a pediatric study, 11 patients ranging from 2 to 15 years old and weighing between 11 and 60 kg, diagnosed with vasoplegic shock and acute kidney injury (AKI) were treated using AN69 oXiris® therapy25. Among these patients, seven were treated for septic shock and six for liver failure, receiving a total of 13 oXiris® therapy sessions. This study demonstrated mixed results, with five patients showing improvement in their condition, while eight patients did not, resulting in a survival rate of 37.5%25. Among the former, their inotropic support decreased by 50% within 24 h; but only four of them ultimately survived25. These findings raise questions about the potential impact of AN69 oXiris® therapy in pediatric patients.
The success of AN69 oXiris® hinges on accurate patient selection, specifically targeting those with significant levels of endotoxins (in Broman's Study: > 0.03 EU/ml)15. Further research, including RCT, is necessary to evaluate the impact and effectiveness of AN69 oXiris® therapy, especially in pediatric populations.
CHALLENGES IN AN69 OXIRIS® THERAPY: WHY HAS IT FAILED?
The efficacy of AN69 oXiris® therapy has been hindered by several key challenges. First, choosing the appropriate assay is crucial, with the EAA proving superior to the LAL assay16. Second, focusing treatment on patients with optimal EAA levels (0.6-0.89) is essential for maximizing therapeutic benefits26. Blindly treating Gram-negative sepsis has shown limited success, necessitating a more tailored approach based on specific pathogens. In addition, the lack of clear guidelines for AN69 oXiris® filter replacement frequency poses a challenge; only one randomized cross-over study exists on this topic, but its limited statistical power prohibits conclusive assessment of mortality outcomes15. Further research and consensus are needed to determine the ideal intervals for filter replacement, ensuring maximum therapeutic benefit. Addressing these challenges through refined patient selection, improved endotoxin assessment, tailored treatment strategies, and evidence-based guidelines for filter replacement will enhance the efficacy of AN69 oXiris® therapy and improve patient outcomes.
HEMOFILTRATION WITH A NON-SPECIFIC ADSORPTION MEMBRANE FOR CYTOKINES (AN69 -ST, PMMA…..)
AN69-ST is designed to have enhanced biocompatibility and reduced activation of the immune system compared to the original AN69 polymer. The surface treatment process in AN69-ST aims to minimize the adsorption of blood components and decrease the activation of inflammatory pathways.
Studies using AN69-ST are relatively limited, except when targeting specific substances like High-Mobility Group 1 proteins (HMGB-1)27. Notably, the AN69-ST membrane did not exhibit saturation and rapidly decreased circulation when repeatedly exposed to HMGB-1 (approximately 30,000 Da) during hemofiltration27.
In contrast, PMMA membranes have a porous structure that offer distinct advantages due to their larger and longer pores, as well as a highly specific surface area dedicated, almost exclusively, to trapping high molecular weight substances (Fig. 2)28. In a recent investigation, Matsuda et al. compared PMMA-CVVHDF treatment to standard polyacrylonitrile hemofilters in 43 septic shock patients with AKI29. Following 24 h of treatment, the study demonstrated improved urine output, and hemodynamic stability29. Similarly, a study by Sakamoto et al. demonstrated the superior efficacy of PMMA in removing several cytokines, reducing systemic inflammation, and improving hemodynamic stability and renal function in critically ill patients30,31. These two groundbreaking findings make PMMA membranes make them a game-changer in blood-purification techniques.
Figure 2. Polymythylmetacryllate, hemofiltration with a non-specific adsorption membrane for cytokines. Hollow fiber membrane with uniform internal structure and nanopores contributing to adsorption of molecules between 10 kDa and 30 kDa (modified and adapted from Kishikawa et al., 2022)28.
