1. Introduction
Nanocomposite materials have been a major area of research interest owing to their distinct electrochemical properties that differ significantly from those of their constituent components [1]. Similarly, devices based on polymer nanocomposites have received an enormous attention owing to their ability to solve both energy and environmental problems. Polyvinylpyrrolidone (PVP) is one of the water-soluble low-cost polymers that possess non-ionic and nontoxic properties with pore-forming macromolecules, good film forming stability, and appealing chemical properties [2]. PVP is widely used in the design of materials for various applications. Nanocomposite technology based on nanofillers such as graphene derivatives like graphene oxide (GO), and reduced graphene oxide (rGO) has already been considered to be an effective method for producing novel materials with excellent properties and high performance [3]. Because of the presence of oxygen functional groups on its basal planes and edges, GO is thought to be a functionalized graphene [4].The functional groups in GO permit non-covalent and covalent chemical functionalization, which is particularly useful in the formation of nanocomposites [5]. Interestingly, GO has gotten a lot of attention because of its ability to function as a precursor to some conductive materials [6-8]. More specifically, because of the high compatibility of PVP and GO and their good miscibility in water, the growth of films from such components is expected to be of great interest [3]. Nonetheless, despite recent advances in polymer graphene oxide nanocomposites, incorporation of metals or metal oxides, carbon nanomaterials, and polymers into the polymer matrix has been sought to improve their properties because of synergistic effects [9,10]. These hybrid nanocomposites also help to minimize some of the drawbacks experienced in polymer based devises like low chemical stability and mechanical properties [11,12]. Because of its low cost, availability, potency, electrochemical properties, good anti-interference potential, and processability into nanosized material, copper is an intriguing metal choice. Copper, in particular, has been used in solar cells and electronic materials, making it very appealing to researchers [13]. In this study, PVP polymer, copper nanoparticles and graphene oxide (GO) nanoparticles were incorporated together for the electrochemical deposition of ternary nanocomposite PVP/Cu/GO films. The current study sought to ascertain the effect of GO addition on the optical, morphology, and structural properties of PVP/Cu/GO nanocomposite films.
2. Experimental section
2.1. Chemicals and materials
In this study, we used analytical grade polyvinylpyrrolidone (PVP), copper sulfate pentahydrate (CuSO4.5H2O), graphite powder; sulphuric acid (H2SO4), potassium permanganate (KMnO4) and fluorine doped tin oxide (FTO) substrate. These chemicals were used as received from commercial suppliers with no additional treatment, while deionized water (DIW) was utilized throughout the experiment.
2.2. Synthesis of PVP/Cu/GO nanocomposite
Graphene oxide (GO) was first synthesized from natural graphite using a modified Hummers procedure as described in the literature [14]. Then, 0.5 g/mol of synthesized GO nanoparticle was dissolved in 100 mL of DIW and stirred for 1 hour in a beaker with a magnetic stirrer to obtain a homogeneous GO aqueous solution, which was used to prepare PVP/Cu/GO nanocomposites. Following that, a 0.01 M PVP solution was made by dissolving 0.11 g/mole in 100 mL of DIW and stirring for 1 hour. Similarly, 1.25 g/mol CuSO4.5H2O (0.01 M) solution was made by dissolving it in 500 mL of DIW. 15 mL of 0.01 M PVP and 10 mL of 0.01 M Cu were filled in a beaker and stirred for 2 hours to obtain homogeneous PVP and Cu dispersions. Finally, the already prepared GO aqueous solution was incorporated into the PVP and Cu solution and stirred for 2 h using a magnetic stirrer to obtain homogeneous PVP/Cu/GO solutions. The GO content was varied to 0.0, 0.4, 0.6 and 0.8 wt% in relation to the amount of PVP and Cu solution. An electrochemical deposition method was employed to form the nanocomposites while the PVP/Cu/GO solutions served as the electrolyte. fluorine doped tin oxide (FTO) substrate used for the deposition were cleaned and clipped in between two-electrode cell while a large-area platinum sheet acts as the counter electrode and a pseudo-reference electrode. The substrates were then immersed in the electrolyte for 60 seconds at a constant voltage of 15 volts in an electrochemical setup. The current and potential drop passing through the films were assessed with the aid of a sensitive ammeter and a voltmeter. Finally, composite-coated glasses were obtained and washed with deionized water after electrodeposition to remove any unreacted substances before being dried at room temperature.
2.3. Characterizations
Some spectroscopic methods were used in analyzing the optical, structural, morphological and compositional properties of the synthesized nanocomposite films. They include; UV-1800 visible spectrophotometer in the wavelength range of 300-1100 nm, Bruker D8 Advance XRD with a Cu K line (λ = 1.54056 Å) in 2theta ranges from 10° to 90°, SEM, EDX, while the electrical properties of the nanocomposite films were studied using the Jandel four-point probes technique (model T345).
