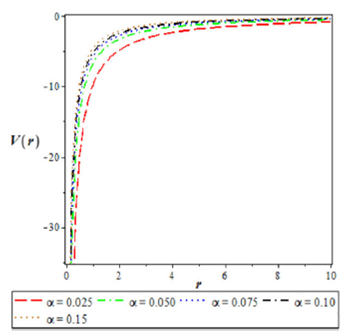
1.Introduction
In the earlier study of quantum mechanics, the Schrödinger equation was introduced as
a second-order differential equation capable of describing the properties of a
non-relativistic Quantum system1-2. Studies have revealed that exact solutions of the
Schrödinger equation are available only for a limited set of physical and chemical
quantum mechanical systems 3-4. Hence, with an approximation to the centrifugal term,
interest is geared towards arbitrary ℓ-solutions of the radial Schrödinger equation.
Arbitrary
The Hulthén potential 26 is one of the essential short-range potentials in physics. Its application to diverse areas of physics, including nuclear and particle physics, atomic physics, condensed matter physics, and chemical physics, has been of great interest in recent times 27-29. The study of this potential is essential in investigating the interaction existing between two particles 30. The superposition of Yukawa and Coulomb potential, otherwise known as Hellmann potential 31, has been extensively used by many authors to obtain the energy of the bound state in atomic, nuclear, and particle physics 32-41. The Hellmann potential is useful in the study of transitions of alkali metal molecules in condensed matter physics.
Recently, a lot of interest has been directed towards combining two or more potentials in relativistic and non-relativistic regimes 35. The essence of combining two or more physical potential models is to have a broader range of applications 36. Hence, in the present study, we attempt to study the radial Schrödinger equation with a newly proposed potential, obtained from a combination of Hulthén and Hellmann’s potential (HHP). The potential is of the form:
where r represents the inter-nuclear distance;
2.Review of Nikiforov-Uvarov method
In this section, we briefly introduce the NU method. The details can be found in the Nikiforov-Uvarov handbook 42. This method is useful to solve the second-order differential wave equation of the hypergeometric type:
where
leading to a hypergeometric-type equation
The first part of the wave functions
where
and λ in Eq. (4) is the parameter defined as
The
The second part of the wave functions in Eq. (3) is a hypergeometric-type function obtained by Rodrigues relation:
where Bn(s) is a constant related to normalization, and the weight function p(s) can be found by 42
We define the function
and
where
The discriminant under the square root sign in Eq. (10) must be set to zero and then has to be solved for k 42. Finally, by solving Eqs. (7) and (12), the energy eigenvalue equation can be obtained.
2.1.Arbitrary l-solution to the radial Schrödinger equation
To obtain the solution of the Schrödinger-like equation given in Eq. (2), we write the radial Schrödinger equation of the form 15,41,43
where we have defined μ as the reduced mass, Enl as the energy
spectrum,
Inserting the potential in Eq. (1) and applying the approximation scheme in Eq. (14), one can obtain
By using the change of variable from
We substitute Eq. (16) into Eq. (15) and after some simplifications, we have:
where
Comparing Eqs. (17) and (2), we obtain the relevant polynomials as:
Inserting the polynomials given by Eq. (19) into Eq. (11) gives the polynomial:
where
According to the NU method, the quadratic form under the square root sign of Eq.