CHALLENGES IN THE EFFICACY OF NON-SPECIFIC ADSORPTION TECHNIQUES
The effectiveness of non-specific adsorption techniques in hemofiltration has encountered persistent challenges. One major limitation lies in the lack of clear indications for the removal of pro- or anti-inflammatory mediators. The optimal cut-off point for the elimination of mediators remains unknown, leading to the presence of many mediators that surpass the defined threshold and remain unremoved27. Another unresolved issue is the timing of initiating non-specific adsorption therapy. The appropriate moment to commence treatment has yet to be determined, leaving a critical question unanswered. Additionally, the lack of large-scale RCTs makes it difficult to assess the overall efficacy of these techniques. To address these challenges, it is suggested to focus on patients with interleukin-6 levels exceeding 5,000 pcg/ml or those in septic shock as a preliminary threshold for inclusion. By establishing this hypothetical threshold, further investigation and refinement of patient selection criteria can be pursued.
HEMOFILTRATION WITH A SPECIFIC SORBENT FOR ENDOTOXINS (POLYMYXIN B)
Polymyxin B, incorporated into a hemofilter, has a high affinity for endotoxins making it an effective binding and eliminating agent32 (Fig. 3). As stated earlier, measuring endotoxin and interleukin-6 in the bloodstream is of upmost importance. It is imperative to transition from less reliable assays, such as LAL assays, and instead prioritize the use of the more accurate and dependable EAA14,15. Thus, as of 2023, to adopting the EAA as the standard assay for endotoxin measurement is becoming an urgency14,15.
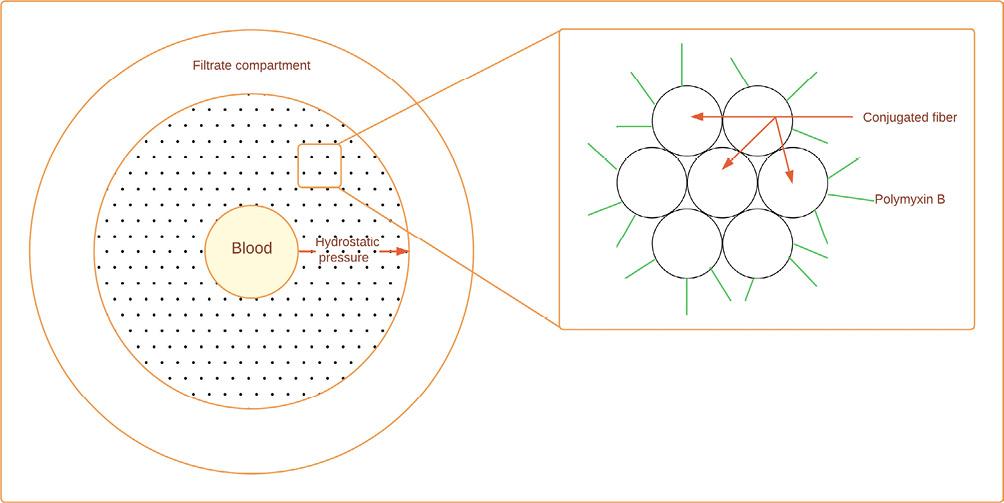
Figure 3. Polymyxin B, hemofiltration with a specific sorbent for endotoxins. Polymyxin B is a polycationic anti-biotic which binds the lipid A portion of endotoxin and neutralizes its toxicity. Due to nephrotoxicity and neurotoxicity, polymyxin B is covalently immobilized as a ligand on the surface of a fibrous material. This fibrous adsorbent is composed of conjugated fibers derived from polystyrene (modified and adapted from Shoji et al., 2021)32.
Furthermore, noteworthy studies have investigated hemoperfusion with polymyxin B, with the study evaluating the use of polymyxin B hemoperfusion in a RCT of Adults Treated for Endotoxemia and Septic Shock (EUPHRATES) standing out as a prominent example33. This multicenter, randomized clinical trial involved 450 critically ill adults with septic shock and high endotoxin activity (EAA levels ≥ 0.60) across 55 tertiary hospitals in North America33. The study concluded that polymyxin B hemoperfusion, in addition to conventional medical therapy, did not reduce 28-day mortality amongst these patients compared to the sham treatment32.