3. Results and discussion
3.1. Optical properties of PVP/Cu/GO nanocomposites
The interaction of photon energy with the surface of a material results to absorption, transmission and reflection [15,16]. These three properties are related by Eq. (1). However, the understanding of transmittance aids in determining the energy band gap and band structure of materials.
where A absorbance, T transmittance, and R reflectance.
Absorbance however is the negative logarithm to base 10 of the transmittance,
It follows from Eq. (2) that the transmittance and absorbance are related by
Figure 1a) illustrates the dependence of the absorbance on the wavelength of the PVP/Cu/GO films in the range 300-1100 nm. According to the diagram, all of the films have nearly the same absorbance spectral, with the highest absorbance in the UV region of ≈ 450 nm, but decreased significantly as they moved into the NIR region of the spectrum. This behavior is attributed to the possible interactions that exist between the GO and long aromatic rings of the polymer chain [17], which could be responsible for the improvement of light absorption of the films in the UV-Vis region, thereby enhancing some photovoltaic properties such as photocatalytic efficiency and photoluminescence of the films. However, as the GO loading increases, the absorption remains relatively constant. The transmittance T versus wavelength plots as calculated from Eq. (3) indicates that the PVP/Cu/0.0% shows transmittance of ≈ 30% which increased with increasing GO loading from (0.4% to 0.8%) to about 70% as revealed in Fig. 1b). Nonetheless, all the films have low transmittance (≈ 400 nm) in the UV region of the electromagnetic spectrum, which increases with increasing wavelength up to the NIR region. The low transmissions of the films reflect the change observed in the optical band gap, which was a consequence of the change in the band structure of PVP matrix. The films’ low transmittance makes them useful in solar thermal energy collectors and active layers for solar cell applications [18], thus, these absorbance and transmittance results suggest the potential application of the nanocomposite in energy-related devices. Figure 1c) shows that the films’ reflectance R is generally low across the electromagnetic spectrum; an all-out of 20% in the UV region.
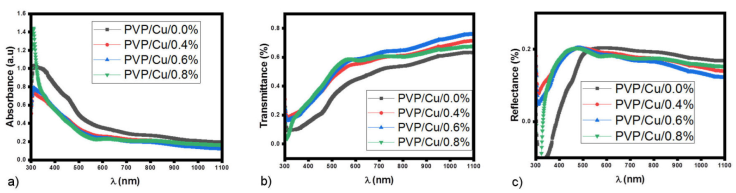
Figure 1 a) absorption spectra versus wavelength, b) transmittance versus wavelength, and c) reflectance versus wavelength plots of PVP/Cu/GO nanocomposites with varying GO loadings.
The refractive index n is the ratio of radiation speed between two media; this property aids in the evaluation of a material for use in anti-reflective coatings for solar cells. It is related with the optical reflectance according to Eq. (4) [19,20].
where n refractive index and R reflectance,
where α absorption coefficient and λ wavelength.
The variation of the refractive index of the films with the photon energy as shown in Fig. 2a), exhibits a non-linear feature which
indicates a departure from the films normal dispersion behavior hence, for all
films, high values of refractive index are observed which indicates that the
films deposited are of high density which consequently lower the inter-atomic
spacing as was observed from the XRD results [21]. Owing to the high values of the refractive index of the
deposited films, they can be utilized in the design of optical waveguides [22]. The extinction coefficient
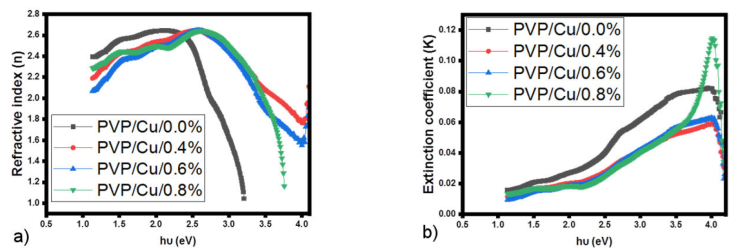
Figure 2 a) Refractive Index versus hv and b) Extinction Coefficient versus hv plots of PVP/Cu/GO nanocomposites with varying GO loading.
To get an insight about the optical shift which occurs when a valence electron migrates to the conduction band of a material, we explore the correlation between the absorption coefficient α and the photon energy hv [24,25]. When a photon of known energy in transmitted radiation is absorbed, an electron moves from the material’s valence to conduction band. Changes in transmitted radiation provide information about the types of electron transitions that may occur. The excited - state transition is known as fundamental or basic absorption, and it is characterized by a rapid increase in absorption known as the absorption edge, which is used to calculate the optical band gap from [27] Eq. (6).
where E g band gap energy, α absorption coefficient, B constant which is dependent on the sample structure, v frequency of the incident light and h Planck’s constant.