(20) must be solved by setting the discriminant of this quadratic equation equal
to zero:
Since
using Eq. (22). Therefore, we obtain
where
where n is the number of nodes in the radial wavefunctions
We can obtain the other part of the wave function
Using Eq. (30), the Rodrigues relation in Eq. (9) is written as:
where Bn is the Jacobi polynomial; hence, the wave function in Eq. (3) becomes:
where N nl is the normalization constant. Using the normalization condition, we obtain the normalization constant as follows:
where
Comparing Eq. (33) with the standard integral of the form of Eq. (37) of 41,
Hence, we write the normalization constant as
3.Discussion
In Table I, we have reported some numerical
results of the energy (eV) levels of Hulthén-Hellmann potential for various values
of the potential strength: Λ1=0.025, Λ2=2, Λ3=1;
Λ1=0.050, Λ2 =2; Λ3= -1 and Λ1=0.15,
Λ2 =2, V2=-2 , respectively, as a function of the
screening parameter for
Table I Energy eigenvalues (eV) of the Hulthén-Hellmann potential with
| state | α | Λ1 = 0.025, Λ2 = 1, Λ3 = −1 | Λ1 = 0.050, Λ2 = 2, Λ3 = −2 | Λ1 = 0.015, Λ2 = 4, Λ3 = −4 |
| 1s | 0.025 | -2.237656250 | -8975156250 | -48.92515625 |
| 0.050 | -1.550625000 | -6.225625000 | -30.17562500 | |
| 0.075 | -1.350017362 | -5.420850695 | -2492640624 | |
| 0.10 | 1.255625000 | -5.040000000 | -22.49000000 | |
| 0.15 | -1.166736110 | -4.675069442 | -20.18062500 | |
| 2s | 0.025 | -0.5506250000 | -2.225625000 | -12.17562500 |
| 0.050 | -0.3806250000 | -1.540000000 | -7.49000000 | |
| 0.075 | -0.3334027780 | -1.341736111 | -4333402775 | |
| 0.10 | -0.3139062500 | -1.250625000 | -5.575625000 | |
| 0.15 | -0.3034027778 | -1.171111110 | -5.010000000 | |
| 2p | 0.025 | -0.5314062500 | -2.187656250 | -12.08765625 |
| 0.050 | -0.3475000000 | -1.475625000 | -7.350625000 | |
| 0.075 | -0.2854340280 | -1.250017360 | -5.988906248 | |
| 0.10 | -0.2501562500 | -1.130625000 | -5.330625000 | |
| 0.15 | -0.2052777777 | -0.9917361105 | -4.655625000 | |
| 3s | 0.025 | -0.2389062500 | -0.9764062500 | -5.370850695 |
| 0.050 | -0.1667361111 | -0.67506494445 | -3.291736110 | |
| 0.075 | -0.1513908180 | -0.5925945218 | -2.715434026 | |
| 0.10 | -0.1506250000 | -0.5600000000 | -2.454444445 | |
| 0.15 | -0.1685262345 | -0.5472299380 | -2.225625000 | |
| 3p | 0.025 | -0.2300173611 | -0.9591840278 | -5.331406250 |
| 0.050 | -0.1506250000 | -0.645069445 | -3.228402778 | |
| 0.075 | -0.1269463735 | -0.5487056328 | -2.627100694 | |
| 0.10 | -0.1167361111 | -0.5011111110 | -2.340000000 | |
| 0.15 | -0.1124151234 | -0.4550077159 | -2.055625000 | |
| 3d | 0.025 | -0.2126562500 | -0.9251562500 | -5.252934028 |
| 0.050 | -0.1200694444 | -0.5867361110 | -30103402778 | |
| 0.075 | -0.08180748460 | -0.4646778548 | -2.454184026 | |
| 0.10 | -0.05562500000 | -0.3900000000 | -2.117777778 | |
| 0.15 | -0.01519290124 | -0.2855632714 | -1.730625000 | |
| 4s | 0.025 | -0.13062500000 | -0.5400000000 | -2.990000000 |
| 0.050 | -0.09515625000 | -0.3756250000 | -1.825625000 | |
| 0.075 | -0.09506944448 | -0.3377777778 | -1.509999999 | |
| 0.10 | -0.1066015625 | -0.3314062500 | -1.375156250 | |
| 0.15 | -0.1508506944 | -0.3584027777 | -1.280625000 | |
| 4p | 0.025 | -0.1253515625 | -0.5300390625 | -2.967539062 |
| 0.050 | -0.08500000000 | -0.3576562500 | -1.788906250 | |
| 0.075 | -0.07885850696 | -0.3106293402 | -1.457851562 | |
| 0.10 | -0.08316406250 | -0.2939062500 | -1.306406250 | |
| 0.15 | -0.1094444445 | -0.2966840277 | -1.175156250 |
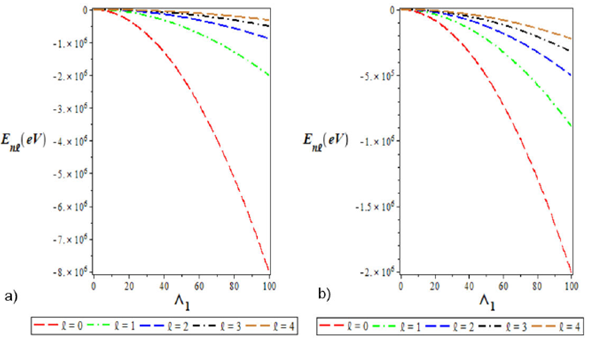
Figure 2 a). Variation of the ground state energy spectra for various l as a function of Λ1 . b). The plot of the first excited state energy spectra for different l as a function of Λ1. We choose Λ1 = 0:10, Λ2 = 4, Λ3 = -4, and a = 0:025 for the ground and excited states.