In the assessment of polymyxin B hemoperfusion as a therapeutic intervention for septic shock, analyzing endotoxin activity has released valuable insights. The authors of the EUPHRATES trial identified a subgroup of patients with extremely high endotoxin activity (EAA ≥ 0.9), which exceeded the capacity of the polymyxin B hemoperfusion cartridge used in this trial33. This suggests a possible benefit of polymyxin B hemoperfusion in patients with non-extreme endotoxin activity. A subsequent post hoc analysis focused on patients with septic shock and EAA levels between 0.6 and 0.89 to investigate the impact of polymyxin B use in this specific subgroup25. An additional, ongoing post hoc analysis, the TIGRIS study, aims to replicate the EUPHRATES trial while modifying the eligibility criteria to include patients with EAA levels of 0.6-0.89, thus expanding the available data33. The TIGRIS study shows promise in further enhancing our understanding of polymyxin B hemoperfusion therapy34.
CHALLENGES IN POLYMYXIN B THERAPY
The use of polymyxin B therapy in septic shock has faced challenges, despite a compelling rationale focused on targeting endotoxins. One crucial factor is the need to restrict the patient population to those with endotoxin levels between 0.6 and 0.9, as highlighted by previous research26. In addition, selecting the appropriate assay for endotoxin detection is essential, with the EAA showing superiority over the LAL assay16. The EUPHRATES study provided insights into the benefits of polymyxin B therapy, particularly in patients with endotoxin levels above 0.6 and sterile cultures, indicating that gut translocation plays a role in endotoxin release33. Considering these findings, there is a rationale to focus on this specific patient group. The TIGRIS study holds great promise in shedding further light on the potential of polymyxin B therapy and may provide us with the best chance of success in addressing these challenges.
HEMOFILTRATION WITH A NON-SPECIFIC SORBENT FOR MEDIATORS
CytoSorb®
CytoSorb® is a device made from biocompatible porous polymer beads that are designed to adsorb various hydrophobic, lipophilic, and hydrophylic substances (Fig. 4)35. In a recent study, Cytosorb® was investigated for its effects on plasma cytokine levels35. The study employed the repeated human experimental endotoxemia model, a standardized human in vivo model of systemic inflammation and immunological tolerance induced by administering bacterial lipopolysaccharide, to assess the impact of Cytosorb® hemoperfusion35. The findings of the study revealed that CytoSorb® hemoperfusion effectively attenuates circulating cytokine concentrations during systemic inflammation in humans, without compromising long-term immune function35. A recent meta-analysis by Becker et al. Compared mortality in patients treated with Cytosorb®; they divided the patients into three subgroups: sepsis, cardiopulmonary bypass surgery, other severe illness, SARS-CoV-2 infection, and recovery from cardiac arrest36. This meta-analysis revealed that Cytosorb® intervention did not lower mortality; and in patients with cardiac arrest, there was even a significant survival advantage if patients were untreated. There were also no differences in ICU length of stay, lactate levels, or IL-6 levels after treatment36. Consequently, the use of CytoSorb® therapy may hold potential in conditions characterized by excessive cytokine release, providing a valuable approach for managing such conditions35.
CHALLENGES IN THE EFFICACY OF CYTOSORB®
The efficacy of this therapy has encountered persistent challenges and limitations that contribute to its current shortcomings. First, the therapy's non-specific removal mechanism raises concerns regarding its selectivity and targeted action34. In addition, the optimal timing for administering the therapy remains uncertain, emphasizing the need to establish appropriate treatment windows for maximum efficacy34. Furthermore, the frequency of cartridge changes, whether every 6, 8, 12, or 24 h, lacks clear guidelines and requires further investigation36. Identifying the appropriate patient population for treatment presents another hurdle, with IL-6 levels above 5,000 pg/ml or procalcitonin levels above 3 ng/ml suggested as potential indicators37. The optimal number of cartridges needed for effective treatment is yet to be determined, highlighting the need for additional research in this area34. Finally, the absence of mortality data from RCTs presents a gap in understanding the therapy's impact on patient outcomes, necessitating further mechanistic studies before launching an RCT34.