For direct band gap materials, Eq. (6) determines the absorption coefficient and photon energy [26]. However, the absorption coefficient, the absorbance and the films’ thickness d are related by Eq. (7).
where α absorption coefficient, A absorbance and d films thickness.
Nonetheless, the optical band gap of pure PVP has been reported elsewhere as 3.88 eV [28].
Hence, in the present study, the optical band gap of PVP/Cu/0.0% nanocomposites before
incorporation of GO was found to be 3.51 eV, as shown in Fig. 3a). This reduction in optical band gap of the
PVP/Cu/0.0% can be attributed to the presence of chemical bonding that exists
between the PVP long aromatic rings and Cu nanoparticles. This behavior shows
that Cu nanoparticles will influence more localized energy states which will
control the optical and electrical characteristics and hence modify the band
structure of PVP matrix. However, upon interaction with GO at various loadings
(0.4% to 0.8%), the direct band gap narrowed to a value of 3.51 to 2.02 eV. This
result indicates that the introduction of the GO further resulted to addition of
energy levels inside the band structure of the films, and consequently reduced
the optical band gap. Thus, by simply changing the GO content, the optical band
gap of the nanocomposites can be controlled. The optical band gap
(E
g
) values of the films are obtained by extrapolating the linear region of
the
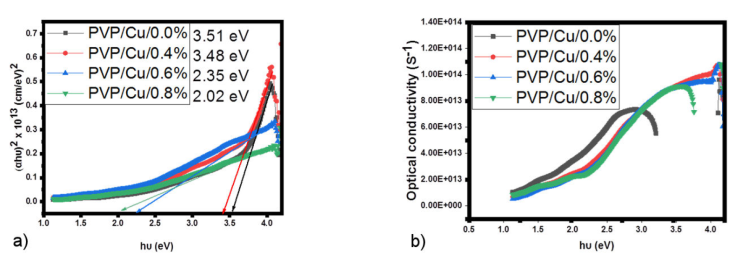
Figure 3 a) (αhv)2 versus hv b) optical conductivity versus hv plots of PVP/Cu/GO nanocomposites with varying GO loading.
Figure 3b) shows an increasing trend for the optical conductivity of all the films by an increasing photon energy, resulting in an increase in the films’ conductivity which suggests that they have good photo-response and could be used as photoconductors [31]. All the films show the same trend within the UV region of the spectrum where the films exhibit optimum optical conductivity. These types of materials are thought to be favorable for various optoelectronic device applications [32].
A material’s dielectric constant ε is a parameter that is used to estimate the degree to which the speed of light is shaded off throughout the material and how much energy is absorbed by a dielectric material due to dipole movement in an electric field [33]. It is shown in Eq. (9). Hence, it turns out that the dielectric constant is of two parts namely; the real ε r and imaginary ε i parts as shown in Eq. (9-12) [34].
where n refractive index, K extinction coefficient, ε r real part that represents the degree of polarization, as the degree of polarization increases, so does ε r . ε i imaginary part which represent dielectric losses.
The dielectric constant is also frequency dependent; in other words, frequency affects the value of the dielectric constant because the polarization mechanisms are unable to keep up with the rapidly changing field, and increasing frequency decreases the value. Plots of the real (RDC) and imaginary (IDC) dielectric constants versus photon energy of the PVP/Cu/GO nanocomposite films are shown in Fig. 4a) and 4b). From the plots, it is evident that the real dielectric possesses much higher values than the imaginary dielectric as all the films show similar pattern in RDC while increasing with a rise in photon energy to a value above 2.2 eV and drastically experiences a drop at higher photon energy values. Meanwhile, the imaginary dielectric from Fig. 4a) exhibits a near linear relationship with photon energy, increasing with increasing photon energy above 2.7 eV for all films. In general, this characteristic suggests a switching capability of the deposited PVP/Cu/GO film materials, and also shows that PVP/Cu/GO nanocomposites can serve as photovoltaic devices.
3.2. X-ray diffraction
X-ray diffraction (XRD) measurements were used to examine the structure of the PVP/Cu/GO ternary nanocomposite films and the synthesized GO. The X-ray diffraction pattern was produced at a (λ = 1.54056) in the 2 theta degree range from 100 - 900. Figure 5a) depicts the XRD of the synthesized GO which shows a strong peak at 2θ = 10.21, corresponding to the miller index (002) reflection peak. As reported in the literature [35], GO has its highest intensity peak at 2 theta between 10-120; thus, the X-ray diffraction pattern confirmed the successful formation of GO. Figure 5b) depicts the XRD curves of the various PVP/Cu/GO films. The plots show that all of the films’ characteristic peaks occurred at the same reflection angles. Only typical CuO peaks are visible at 2θ = 27.10, 34.50, 38.20, 52.30, 62.20 and 67.40, which are indexed at (101), (211), (111), (200), (220), and (311), all of which belong to the monoclinic phase of CuO in the JCPDS No 45-0937. These findings suggest that during the film formation process, the Cu nanoparticles may have interacted with the oxygen in the reacting environment, resulting in copper oxidation and the formation of CuO [22]. The lack of a GO diffraction peak in the XRD pattern indicates that the GO sheets are uniformly distributed in the nanocomposites with negligible aggregation [36]. The observed sharp peaks indicate that the synthesized films have good crystallinity [37]. The size of the crystallinity was calculated using Scherrer’s equation [38-42].