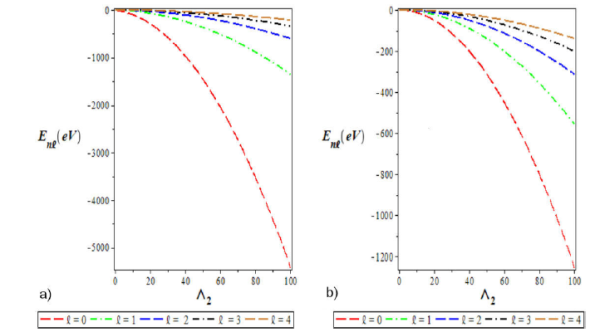
Figure 3 a) Variation of the ground state energy spectra for various l as a function of Λ2. b) A plot of the first excited state energy spectra for various l as a function of Λ2. We choose Λ1 = 0:10, Λ2 = 4, Λ3 = -4, and a = 0:025 for the ground and excited states.
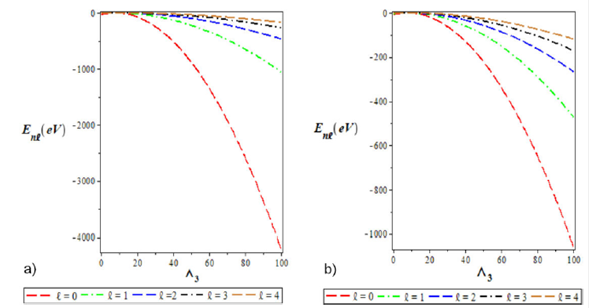
Figure 4 a) Variation of the ground state energy spectra for various l as a function of Λ3. b) The plot of the first excited state energy spectra for various l as a function of Λ3. We choose Λ1 = 0:10, Λ2 = 4, Λ3 = -4, and a = 0:025 for the ground and excited states.
In Fig. 5a) and 5b), we graphically show the variation of energy eigenvalues of HHP as a
function of the screening parameter. Here, the energy eigenvalue increases with a
small screening parameter (
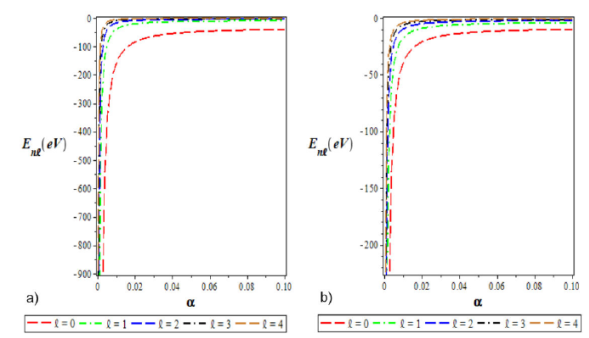
Figure 5 a) Variation of the ground state energy spectra for various l as a function of the screening parameter (a). b) A plot of the first excited state energy spectra for different l as a function of the screening parameter (a). We choose Λ1 = 0:10, Λ2 = 4, Λ3 = -4, and a = 0:025 for the ground and excited states.