Jafron Cartridge
The Jafron Cartridge is a blood purification system similar to CytoSorb®. It consists of a specially designed cartridge that contains adsorptive materials, such as resins or membranes, with high affinity for cytokines capable of capturing and removing them from the bloodstream (Fig. 5). The Jafron Cartridge has recently undergone investigation to assess its efficacy in cytokine removal38. In an in vitro study, researchers compared the performance of two blood purification systems, CytoSorb® and Jafron HA 380, in removing interleukins-6 and 10, tumor necrosis factor-α, and monocyte chemoattractant protein-1 from blood38. Both devices demonstrated the ability to remove cytokines from blood in a benchtop model39. However, the CytoSorb® 300 device exhibited significantly greater efficiency, accomplishing the majority of cytokine removal within the first 120 min38. These intriguing findings shed light on the comparative efficacy of the CytoSorb® and Jafron Cartridge systems in cytokine removal, suggesting the potential superiority of CytoSorb® in terms of efficiency.
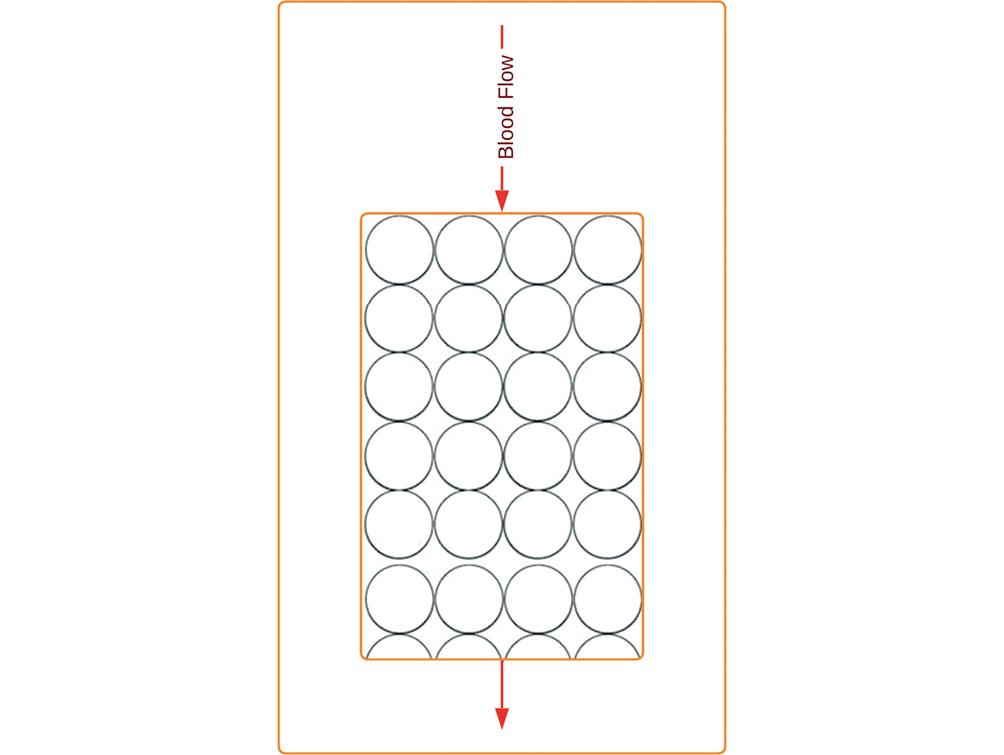
Figure 5. Jafron cartridge, hemofiltration with a non-specific sorbent for mediators. Cartridge containing biocompatible sorbents and neutro-macroporous resin made of styrene-divinylbenzene copolymer with high affinity for cytokines and capable of capturing and removing them from the bloodstream. The adsorbing beads' pore size ranges from 500 Da to 60 kDa, giving them the ability to absorb various medium-sized factors, including interleukins-6 and 10, tumor necrosis factor-α, and monocyte chemoattractant protein-1.