where β is the full width at half maximum (FWHM) of the most strong diffraction peak, and λ wavelength of incident radiation (1.54056). The synthesized nanocomposite has a crystallinity size of 7 nm.
3.3. Morphology study
Using scanning electron microscopy images, surface morphological studies of various PVP/Cu/GO films with varying GO loading were investigated (SEM). Figure 6 compares the morphological characteristics of films. Figure 6a) shows that the PVP/Cu/0.0% nanocomposite had spherical shapes and homogeneous particle distributions due to the compatibility interface between the PVP matrix and the Cu nanoparticles. However, as shown in Figure 6b)-d), the surface morphology of the PVP/Cu/GO nanocomposites has changed significantly after the addition of GO at various loadings (0.4%, 0.6%, and 0.8%), resulting in relatively large nano fine-grained grains; this behavior can confirm the required conductivity for the nanocomposite films [43].
3.4. EDX analysis
Figure 7 is a representative spectrum of the ternary PVP/Cu/GO nanocomposite with a graphene oxide load of 0.8%, similar spectra were obtained at other graphene oxide loads. The figure depicts the elemental compositions of the PVP/Cu/GO nanocomposite films. The graph clearly shows that the films contain the expected elements of carbon, nitrogen, copper, and oxygen as the main constituents of the deposited films. The EDX result validates the synthesis of a new PVP/Cu/GO nanocomposite.
3.5. Electrical properties of PVP/Cu/GO nanocomposite
The electrical conductivity level of the PVP/Cu/GO nanocomposite films was estimated using a four-point probe system. The particles nanostructure of a nanocomposite film plays an important role in determining its electrical characteristics [44-46]. To ensure good ohmic contact with the films, the voltage across the transverse distance of the films and the corresponding current values were measured using silver paste in a typical setup. The resistance values of the deposited films were investigated. The four point probes were linked to a current supply, while the inner probes were linked to a voltmeter. The voltage drop across the probes is measured as current flows between the outer probes. The thickness, resistivity, and conductivity values of PVP/GO/Cu films are summarized in Table I. The results show that the material deposited with different GO loading increases in thickness from 102.02 to105.02 nm with a corresponding decrease in resistivity from
Table I Electrical measurements of PVP/Cu/GO nanocomposite
| Samples | Thickness t (nm) | Conductivity σ(Ωm/cm)-1 | Resistivity ρ(Ωcm) |
|---|---|---|---|
| PVP/Cu/0.0% | 102.02 | 8.711 × 10-8 | 1.147 × 10-9 |
| PVP/Cu/0.4% | 104.02 | 8.729 × 10-8 | 1.145 × 10-9 |
| PVP/Cu/0.6% | 105.01 | 8.734 × 10-8 | 1.144 × 10-9 |
| PVP/Cu/0.8% | 105.02 | 8.752 × 10-8 | 1.142 × 10-9 |
4. Conclusions
The electrochemical deposition method was successfully utilized for the synthesis of polymer, copper, graphene oxide nanocomposites (PVP/Cu/GO) with different GO loading. Various complementary techniques; UV-Vis, XRD, SEM, EDX, and a four-point probe were used to investigate the effect of GO loading on the optical, structural, morphological, compositional, and electrical properties of the different films for applications in photovoltaic devices. It was observed that the GO loading reduced the optical band gap of the nanocomposites from 3.51 to 2.02 eV. The optical results also showed highest absorbance in the UV region ≈ 450 nm. The XRD pattern reveals that the deposited films were crystalline in nature, exhibiting major reflection peaks at (101), (211), (111), (200), (220) and (311) lattice planes. With increase in GO loading, the SEM micrograph showed relatively large nano fine-grained grains indicating uniform deposition. The presence of carbon, nitrogen, copper, and oxygen as major elemental constituents of the films was confirmed by the EDX results. The four point probe results revealed that the GO content improved the nanocomposite’s conductivity thereby confirming the semiconducting nature of the films. Owing to these results, the PVP/Cu/GO nanocomposite has great potential in photovoltaic applications.











 nueva página del texto (beta)
nueva página del texto (beta)