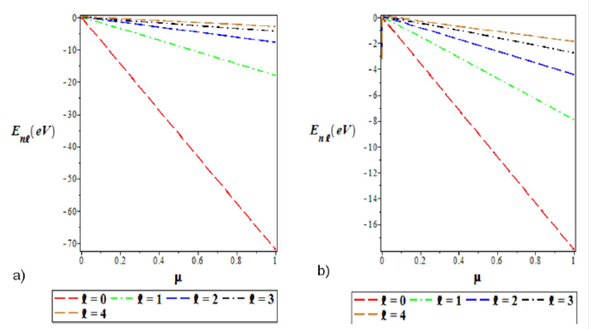
Figure 6 a) Variation of the ground state energy spectra for various l as a function of the screening parameter (μ). b) A plot of the first excited state energy spectra for different l as a function of the reduced mass (μ). We choose Λ1 = 0:10, Λ2 = 4, Λ3 = -4, and a = 0:025 for the ground and excited states.
4.Special cases
4.1.Hellmann potential
If we set the parameter Λ1 = 0, the Hulthén-Hellmann potential in Eq. (1) reduces to Hellmann potential of the form
hence, its corresponding energy eigenvalue is of the form
Equation (38) is in agreement with Eq. (39) of 15. In Table II and Table III, we present the numerical energy eigenvalues for Hellman potential for (Λ1 = 0, Λ2 = 2, Λ2 = 1) and (Λ1 = 0, Λ2 = 5, Λ3 = -1), respectively. The results showed good agreement with the earlier results of 19 with NU, AP, and SUSY methods of 45 and PT method of 46.
Table II Comparison of energy eigenvalues (eV) for a particular case of
Hellmann potential as a function of the screening parameter α with
| State | α | Present method | (SUSY) [45] | (NU) [19] | (AP) [19] | (PT) [46] |
| 1S | 0.001 | -0.2515002500 | -0.251500 | -0.251500 | -0.250969 | -0.250999 |
| 0.005 | -0.2575062500 | -0.257506 | -0.257506 | -0.254933 | -0.254963 | |
| 0.01 | -0.2650250000 | -0.265025 | -0.265025 | -0.259823 | -0.289852 | |
| 2S | 0.001 | -0.06400100000 | -0.064001 | -0.064001 | -0.063243 | -0.063494 |
| 0.005 | -0.07002500000 | -0.070025 | -0.070025 | -0.067106 | -0.067353 | |
| 0.01 | -0.07760000000 | -0.077600 | -0.077600 | -0.071689 | -0.071928 | |
| 2P | 0.001 | -0.06375025000 | -0.063750 | -0.064000 | -0.063495 | -0.063495 |
| 0.005 | -0.06875625000 | -0.068756 | -0.070000 | -0.067377 | -0.067377 | |
| 0.01 | -0.07502500000 | -0.075025 | -0.077500 | -0.072020 | -0.072020 | |
| 3S | 0.001 | -0.02928002778 | -0.029280 | -0.029280 | -0.028283 | -0.028764 |
| 0.005 | -0.03533402778 | -0.035334 | -0.035334 | -0.031993 | -0.032457 | |
| 0.01 | -0.04300277778 | -0.043003 | -0.043003 | -0.036142 | -0.036557 | |
| 3P | 0.001 | -0.02916802778 | -0.029169 | -0.029279 | -0.028765 | -0.028765 |
| 0.005 | -0.03475625000 | -0.034756 | -0.035309 | -0.032480 | -0.032480 | |
| 0.01 | -0.04180277778 | -0.041803 | -0.042903 | -0.036648 | -0.036644 | |
| 3d | 0.001 | -0.02894469445 | -0.028945 | -0.029388 | -0.028767 | -0.250833 |
| 0.005 | -0.03361736111 | -0.033617 | -0.035817 | -0.032526 | -0.254151 | |
| 0.01 | -0.03946944445 | -0.039469 | -0.043825 | -0.036813 | -0.258269 | |
| 4S | 0.001 | -0.01712900000 | -0.017129 | -0.029280 | -0.016130 | -0.016601 |
| 0.005 | -0.02322500000 | -0.023225 | -0.035334 | -0.019646 | -0.020077 | |
| 0.01 | -0.03102500000 | -0.031025 | -0.043003 | -0.023289 | -0.023551 | |
| 4p | 0.001 | -0.01706556250 | -0.017066 | -0.017128 | -0.016602 | -0.016602 |
| 0.005 | -0.02288906250 | -0.022889 | -0.023200 | -0.020100 | -0.020098 | |