CHALLENGES REGARDING THE JAFRON CARTRIDGE
Unlike Cytosorb®, there is limited literature and research available, specifically regarding its use in sepsis and mechanisms of action. However, similarly to Cytosorb®, there are still numerous unanswered questions regarding the effectiveness and optimal utilization of the Jafron cartridge in sepsis therapy. These questions include issues such as the specificity of the removal, the timing and duration of treatment, the determination of the patient population that would benefit the most, and the number of cartridges to be used. Without comprehensive mechanistic studies and a sufficient body of literature, the understanding of how the Jafron cartridge performs in sepsis treatment remains limited.
NEW POLYMYXIN B DEVICE (KCEA)
In a recent study, the authors aimed to investigate the safety and efficacy of direct hemoperfusion using a newly developed polymyxin B-immobilized resin column, known as the disposable endotoxin adsorber (KCEA), in a sepsis model induced by endotoxins/lipopolysaccharides39. The KCEA functions by directly adsorbing and neutralizing endotoxins, thereby mitigating their harmful effects in septic conditions.
The study findings demonstrated that KCEA hemoadsorption effectively detoxified circulatory endotoxin and inflammatory mediators, leading to a notable reduction in the mortality rate among septic beagles39. This suggests that the KCEA device holds promise as a valuable tool for sepsis management, offering the potential to improve patient outcomes by targeting the underlying endotoxin-mediated inflammatory response. Further research and clinical studies are warranted to validate the efficacy and safety of the KCEA device in human sepsis cases and to explore its potential as a therapeutic intervention in a clinical setting.
NEW POLYMYXIN B DEVICE (KCEA): INSUFFICIENT DATA
Indeed, insufficient data to evaluate the challenges.
HEMOFILTRATION WITH A NON-SPECIFIC SORBENT FOR BACTERIA AND VIRUSES
Seraph® 100
The Seraph® 100, a novel sorbent (Fig. 6), has demonstrated the ability to capture both bacteria and viruses, including SARS-CoV-240-42. In a RCT conducted by Chitty et al., COVID-19 patients treated with Seraph® 100 (n = 53) showed a lower mortality rate compared to the control group (n = 53) (32.1% vs. 64.2%; p = 0.001)43. This difference remained significant even after adjustment for confounding factors43. While a post hoc analysis utilizing an external control group did not show a significant difference in mortality, in the overall cohort of 86 treated patients, only three experienced serious adverse events in the 177 total treatments43. Although the study did not consistently demonstrate significant clinical benefits in all endpoints and comparisons, the results suggest that the use of broad-spectrum, pathogen-agnostic blood purification, such as Seraph® 100, can be safely employed as a response to emerging pathogen threats while awaiting targeted therapies and vaccines22.
CHALLENGES IN THE USE OF SERAPH® 100
The failure of Seraph® 100 therapy in effectively combatting SARS-CoV-2 infection can be attributed to several factors. Researchers estimated the total number and mass of SARS-CoV-2 virions in body fluids and host tissues in an infected person at 109-1011 virions during the peak of infection, with a total mass ranging from 1 μg to 100 μg44. This amounts to roughly 100 billion virions, corresponding to the capacity of a single Seraph® 100 filter44,45. However, this calculation does not take into account the regeneration of the virus. Based on the estimated "resident extracellular time" of 8 h and the peak RNA concentration occurring at approximately 2.7 days, it is projected that SARS-CoV-2 production during this period would be approximately 30 times higher than the observed peak and the capacity of a single Seraph® 100 filter46. The quantity of filters required to clear a patient of SARS-CoV-2 over few days raises doubts about the practicality and efficiency of the therapy47.