| 0.01 | -0.03030625000 | -0.030306 | -0.030925 | -0.023711 | -0.023641 | |
| 4d | 0.001 | -0.01693906250 | -0.016939 | -0.017189 | -0.016604 | -0.016604 |
| 0.005 | -0.02222656250 | -0.022227 | -0.023464 | -0.020142 | -0.020142 | |
| 0.01 | -0.02890625000 | -0.028906 | -0.031356 | -0.023857 | -0.023814 | |
| 4f | 0.001 | -0.01675025000 | -0.016750 | -0.017311 | -0.016607 | -0.016607 |
| 0.005 | -0.02125625000 | -0.021257 | -0.024027 | -0.020206 | -0.020206 | |
| 0.01 | -0.02690000000 | -0.026900 | -0.032356 | -0.024072 | -0.024056 |
TableIII Comparison of energy eigenvalues (eV) for a particular case of
Hellmann potential as a function of the screening parameter α with
| State | α | Present method | (NU) [19] | (AP) [19] | (PT) [46] |
| 1S | 0.001 | -2.250500250 | -2.250500 | - 2.248981 | - 2.24900 |
| 0.005 | -2.252506250 | -2.252506 | - 2.244993 | - 2.24501 | |
| 0.01 | -2.255025000 | -2.255025 | - 2.240030 | - 2.24005 | |
| 2S | 0.001 | -0.5630010000 | - 0.563001 | - 0.561502 | - 0.561502 |
| 0.005 | -0.5650250000 | - 0.565025 | - 0.557549 | - 0.557550 | |
| 0.01 | -0.5676000000 | - 0.567600 | - 0.552697 | - 0.552697 | |
| 2P | 0.001 | -0.5622502500 | - 0.563000 | - 0.561502 | - 0.561502 |
| 0.005 | -0.5612562500 | - 0.565000 | - 0.557541 | - 0.557541 | |
| 0.01 | -0.5600250000 | - 0.567500 | - 0.552664 | -0.552664 | |
| 3S | 0.001 | -0.2505022500 | - 0.250502 | - 0.249004 | - 0.249004 |
| 0.005 | -0.2525562500 | - 0.252556 | -0245110 | - 0.245111 | |
| 0.01 | -0.2552250000 | - 0.255225 | -0.240435 | - 0.240435 | |
| 3p | 0.001 | -0.2501680278 | -0.250501 | - 0.249004 | - 0.249004 |
| 0.005 | -0.2508673611 | -0.252531 | -0.245102 | -0.245103 | |
| 0.01 | -0.2518027778 | -0.255125 | -0.240404 | -0:240404 | |
| 3d | 0.001 | -0.2495002500 | -0.250833 | -0.249003 | -0.249003 |
| 0.005 | -0.2475062500 | -0.254151 | -0.245086 | -0.245086 | |
| 0.01 | -0.2450250000 | -0.258269 | -0.240341 | -0.240341 | |
| 4S | 0.001 | -0.1411290000 | -0.141129 | -0.139633 | -0.139633 |
| 0.005 | -0.1432250000 | -0.143225 | -0.135819 | -0.135819 | |
| 0.01 | -0.1460250000 | -0.146025 | -0.131380 | -0.131381 | |
| 4p | 0.001 | -0.1409405625 | -0.141128 | -0.139632 | 0.139633 |
| 0.005 | -0.1422640625 | -0.143200 | -0.135811 | 0.135811 | |
| 0.01 | -0.1440562500 | -0.145925 | -0.131350 | -0.131351 | |
| 4d | 0.001 | -0.1405640625 | -0.141314 | -0.139632 | -0.139632 |
| 0.005 | -0.1403515625 | -0.144089 | -0.135795 | -0.135796 | |
| 0.01 | -0.1401562500 | -0.147606 | -0.131290 | -0.131290 | |
| 4f | 0.001 | -0.1400002500 | -0.141686 | -0.139631 | -0.139631 |
| 0.005 | -0.1375062500 | -0.145902 | -0.135772 | -0.135772 | |
| 0.01 | -0.1344000000 | -0.151106 | -0.131200 | -0.131200 |
4.2.Hulthén potential
If we set Λ2 = Λ3 = 0, the Hulthén-Hellmann potential in Eq. (1) reduces to Hulthén potential
where
Equation (40) is in agreement with Eq. (41) of 47, Eq. (32) of 48; Eq. (24) of 49; Eq. (28) of 32; Eq. (37) of 50 and Eq. (20) of 16. Table IV
is the numerical energy eigenvalues of Hulthén potential for 2p, 3p, 3d, and 4p
with
Table IV Comparison of energy eigenvalues (eV) of the Hulthén potential as
a function of the screening Parameters α for 2p, 3p, 3d, and 4p
states and for Z = 1 in atomic units (
| State | α | Present (NU) | AIM [43] | EQR [51] | SUSY [52] |
| 2p | 0.025 | -0.1128125000 | -0.1128125 | -0.1128125 | -0.1127605 |
| 0.050 | -0.1012500000 | -0.1012500 | -0.1012500 | -0.1010425 | |
| 0.075 | -0.09031249994 | -0.0903125 | -0.0903125 | -0.0898478 | |
| 0.10 | -0.08000000000 | -0.0800000 | -0.0800000 | -0.0791794 | |
| 0.15 | -0.06124999998 | -0.0612500 | -0.0612500 | -0.0594415 | |
| 3p | 0.025 | -0.04070312500 | -0.0437590 | -0.0437590 | -0.0437068 |
| 0.050 | -0.03336810000 | -0.0333681 | -0.0333681 | -0.0331632 | |
| 0.075 | -0.02438370000 | -0.0243837 | -0.0243837 | -0.0239331 | |
| 0.10 | -0.01680560000 | -0.0168056 | -0.0168056 | -0.0160326 | |
| 0.15 | -0.00586810000 | -0.0058681 | -0.0058681 | -0.0043599 | |
| 3d | 0.025 | -0.04360440000 | -0.0437587 | -0.0437587 | -0.0436030 |
| 0.050 | -0.03275080000 | -0.0333681 | -0.0333681 | -0.0327532 | |
| 0.075 | -0.02299480000 | -0.0243837 | -0.0243837 | -0.0230306 | |
| 0.10 | -0.01433640000 | -0.0162600 | -0.0162600 | -0.0144832 | |
| 0.15 | -0.00031240000 | -0.0058681 | -0.0058681 | -0.0132820 | |
| 4p | 0.025 | -0.01994860000 | -0.0200000 | -0.0200000 | -0.0199480 |
| 0.050 | -0.01104420000 | -0.0112500 | -0.0112500 | -0.0110430 | |
| 0.075 | -0.00453700000 | -0.0050000 | -0.0050000 | -0.0045385 | |
| 0.10 | -0.00042690000 | -0.0012500 | -0.0012500 | -0.0004434 |
4.3.Hulthén-Yukawa potential
If we set Λ2 = 0 the Hulthén-Hellmann potential in Eq. (1) reduces to Hulthén-Yukawa potential
Similarly, Eq. (27) reduces to the energy equation for the Hulthén-Yukawa potential
Equation (42) is in agreement with Eq. (24) of 43.
4.4.Hulthén-Coulomb potential
If we set Λ3 = 0, the Hulthén-Hellmann potential in Eq. (1) reduces to Hulthén-Coulomb potential
Equation (27) reduces to energy eigenvalues of Hulthén-Coulomb potential.
4.5.Yukawa Potential
When the potential strength of Hulthén and Coulomb potentials are equal to zero, i.e., Λ1 = Λ2 = 0 the Hulthén-Hellmann potential in Eq. (1) reduces to the Yukawa potential
with the corresponding energy eigenvalues obtained from Eq. (27) as
Equation (46) is in agreement with Eq. (34) of 16.
4.6.Coulomb potential
If we set Λ1 = Λ2 = a =0, the Hulthén-Hellmann potential in Eq. (1) reduces to the familiar Coulomb potential
and we write the energy eigenvalues as
where the principal quantum number, n
p
= (n + l + 1), and
5.Conclusion
In this work, we have studied the bound state solutions of the Schrödinger equation
for any arbitrary











 nueva página del texto (beta)
nueva página del texto (beta)