Furthermore, the timing of therapy and the stages of SARS-CoV-2 infection play a crucial role. The infection progresses through three distinct phases (Fig. 7) starting with the asymptomatic viral replication in the lungs (stage I), followed by pulmonary involvement and potential hypoxemia (stage II), and ultimately systemic inflammation with severe hypoxemia (stage III)48,49. During the initial phase, patients who are paucisymptomatic but test positive for the virus exhibit specific biological changes, including lymphopenia, elevated D-dimer concentrations, and increased prothrombin time. Notably, C-reactive protein and IL-6 levels remain unaltered, and chest X-rays appear normal49. Moving to stage IIA, patients do not experience hypoxemia, but pulmonary infiltrates become visible on chest X-rays, indicating progressive lung involvement. As the infection progresses to stage IIB, patients develop hypoxemia and require hospitalization, although viremia levels tend to be relatively low49. In stage III, the majority of patients necessitate intensive care unit admission. These individuals face critical conditions that warrant close monitoring and advanced medical interventions49 (Fig. 7).
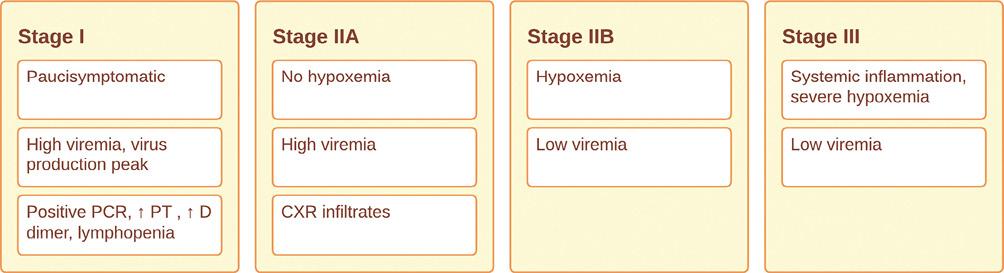
PCR: polymerase chain reaction; PT: prothrombin time; CXR: chest X-ray; RCT: randomized controlled trial; SOFA: sequential organ failure assessment.
Figure 7. Evolution of the four stages of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and proposed ideal timing for Seraph 100 treatment.
The study by Chitty et al., which evaluated the use of Seraph® 100, primarily included patients in stages IIB and III, characterized by low viremia43. As a result, the lack of clinical improvement observed in the study may be attributed to the stage of infection at which the therapy was administered. To better understand the effects of Seraph-100, future RCTs focusing on patients in the early stages of infection (stage I and IIA) with high viral loads would be beneficial50.
GARNETTM Filter
The GARNETTM Filter utilizes an innovative approach by coating the inner surface of its polysulfone fibers with an engineered protein that possesses pathogen-binding capabilities22. This protein, known as mannose-binding lectin (MBL), is a crucial component of the human innate immune system51. MBL has the ability to recognize and bind to various carbohydrate patterns present on the surface of different pathogens, including bacteria, viruses, fungi, and parasites51.
The GARNETTM Filter takes advantage of this innate immune mechanism by incorporating a genetically engineered recombinant mannose-binding lectin, which is fused with the Fc portion of a human immunoglobulin51. This fusion protein allows for direct binding of pathogens and various pathogen-associated molecular patterns. By specifically targeting mannose, which is universally present on the surface of all pathogens, the GARNETTM Filter effectively captures and removes a wide range of pathogens from the blood during hemofiltration51,52.
CHALLENGES REGARDING THE GARNETTM Filter
The effectiveness of the GARNETTM Filter in therapy is still being evaluated, and its potential limitations have not yet been fully determined. A prospective single-arm, multicenter, human study has been initiated to assess the safety and feasibility of hemodialysis with the GARNETTM Filter in chronic hemodialysis patients with bloodstream infections (NCT 04658017). It is important to note that the results of this ongoing study are not yet available, and it is premature to draw definitive conclusions regarding the success or failure of this therapy.
The Hemopurifier®
The Hemopurifier® by Aethlon Medical is a specialized lectin affinity plasmapheresis device53 (Fig. 8) that specifically targets viruses, including enveloped viruses such as coronaviruses and filoviruses. This device utilizes a lectin protein derived from the common snowdrop, which exhibits a strong affinity for glycoproteins (GPs) found on the surface of viruses54,55. In addition, it can bind soluble GPs shed from virus-infected cells, further enhancing its effectiveness55. The Hemopurifier® has shown promise in the treatment of critically ill patients, including those with severe Ebola virus disease54. Furthermore, in combination with dialysis, it has demonstrated a significant reduction in hepatitis C virus viral load (57% in 1 week)56. These findings highlight the potential of the Hemopurifier® in managing virus-related diseases and underscore its role as a valuable therapeutic tool.
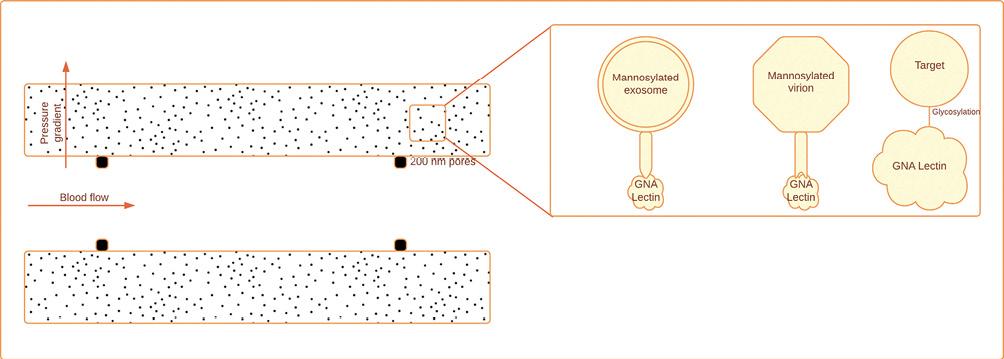
Figure 8. Hemopurifier, hemofiltration with a non-specific sorbent for bacteria and viruses. Plasma separation and size exclusion through pores of 200 nm. Galanthus nivalis (GNA) lectin binds with mannosylated exosomes. GNA binds with mannosylated virions via spike mannosylations. GNA lectin binds to high mannose glycans with alpha 1-3 linkage (modified and adapted from Amundson et al. 2021)53.
CHALLENGES IN THE USE OF THE HEMOPURIFIER®
Amidst the ongoing COVID-19 pandemic, the Hemopurifier® therapy holds promise as a potential option for managing the disease. A clinical trial (NCT04595903) is anticipated to begin recruiting patients soon, aiming to evaluate the therapy's efficacy. However, there is a concern regarding the timeliness of patient recruitment, which may hinder the availability of sufficient data for conclusive statements. The early stage of the trial necessitates cautious interpretation.
CONCLUSIONS
The extensive research and innovation invested in blood purification techniques as an adjunctive treatment for septic shock demonstrate remarkable advancements in the field. Despite these efforts, the clinical outcomes have remained largely unsatisfactory. Several factors contribute to this limited success. A crucial aspect is the need for better patient selection by employing bedside measurements of interleukin and endotoxin levels. The choice of assay is also pivotal, with the EAA proving superior to other forms of LAL assays for endotoxin detection. Timing poses a significant challenge, both in sepsis management and the administration of blood purification therapies, as incorrect timing may potentially harm patients. Mechanistic studies are lacking for most devices, leaving us to treat patients with limited understanding, except in the case of endotoxin removal. The complexity of viral multiplication, particularly evident in viruses like COVID-19, highlights the need for further exploration to thus enhance the effectiveness of these techniques. The identified failures associated with each device should be regarded as valuable insights to inspire improvements. Nonetheless, progress is being made in both clinical and experimental knowledge. While the journey may still be long, there is hope that with continued advancements, we will ultimately triumph in our fight against sepsis and viral infections.











 nueva página del texto (beta)
nueva página del texto (beta)